
Feasibility and Usability of Wearable Devices for Ambulatory
Monitoring of the Rehabilitation Process of Older Patients after Hip
Fracture Surgery
Dieuwke van Dartel
1,2 a
, Johannes H. Hegeman
1,2
and Miriam M. R. Vollenbroek-Hutten
1,3
1
Biomedical Signals and Systems Group, University of Twente, Enschede, The Netherlands
2
Department of Trauma Surgery, Ziekenhuisgroep Twente, Almelo, The Netherlands
3
ZGT Academy, Ziekenhuisgroep Twente, Almelo, The Netherlands
Keywords: Wearable Devices, Ambulatory Monitoring, Fitbit, MOX, Feasibility, Usability, Older Patients, Hip Fracture.
Abstract: Objective: To assess the feasibility and usability of wearable devices for ambulatory monitoring of older
patients during geriatric rehabilitation after hip fracture surgery.
Methods: Patients (≥70 years) who were surgically treated for a hip fracture wore the Fitbit Charge 2/HR and
the MOX device. Feasibility was assessed by investigating whether real world data gathering revealed
sufficient high-quality data. Usability was assessed by 1) evaluating whether changes in the device parameters
correlated with changes in clinimetric tests and 2) determining whether the wearable devices properly
measured activity.
Results: Data from 67 patients was used to assess feasibility; all patients wore the Fitbit and 33 the MOX.
The mean amount of high-quality data was 88.1% for the Fitbit and 93.6% for the MOX. Data from 42 patients
was used to assess usability; all patients wore the Fitbit and 14 the MOX. A positive progression in clinimetric
tests was correlated with an increase in activity parameters. However, the Fitbit often miscalculated the
number of steps and the MOX algorithm often misclassified slow walking as standing.
Conclusions: Ambulatory monitoring using the Fitbit and MOX is feasible in older patients with a hip fracture.
Concerning the usability, the Fitbit often miscalculated the number of steps. The MOX was more adequate
but the activity classification algorithm often misclassified slow walking based on which it is recommended
to use the raw data instead.
1 INTRODUCTION
The ultimate goal of hip fracture treatment in older
patients is functional recovery, which is defined as the
patient regaining the premorbid level of functioning
(Ceder, 2005; Folbert et al., 2011; Zuckerman, 1996).
To achieve this, adequate post-operative
rehabilitation during and after the patient’s hospital
stay is essential (Prestmo et al., 2015). Clinimetric
tests are often used to obtain insight into the patient’s
progress during rehabilitation. These tests assess the
patient’s physical function, mobility, and cognitive
impairment. Scientific studies have used clinimetric
tests to provide insight into the rehabilitation process
and identify predictive factors for a positive outcome.
However, although clinimetrics provide helpful
a
https://orcid.org/0000-0002-3556-4522
information, they are also static and administered
infrequently. Furthermore, it is not always possible to
perform a clinimetric test, as patients need a certain
level of mobility (Benzinger et al., 2014;
Hershkovitz, Beloosesky, & Brill, 2012; Nygard,
Matre, & Fevang, 2016). As a result, important
information about patient recovery during
rehabilitation might be missed, with the consequence
that treatment is not adjusted at the right time and
recovery is suboptimal. Therefore, there is a need for
a better, continuous, and accurate way to monitor
older hip fracture patients during rehabilitation.
One possible solution is the use of wearable
devices. Wearable devices are small, portable, body-
fixed sensors that can be used for continuous
ambulatory monitoring of bodily signals. In the case
of hip fracture rehabilitation, most ambulatory
van Dartel, D., Hegeman, J. and Vollenbroek-Hutten, M.
Feasibility and Usability of Wearable Devices for Ambulatory Monitoring of the Rehabilitation Process of Older Patients after Hip Fracture Surgery.
DOI: 10.5220/0010522500590066
In Proceedings of the 18th International Conference on Wireless Networks and Mobile Systems (WINSYS 2021), pages 59-66
ISBN: 978-989-758-529-6
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
59

monitoring is performed in the physical activity
domain. Some scientific studies of the rehabilitation
process in older hip fracture patients have already
investigated the added value of physical activity
monitoring. However, these studies monitored
patients for only a few days during hospital stay or
rehabilitation instead of continuously throughout the
whole rehabilitation process, which lasts for several
weeks (Bakker, Blokhuis, Meeks, Hermens, &
Holtslag, 2014; Benzinger et al., 2014; Davenport et
al., 2015; Fleig et al., 2016; Keppler et al., 2020;
Schmal et al., 2018; Talkowski, Lenze, Munin,
Harrison, & Brach, 2009; Taylor, Peiris, Kennedy, &
Shields, 2016). Other studies measured physical
activity in older patients who returned to the
community setting, but again only for a few days
(Fleig et al., 2016; Resnick et al., 2011; Taraldsen et
al., 2015). There is limited information on whether it
is feasible or useful to monitor patients throughout the
entire rehabilitation period using wearable devices.
