
Toward a Multimodal Multitask Model for Neurodegenerative Diseases
Diagnosis and Progression Prediction
Sofia Lahrichi, Maryem Rhanoui, Mounia Mikram and Bouchra El Asri
IMS Team, ADMIR Laboratory, Rabat IT Center, ENSIAS, Mohammed V University, Rabat, Morocco
mrhanoui@esi.ac.ma, mmikram@esi.ac.ma
Keywords:
Alzheimer’s Disease, Multimodal Multitask Learning, Machine Learning, Deep Learning, Progression
Detection, Time Series.
Abstract:
Recent studies on modelling the progression of Alzheimer’s disease use a single modality for their predictions
while ignoring the time dimension. However, the nature of patient data is heterogeneous and time dependent
which requires models that value these factors in order to achieve a reliable diagnosis, as well as making it
possible to track and detect changes in the progression of patients’ condition at an early stage. This article
overviews various categories of models used for Alzheimer’s disease prediction with their respective learning
methods, by establishing a comparative study of early prediction and detection Alzheimer’s disease progres-
sion. Finally, a robust and precise detection model is proposed.
1 INTRODUCTION
Alzheimer’s disease (AD) is a progressive, irre-
versible neurodegenerative disease characterized by
an abnormal build-up of amyloid plaques and neu-
rofibrillary tangles in the brain, resulting in memory,
thinking and behavior issues. AD is the most common
form of dementia characterized by a slow and asymp-
tomatic progress of the disease.
AD is clinically very heterogeneous, varying from
patient to patient in terms of cognitive symptoms, test
results, and rate of progression. Indeed, several recent
therapeutic trials have shown variable efficacy from
one subset of patients to another. Currently available
treatments only slow the progression of AD, and no
definitive cure has been developed yet. When it comes
to AD, patient data (Zhang et al., 2012) is heteroge-
neous, but complementary, of different types; mag-
netic resonance imaging (MRI), positron emission to-
mography (PET), genetics, cerebrospinal fluid (CSF),
etc. The combination of multimodalities (Weiner
et al., 2013) facilitates the detection of changes in the
patient’s states and constitutes a reliable diagnosis.
Patient data is gathered from different visits and
from continuous patient monitoring. The state of the
disease at any given time is not independent of the
condition at a previous time. Therefore, AD data are
not only multimodal but could also be considered as
time series and longitudinal series.
In the practical diagnosis of AD (Jo et al., 2019),
the most widely used neuroimaging dataset comes
from the Alzheimer’s Disease Neuroimaging Initia-
tive (ADNI) which contains (Jack Jr et al., 2008)
socio-demographic data (gender, education level), the
APOE genotype and five neuropsychological test re-
sults: MMSE (mini-mental state examination), CDR-
SB (the sum of boxes of clinical dementia rating),
ADASCog (Alzheimer’s Disease Assessment Scale
cognitive sub-scale), LMT (Logical Memory Test )
and RAVLT (Rey Auditory Verbal Learning Test).
Several learning methods have been developed for
the classification and prediction of MCI (Mild Cogni-
tive Impairment) to AD conversion, namely Machine
Learning and Deep Learning.
After extensive bibliographical and analytical
work on various articles, we will give an overview of
the categories of approaches used for the prediction of
Alzheimer’s disease, then we will study the different
learning techniques for each approach and establish
a comparative table of this last. This categorization
was essential to us because it will allow us at the end
of the study to propose a stable and precise detection
model.
The remainder of the article will consist of three
main parts; the first will be the most important be-
cause through it, we will establish the bases of our
study by defining the concepts of multimodal and
multitask learning. The second part will be devoted
322
Lahrichi, S., Rhanoui, M., Mikram, M. and El Asri, B.
Toward a Multimodal Multitask Model for Neurodegenerative Diseases Diagnosis and Progression Prediction.
DOI: 10.5220/0010600003220328
In Proceedings of the 10th International Conference on Data Science, Technology and Applications (DATA 2021), pages 322-328
ISBN: 978-989-758-521-0
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

to the comparative study of the different models for
predicting the progression of AD. And in the last part,
we will propose a model grouping together all the ad-
equate criteria for a better detection of the disease.
2 BACKGROUND
In this section, we introduce the main notions neces-
sary to understand the context of our proposal.