Armitage et al. recently assessed the feasibility
and acceptability of an activity tracker worn as a
pendant for the continuous monitoring of older
patients (Armitage et al., 2020). In that study, patients
discharged to their home after surgery and patients
temporarily discharged for geriatric rehabilitation
were continuously monitored for 16 weeks. Results
showed that the activity tracker was able to monitor
meaningful activity data. However, many patients
were unwilling to wear it and, therefore, patient
recruitment and retention was low. Therefore, the aim
of this study was to assess the feasibility and usability
of wrist-worn and thigh-worn wearable devices for
the continuous monitoring of older patients during the
entire rehabilitation period after hip fracture surgery.
Feasibility will be assessed by determining whether
real world data gathering revealed sufficient high-
quality data to monitor a patient’s rehabilitation
progression. Usability will be assessed by 1)
evaluating whether changes measured with wearable
devices correlate with changes in the standard
clinimetric tests and 2) by determining whether the
wearable devices properly assess different activities
by comparing recorded data with observations made
during therapy sessions.
2 METHODS
2.1 Subjects
This study included patients aged 70 years or older
who received surgery for their hip fracture at the
department of Trauma Surgery in Ziekenhuisgroep
Twente (ZGT). Patients with severe cognitive
impairment, total hip replacement, a pathological or
periprosthetic fracture, terminal illness, or contact
isolation were excluded. Where possible, patients
were enrolled in the study one day post-surgery; if not
possible, inclusion took place one day before the
patient was discharged for rehabilitation to one of the
three collaborating skilled nursing homes
(TriviumMeulenbeltZorg, Carintreggeland, and
ZorgAccent). If an included patient was not admitted
to one of three collaborating nursing homes,
measurements were only taken during the hospital
stay. This study was part of the “Up&Go after a hip
fracture” project. All patients gave written informed
consent to participate. This study was approved by the
ethical review committee of ZGT and the Medical
Research Ethics Committee Twente.
2.2 Continuous Monitoring
Patients were continuously monitored during their
entire hospital stay and/or rehabilitation stay at the
nursing home. We initially started monitoring
patients with the Fitbit Charge 2 / HR (Fitbit Inc., San
Francisco, CA, USA), which are wrist-worn
community-based activity trackers that were
preferably placed on the patient’s non-dominant
wrist. The Fitbit contains a 3D-accelerometer and
photoplethysmography in order to measure the
number of steps a patient takes and the patient’s heart
rate, respectively. The Fitbit was connected via
Bluetooth to the Fitbit App on a mobile phone to
access the step count and heart rate data.
After a few months of the study, we began
monitoring any newly enrolled patient with a MOX
device in addition to a Fitbit, since the Fitbit is not
able to monitor time spent in different postures. The
MOX (model MMOXX1) is a small, single-unit,
dust- and waterproof device (35x35x10mm) that
continuously monitors physical activity throughout
the day (Maastricht Instruments BV, the
Netherlands). The MOX contains a 3D-
accelerometer, has a sample frequency of 25 Hz, and
was attached to the anterior thigh, 10 cm above the
knee of the fractured leg, with a plaster. We used the
IDEEQ software provided by Maastricht Instrument
BV to download the raw acceleration data from the
MOX and convert it into continuous activity data, i.e.
the number of active minutes (walking) and the
number of sedentary minutes (sitting and lying).
2.3 Assessment of Feasibility
Feasibility was assessed by calculating the amount of
WINSYS 2021 - 18th International Conference on Wireless Networks and Mobile Systems
60

high-quality data that was available when older hip
fracture patients were continuously monitored. For
each patient, we first calculated the amount of
missing data for the MOX and Fitbit during daytime
(7.00 am to 10.00 pm) by calculating the number of
missing minutes for each hour. There are no
guidelines in the literature for how to handle missing
data from these devices, so based on our best
judgement, we considered an hour as “missing” if
more than 10 minutes of data were missing. When
more than three hours were missing on a given day,
we considered the day as a missing day. Based on the
number of missing days, we then calculated the
percentage of available data for each patient and the
mean percentage across all patients. The first and last
day of the measurement period were excluded for all
patients because these days were not full
measurement days. Data was analysed with
MATLAB R2017b (MathWorks, Natick, MA, USA).
2.4 Assessment of Usability
Usability was assessed by determining whether the
changes in activity parameters correlated with
changes measured in clinimetric tests, which are
considered the gold standard for evaluating patient
recovery. For this part of the study, we only used data
of the patients monitored during rehabilitation at the
nursing home. For each patient the number of active
minutes, the number of sedentary minutes, and the
number of steps per day were calculated. The
parameters were then used in linear regression, with
time as a dependent variable, to calculate the slope.
The slope was used to determine if the patient’s
progression was positive or negative for each activity
parameter. “Positive progression” was defined as
cases where the number of active minutes and the
number of daily steps have a positive slope and the
number of sedentary minutes a negative slope.
Activity progression was compared to results
from the following clinimetric tests: Timed Up and
Go test (TUG), 10 Meter Walk Test (10MWT),
Functional Ambulation Categories (FAC), Katz
Index of Independence in Activities of Daily Living
(Katz-ADL) and Barthel Index (BI).
The TUG and 10MWT are both functional
capacity tests. For the TUG patients were instructed
to stand up from a chair, walk three meters, turn
around, walk back to the chair, and sit down again.
The time (in seconds) that it took to perform the test
was used as an outcome measure. For the 10MWT the
patient’s gait speed (m/sec) was assessed over a 10-
meter distance and used as an outcome measure.
The FAC, Katz-ADL and BI are functional perfor-
mance tests. The FAC assessed the patient’s ability to
walk and ranged from 0 (not functionally able to
walk) to 5 (walk independently). The TUG and
10MWT tests could only be performed with FAC 3.
The Katz-ADL and BI assessed the patient’s
independence in activities of daily living (ADL). The
Katz-ADL ranged from 0 (completely independent)
to 6 (completely dependent) and the BI from 0
(completely dependent) – 20 (completely
independent).
To calculate patient progression for each
clinimetric test, we calculated the difference between
the test score obtained at discharge from the
rehabilitation department and the test score obtained
at admission to the rehabilitation department.
Differences were expressed as a percentage of the
initial (admission) score, resulting in measurements
for TUG, 10MWT, FAC, Katz-ADL, and BI.
A patient exhibited a “positive progression” during
rehabilitation if 10MWT, FAC, and BI were
positive and TUG and Katz-ADL were negative.
Subsequently, we calculated Pearson’s or Spearman’s
correlation coefficient between the slope of the
activity parameters over time and TUG, 10MWT,
FAC, Katz-ADL, and BI to assess how well
results from continuous sensors correlated with
results from clinimetric tests.
To assess whether the Fitbit and the MOX
correctly identified patient activity as “activity” a
researcher observed weekly therapy sessions at the
rehabilitation department, with 10 patients observed
for a total of 37 sessions. The observer noted the start
and end time for each activity (sitting, standing, and
walking) and manually counted the number of steps
when patients were walking. Results from these direct
observations were compared with the activity-data
logged by the Fitbit and the MOX. Deviations
between the observed and the monitored values were
expressed as percentages.
3 RESULTS
3.1 Subjects
A total of 86 patients were enrolled in this study. Of
these patients, 19 did not complete the study; reasons
for non-completion included problems with
synchronizing the Fitbit (n=6), choosing not to
complete the study (n=3), not wearing the Fitbit
(n=2), discomfort of the Fitbit (n=1), an allergic
reaction to the MOX plaster (n=1), overall decline in
health status (n=1), contact isolation (n=1), death
Feasibility and Usability of Wearable Devices for Ambulatory Monitoring of the Rehabilitation Process of Older Patients after Hip Fracture
Surgery
61
during rehabilitation (n=1), or unknown reasons
(n=3).
The sensor data from the remaining 67 patients
was used to assess the feasibility of the sensors. All
67 patients wore the Fitbit. The median measurement
period was 24 days (min: 2 days, max: 75 days).
Because the MOX measurements were added later in
the study, only 33 of the 67 patients also wore the
MOX. The median measurement period was 6 days
(min: 2 days, max: 75 days).
Data from 42 of the 67 patients was used to assess
the usability of the wearable sensors. All 42 patients
wore the Fitbit, and 14 of these patients also wore the
MOX. The median measurement period was 29 days
(min: 11 days, max: 71 days) and 27 days (min: 11
days, max 67 days) for the Fitbit and MOX,
respectively. The mean age of the 42 patients was 82
6 years, and 83% of the patients were female. Prior
to the hip fracture, 69% of the patients lived
independent and 57% of the patients were able to
walk independently. The mean age of the ten patients
whose therapy sessions were observed by a researcher
was 83 ± 3 years, and 70% of the observed patients
were female.
3.2 Feasibility
The percentage of available data varied among
patients, with a maximum of 100% data availability
for both the Fitbit and the MOX and a minimum of
20% data availability for the Fitbit and 74% for the
MOX (Figure 1). The mean percentage of available
data across all patients were 88.1% and 93.6% for the
Fitbit and MOX, respectively. Data availability was
more variable among patients monitored with the
Fitbit compared with the MOX (Figure 1).
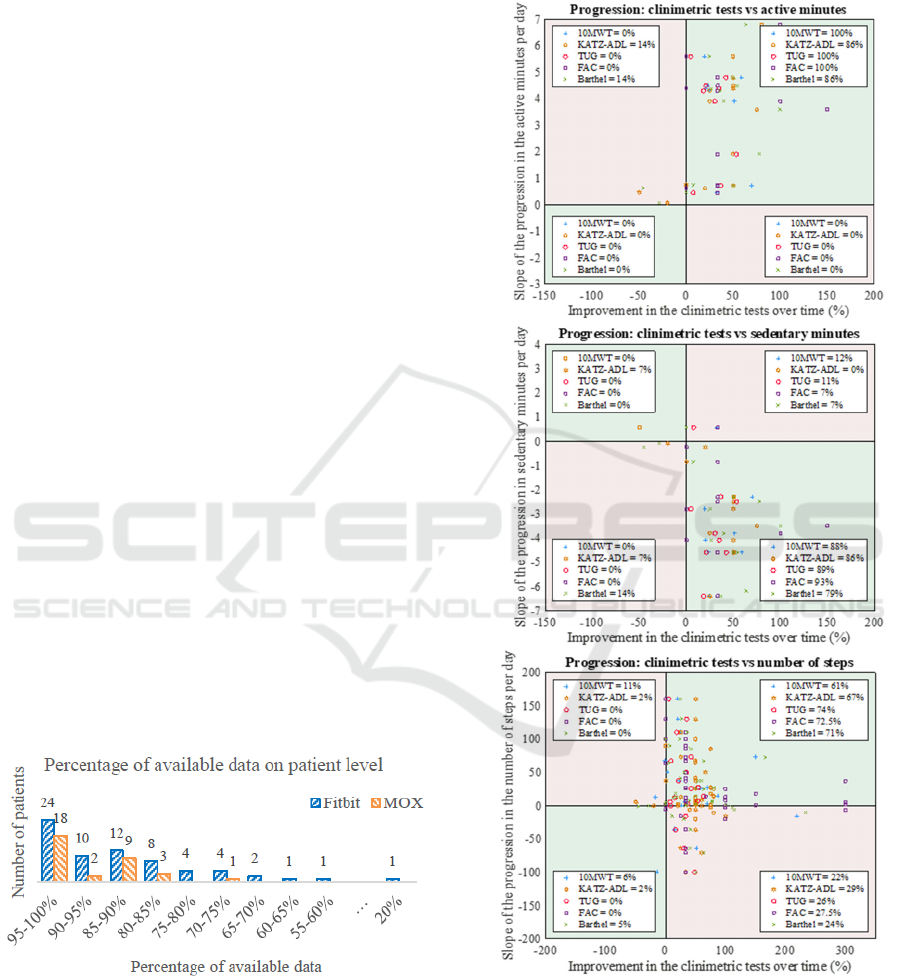
Figure 1: The percentage of available data for each patient
for the Fitbit and the MOX.
3.3 Usability
Results show that most patients show a positive
progression throughout their rehabilitation, measured
both with the clinimetric tests as well as with the
activity parameters. However, 10MWT is missing
for 57% of the patients and TUG for 55%.
Figure 2: This figure provides a scatterplot for each activity
parameter, in which the slope of the activity parameter is
compared against the patient’s progression in clinimetric
tests. The clinimetric data is standardized so that the
progression is shown for all the clinimetric tests. The legend
in every quarter shows the percentage of patients within that
plane.
WINSYS 2021 - 18th International Conference on Wireless Networks and Mobile Systems
62
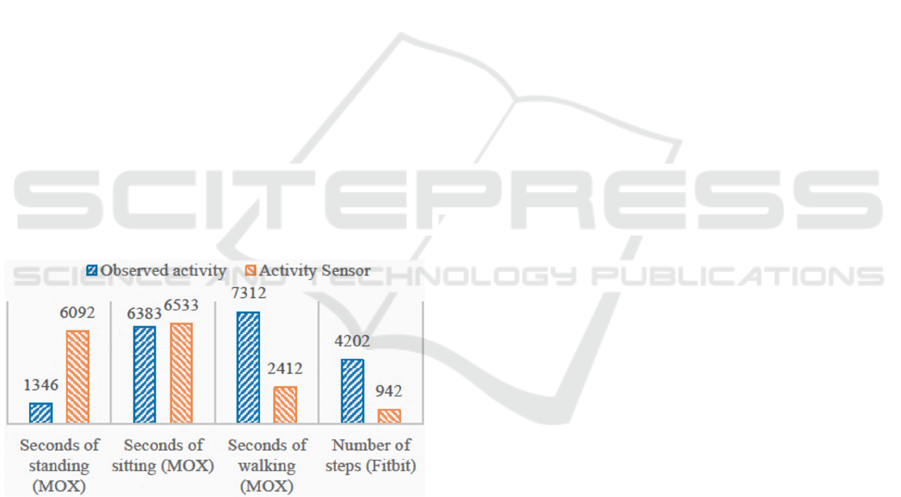
Figure 2 presents the scatterplots to compare each
patient’s progression in the clinimetric tests with their
progression in the activity parameters. In each plot,
each point represents a patient’s result. Different
point shapes represent the different clinimetric tests.
Each scatterplot is divided into four quarters; patients
(points) in the green quarters show the same
progression in their activity parameter as in their
clinimetric tests. The pink quarters represent those
patients with discrepancies between activity
parameter and clinimetric tests.
For most patients, the physical activity parameters
show the same progression as the clinimetric tests.
However, approximately 25% of the patients show a
decrease in the number of steps even though the
clinimetric tests indicate a positive progress.
Results from the correlation tests show that for
ΔKatz-ADL there is a moderate negative correlation
with the slope of the number of active minutes (r = -
0.66, p < 0.05, n=14) and a moderate positive
correlation with the slope of the number of sedentary
minutes (r = 0.67, p < 0.01, n=14). For ΔBI it shows
that there is a moderate positive correlation with the
slope of the number of active minutes (r = 0.54, p <
0.05, n=14) and a moderate negative correlation with
the slope of the number of sedentary minutes (r = -
0.57, p < 0.05, n=14). No other significant correlations
were found between the changes in activity parameter
and changes in clinimetric tests (Table 1).
Figure 3: This figure compares the observed activity of
patients during their therapy sessions and the activity
measured by the Fitbit and MOX. For each measure, the
bars show the sum of the activity measure over all observed
therapy sessions.
Figure 3 shows how well the activities are
measured and recorded by the Fitbit and the MOX
compared to direct observations of patient activity.
Data from 24 of the 37 observed therapy sessions
were used to evaluate the accuracy of the Fitbit’s
measured number of steps. Not all therapy sessions
were used since some sessions did not contain proper
step count observations. A total number of 4,202
steps were observed by the researcher across all 24
sessions; however, the Fitbit only counted 942 steps
(Figure 3), which means that only 22.4% of the
observed number of steps were correctly measured by
the Fitbit. The Fitbit generally counted too few steps
when patients were walking with a walker and too
many when patients were moving around in a
wheelchair.
Data from 33 of the 37 observed therapy sessions
were used to compare the activity measured with the
MOX to observed activity. During these sessions, the
researcher observed a total of 6,383 seconds of
sitting, 1,346 seconds of standing, and 7,312 seconds
of walking, whereas the MOX measured 6,533,
6,092, and 2,412 seconds of sitting, standing, and
walking, respectively (Figure 3). This means that the
MOX overestimated the amount of time spent sitting
and standing by 2.3% and 352.6%, respectively, and
underestimated the seconds of walking, as only 33%
of the observed second
s of walking were also
measured by the MOX.
4 DISCUSSION
The aim of this study was to assess the feasibility and
usability of the Fitbit Charge and the MOX for
continuously monitoring of the physical activity of
older patients throughout their rehabilitation period
after hip fracture surgery. We found that 78% of the
patients adhere to the sensors and approximately 88%
and 94% high quality data was available for the Fitbit
and MOX measurements, respectively. This suggests
that it is feasible to use wearable devices for long-
term monitoring and that these devices record enough
data to obtain insight in a patient’s progression during
rehabilitation. We also found that the clinical
progression measured using the sensor parameters
was generally the same as the progression measured
with the standard clinimetric tests, suggesting that the
data produced by the Fitbit and MOX is also usable.
However, the Fitbit was not always able to properly
count the number of steps, especially in patients using
a wheelchair or walking aids, and the IDEEQ
software for analysing the MOX data often classified
slow walking as standing.
4.1 Feasibility
Patients in our study were generally open to wearing
the Fitbit and the MOX sensors and wore them
correctly. Similar results were found by O’Brien et al.
who also showed a high acceptability of a wristband
activity tracker in older adults (O'brien, Troutman-
Feasibility and Usability of Wearable Devices for Ambulatory Monitoring of the Rehabilitation Process of Older Patients after Hip Fracture
Surgery
63
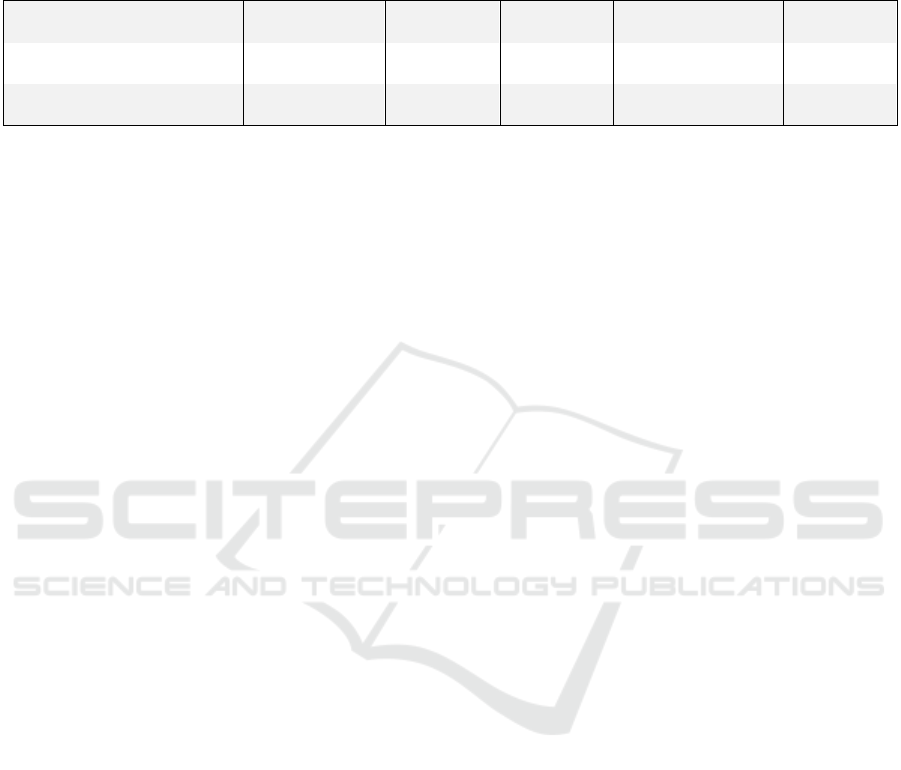
Table 1: Correlation coefficients from the different correlation tests, which tested whether the slope of the activity parameters
of the Fitbit and the MOX were correlated with the patient’s progression in clinimetric tests. The MOX parameters (slope of
the active minutes and slope of the sedentary minutes) were compared to clinimetric tests using Spearman’s correlation. The
slope of the number of steps was tested using Pearson’s correlation with the exception of the comparison with TUG.
10MWT
TUG
FAC
KATZ-ADL BI
Slope number of steps
r=0.02
p
=0.94
r=0.33
p
=0.18
r=-0.31
p
=0.06
r=0.13
p
=0.42
r=-0.14
p
=0.39
Slope active minutes
r=-0.2
p
=0.63
r=0.33
p
=0.38
r=0.07
p
=0.83
r=-0.66
p<0.05
r=0.54
p<0.05
Slope sedentary minutes
r=0.39
p
=0.35
r=-0.08
p
=0.83
r=-0.28
p
=0.35
r=0.67
p<0.01
r=-0.57
p<0.05
Jordan, Hathaway, Armstrong, & Moore, 2015). In
contrast, Raymond et al. and Armitage et al. found a
low acceptability of the sensors used in their study.
However, Raymond et al. used an activity tracker that
consists of two parts connected via an electrical cable
(PAL2) and Armitage et al. used an activity tracker
worn in a pendant (Armitage et al., 2020; Raymond,
Winter, Jeffs, Soh, & Holland, 2018). Both devices
were no compact sensor, and both have cables, which
could explain the discrepancy with our generally high
rate of devices acceptance.
We found that wearable devices resulted in more
available data than was obtained using clinimetric
tests. It is not possible to obtain clinimetric data from
every patient since some patients lack the mobility
necessary to perform a clinimetric test. This was true
in our study, as TUG and 10MLT were missing for
55% and 57% of the patients, respectively. However,
this was not the case for continuous monitoring with
the Fitbit and the MOX, for which approximately
88% and 94% of the data were available, respectively.
This shows the advantage of ambulant sensing as this
reveals a high amount of data that do provide a clear
insight in the progression of the patient and enables
the detection of deterioration at an earlier stage.
Data availabilty was not 100% in all patients. The
main reason for missing data was due to sensor
charging; the MOX and Fitbit had a battery life of 7
days and 3-7 days, respectively. The Fitbit exhibited
more variability in the amount of available data for
each patient, which could also be due to forgetting to
synchronize the Fitbit, or due to disturbances in the
measurements caused by sweat, movement of the
sensor, no proper contact with the skin, or excessive
pressure on the skin (Allen, 2007; Jo, Lewis, Directo,
Kim, & Dolezal, 2016).
4.2 Usability
Regaining the premorbid level of functioning is the
main goal in the rehabilitation of an older hip fracture
patient (Ceder, 2005; Zuckerman, 1996), and one way
to achieve this is by increasing physical activity.
Correlation tests showed that an improvement on the
Katz-ADL and BI tests was correlated with a positive
progress in the number of active minutes per day and
the number of sedentary minutes per day. This
corresponds with previous studies that have shown
that physically active patients need less time to regain
ADL function, instrumental ADL function, and
mobility (Hardy & Gill, 2005; Talkowski et al., 2009;
Willems, Visschedijk, Balen, & Achterberg, 2017).
The correlations between the activity parameters
of the MOX and the other clinimetric tests showed the
same directional association, indicating that the
general progression recorded by each approach was
the same, though these correlations were not
significant. This lack of significance can probably be
explained by the fact that the TUG and 10MWT
are focused on the physical capacity of a patient, i.e.
what a patient is capable of doing, whereas the MOX
is focused on the patient’s physical activity, i.e. what
a patient actually does. These are two different
aspects, and it could occur that a patient is showing
less physical activity than he/she is capable of doing,
where pain and low motivation are great barriers for
being physically active during hip fracture
rehabilitation (Benzinger et al., 2014; Resnick et al.,
2011; Sims-Gould, Stott-Eveneshen, Fleig,
McAllister, & Ashe, 2017; Talkowski et al., 2009). In
addition, the correlation coefficients were assessed on
the results of only 14 patients for whom both MOX
and clinimetric data was available, which is a very
limited sample size.
There were more discrepancies between the
progression in the number of steps monitored with the
Fitbit and the progression in the clinimetric tests, and
none of the correlations were significant.
Approximately 25% of the patients showed a positive
progression in their clinimetric tests but a negative
progression in their number of steps per day. One
potential cause of this discrepancy is that the Fitbit
was not able to properly count the number of steps in
older patients, as the Fitbit calculated too many steps
when a patient was in a wheelchair and too few steps
when a patient walked with a walker. This
WINSYS 2021 - 18th International Conference on Wireless Networks and Mobile Systems
64

miscalculation likely arises because the Fitbit is worn
around the wrist. Moving around in a wheelchair
results in movement of the wrists, so the Fitbit
incorrectly counts this movement as steps. Walking
with a walker results in no movement of the wrists, so
the Fitbit does not count any steps. Schmal et al.
similarly found that step counts were less accurate in
patients using mobility aids (Schmal et al., 2018). It
is also possible that patients in our study walked too
slowly for the Fitbit to accurately count their steps
(Schmal et al., 2018); Treacy et al. showed that the
Fitbit produced an inaccurate step count relative to
the observed step count in a group of slow-walking
participants with a mean age of 80 years (Treacy et
al., 2017). We therefore suggest using a wearable
device located on the lower extremities to monitor the
physical activity of older patients.
The MOX is one such device that can be located
on the lower extremity. However, the IDEEQ
software associated with the MOX device was also
unable to properly detect activity in older patients.
The algorithm for activity classification by the
IDEEQ software was designed based on the activity
of two patient populations with a mean age of
54.216.8 and 609.9 years old (Annegarn et al.,
2011), which is significantly younger than the mean
age of the patients monitored in our study (70 years).
This could explain why slow walking was considered
as standing, as the threshold for “walking” was set too
high to correctly classify it in an older population.
However, the IDEEQ software also provides raw
acceleration data, which could be used to design case-
specific activity classification algorithms.
More broadly, this study showed that continuous
monitoring has several advantages over traditional
clinimetric tests. First, continuous monitoring is not
prone to the ceiling effects common in clinimetric
tests. A “ceiling effect” occurs when patients reach a
high or maximal score on a clinimetric test at the
beginning of rehabilitation, leaving little room for
further improvement. A second advantage of
continuous monitoring is the lack of “floor effects,”
which arise when patients are not able to perform a
clinimetric test. In this study, we found floor effects
for the TUG and the 10MWT, which caused a high
percentage of missing data at admission. In contrast,
physical activity could be monitored in all patients,
despite their level of functioning. Third, continuous
monitoring provides more in-depth information about
the progression of a patient at all times and is
therefore less prone to selectively measuring on a bad
or good day.
5 CONCLUSIONS
Continuous physical activity monitoring with the use
of the Fitbit and the MOX was feasible for older hip
fracture patients throughout their rehabilitation
program. Older patients were largely willing to wear
these devices, resulting in a high amount of available
data, and the rehabilitation progression indicated by
continuous monitoring of physical activity was
similar to the progression measured with clinimetric
tests. Continuous monitoring also provided
information about the patient’s progression, including
fluctuations between days and trajectories over time,
that could not be obtained from clinimetric tests.
However, the Fitbit was less usable than the MOX in
a population of older patients. Because the Fitbit is
worn around the wrist, it often could not properly
measure the number of steps in patients who used
mobility aids. The MOX did not have these
disadvantages, though we recommend developing a
new algorithm that uses the raw accelerometer data to
correctly classify the activity of older patients, as the
MOX could sometimes identify slow walking as
standing. Further research is needed to optimize valid
parameter extraction from continuous monitoring
devices worn by patients with very low physical
activity levels, like those recovering from hip surgery.
ACKNOWLEDGEMENTS
The Up&Go after a hip fracture group: M.M.R.
Vollenbroek–Hutten, J.H. Hegeman, E.C. Folbert, S.
Woudsma, C. de Pagter, M.M. Kemerink op
Schiphorst, S. Gommers, T. Oude Weernink, A.J.M.
Harperink, A.H.S. Oude Luttikhuis, N. den Braber, C.
Pierik, A. Malki, and D. van Dartel.
REFERENCES
Allen, J. (2007). Photoplethysmography and its application
in clinical physiological measurement. Physiological
measurement, 28(3), R1.
Annegarn, J., Spruit, M. A., Uszko-Lencer, N. H., Vanbelle,
S., Savelberg, H. H., Schols, A. M., Meijer, K. (2011).
Objective physical activity assessment in patients with
chronic organ failure: a validation study of a new
single-unit activity monitor. Archives of physical
medicine and rehabilitation, 92(11), 1852-1857. e1851.
Armitage, L. C., Chi, Y., Santos, M., Lawson, B. K., Areia,
C., Velardo, C., . . . Farmer, A. J. (2020). Monitoring
activity of hip injury patients (MoHIP): A sub-study of
the World Hip Trauma Evaluation observational cohort
Feasibility and Usability of Wearable Devices for Ambulatory Monitoring of the Rehabilitation Process of Older Patients after Hip Fracture
Surgery
65

study. Pilot and Feasibility Studies, 6(1).
doi:10.1186/s40814-020-00612-2
Bakker, A., Blokhuis, T. J., Meeks, M. D., Hermens, H. J.,
& Holtslag, H. R. (2014). Dynamic weight loading in
older people with hip fracture. Journal of rehabilitation
medicine, 46(7), 708-711.
Benzinger, P., Lindemann, U., Becker, C., Aminian, K.,
Jamour, M., & Flick, S. (2014). Geriatric rehabilitation
after hip fracture. Zeitschrift für Gerontologie und
Geriatrie, 47(3), 236-242.
Ceder, L. (2005). Predicting the success of rehabilitation
following hip fractures. Disability and rehabilitation,
27(18-19), 1073-1080.
Davenport, S. J., Arnold, M., Hua, C., Schenck, A., Batten,
S., & Taylor, N. F. (2015). Physical activity levels
during acute inpatient admission after hip fracture are
very low. Physiotherapy Research International, 20(3),
174-181.
Fleig, L., McAllister, M. M., Brasher, P., Cook, W. L., Guy,
P., Puyat, J. H., Ashe, M. C. (2016). Sedentary behavior
and physical activity patterns in older adults after hip
fracture: a call to action. Journal of aging and physical
activity, 24(1), 79-84.
Folbert, E., Smit, R., van der Velde, D., Regtuijt, M.,
Klaren, H., & Hegeman, J. (2011). Multidisciplinary
integrated care pathway for elderly patients with hip
fractures: implementation results from Centre for
Geriatric Traumatology, Almelo, The Netherlands.
Nederlands tijdschrift voor geneeskunde, 155(26),
A3197-A3197.
Hardy, S. E., & Gill, T. M. (2005). Factors associated with
recovery of independence among newly disabled older
persons. Archives of Internal Medicine, 165(1), 106-
112.
Hershkovitz, A., Beloosesky, Y., & Brill, S. (2012).
Mobility assessment of hip fracture patients during a
post-acute rehabilitation program. Archives of
Gerontology and Geriatrics, 55(1), 35-41.
Jo, E., Lewis, K., Directo, D., Kim, M. J., & Dolezal, B. A.
(2016). Validation of biofeedback wearables for
photoplethysmographic heart rate tracking. Journal of
sports science & medicine, 15(3), 540.
Keppler, A. M., Holzschuh, J., Pfeufer, D., Neuerburg, C.,
Kammerlander, C., Böcker, W., & Fürmetz, J. (2020).
Postoperative physical activity in orthogeriatric
patients–new insights with continuous monitoring.
Injury.
Nygard, H., Matre, K., & Fevang, J. M. (2016). Evaluation
of Timed Up and Go Test as a tool to measure
postoperative function and prediction of one year
walking ability for patients with hip fracture. Clinical
rehabilitation, 30(5), 472-480.
O'brien, T., Troutman-Jordan, M., Hathaway, D.,
Armstrong, S., & Moore, M. (2015). Acceptability of
wristband activity trackers among community dwelling
older adults. Geriatric Nursing, 36(2), S21-S25.
Prestmo, A., Hagen, G., Sletvold, O., Helbostad, J. L.,
Thingstad, P., Taraldsen, K., . . . Lamb, S. E. (2015).
Comprehensive geriatric care for patients with hip
fractures: a prospective, randomised, controlled trial.
The Lancet, 385(9978), 1623-1633.
Raymond, M. J., Winter, A., Jeffs, K. J., Soh, S.-E., &
Holland, A. E. (2018). Acceptability of physical
activity monitoring in older adults undergoing inpatient
rehabilitation. Aging clinical and experimental
research, 30(8), 1005-1010.
Resnick, B., Galik, E., Boltz, M., Hawkes, W., Shardell, M.,
Orwig, D., & Magaziner, J. (2011). Physical activity in
the post-hip-fracture period. Journal of aging and
physical activity, 19(4), 373-387.
Schmal, H., Holsgaard-Larsen, A., Izadpanah, K., Brønd, J.
C., Madsen, C. F., & Lauritsen, J. (2018). Validation of
Activity Tracking Procedures in Elderly Patients after
Operative Treatment of Proximal Femur Fractures.
Rehabilitation research and practice, 2018.
Sims-Gould, J., Stott-Eveneshen, S., Fleig, L., McAllister,
M., & Ashe, M. C. (2017). Patient perspectives on
engagement in recovery after hip fracture: a qualitative
study. Journal of aging research, 2017.
Talkowski, J. B., Lenze, E. J., Munin, M. C., Harrison, C.,
& Brach, J. S. (2009). Patient participation and physical
activity during rehabilitation and future functional
outcomes in patients after hip fracture. Archives of
physical medicine and rehabilitation, 90(4), 618-622.
Taraldsen, K., Thingstad, P., Sletvold, O., Saltvedt, I.,
Lydersen, S., Granat, M. H., . . . Helbostad, J. L. (2015).
The long-term effect of being treated in a geriatric ward
compared to an orthopaedic ward on six measures of
free-living physical behavior 4 and 12 months after a
hip fracture-a randomised controlled trial. BMC
geriatrics, 15(1), 160.
Taylor, N. F., Peiris, C. L., Kennedy, G., & Shields, N.
(2016). Walking tolerance of patients recovering from
hip fracture: a phase I trial. Disability and
rehabilitation, 38(19), 1900-1908.
Treacy, D., Hassett, L., Schurr, K., Chagpar, S., Paul, S. S.,
& Sherrington, C. (2017). Validity of different activity
monitors to count steps in an inpatient rehabilitation
setting. Physical therapy, 97(5), 581-588.
Willems, E., Visschedijk, J., Balen, R., & Achterberg, W.
(2017). Physical Activity, Physical Function and Fear
of Falling After Hip Fracture. J Orthop Res Physiother,
3, 031.
Zuckerman, J. D. (1996). Hip fracture. New England
journal of medicine, 334(23), 1519-1525.
WINSYS 2021 - 18th International Conference on Wireless Networks and Mobile Systems
66