2.1 Multi-modal Learning
Multimodal learning combines (Zhang et al., 2020)
data from multiple sources that are semantically cor-
related and sometimes provide complementary infor-
mation to each other, resulting in more robust predic-
tions.
The multimodal learning model not only captures
the correlation structure between different modalities
but also allows to recover missing modalities given
those observed.
The multimodal learning model for example (Ba-
heti, 2020) can combine two deep Boltzmann ma-
chines, each corresponding to a modality. An addi-
tional hidden layer is placed on top of the two Boltz-
mann machines to give the common representation.
Multimodal learning is divided into four stages:
(1) Representing inputs and summarize data in a way
that expresses multiple modalities.
(2) Translating (map) the data from one modality to
another.
(3) Extracting important features from information
sources while creating models that best match the
type of data.
(4) Fusion and co-learning. Consists in combining
the information of two or more modalities to make
a prediction. This combination should be normally
weighted.
2.2 Multitask Learning
Multitask learning (MTL) aims to extract useful infor-
mation from multiple tasks and use their correlations
to help learn a more accurate pattern for each task.
Multitask learning has been used successfully in
all applications of machine learning, natural lan-
guage processing(Worsham and Kalita, 2020), speech
recognition(Pironkov et al., 2016), computer vision,
and drug discovery.
MTL (Zhang and Yang, 2018)can be categorized
into several categories, including supervised multi-
task learning(MTSL), unsupervised multitask learn-
ing, semi-supervised multitask learning...
MTSL aims to learn n functions for the n tasks
of the labeled training set, (Each task can be a clas-
sification or regression problem) after it uses fi (•) to
predict the labels of the data instances i from the j the
task. There are three categories of MTSL patterns to
indicate the relationship between stains:
Feature-based MTSL. Which learns common fea-
tures of different tasks in order to share knowledge
and avoid using original representations directly. It
can easily be affected by outliers.
Parameter-based MTSL. Which finds how the
model parameters of different tasks are related, which
leads to ranking. It is more robust to outliers.
Instance-based MTSL. Which aims to find in one
task the instances useful for other tasks. It is not used
too much.
In unsupervised multitask learning, each task, can
be a clustering problem, aims to identify useful pat-
terns contained in a training dataset composed only
of data instances.
In multitask semi-supervised learning, each task
aims to predict the labels of data instances from the
labeled and also unlabeled data.
3 COMPARATIVE STUDY
3.1 Models Categories
After an in-depth bibliographical study on the pro-
gression of AD, and after consulting a large number
of articles that seemed relevant to us, two main ideas
emerge: Research on modeling disease progression
has received special attention from researchers
around the world, and the modeling has been divided
into four categories.
Mono-modal Mono-task Learning. Where the
model optimizes only one objective function based
on a type of data. Through this model, neither the
correlation between the tasks, nor the collective
information between the modalities are explored.
Mono-modal Multitask Learning. Where the tasks
share a few training instances. The relationship be-
tween the spots is modeled assuming that they share
a common representation space or they share certain
parameters. However, they don’t take into account
the relationship between the different modalities of
the same task.
Multi-modal Mono-task Learning. Where several
modalities are taken into consideration in the predic-
tion of a state while ignoring information from other
tasks.
Multi-modal Multitask Learning. Where each
task of the problem has characteristics from several
Toward a Multimodal Multitask Model for Neurodegenerative Diseases Diagnosis and Progression Prediction
323

modalities and or several tasks are linked to each
other in a chronological sequence.
Artificial intelligence is advancing in understand-
ing the world around us and the operating model
that most closely matches our real environment and
provides greater precision is multitask multimodal
learning
3.2 Related Works
In our bibliographical study, we focused on the
following articles which seem to us the most relevant
and which meet our expectations.
Mono-modal Mono-task Learning. (Cui et al.,
2019) proposed a single-task, monomodal model
based on six-step MRI time series data for the
detection of AD progression. They use a stacked
CNN-BGRU (Convolutional Neural Network Bidi-
rectional Gated Recurrent Unit) pipeline. It achieves
an accuracy of 91.33% for AD vs CN (normal
cognitive), and 71.71% for pMCI (progressive MCI)
vs sMCI (stable MCI). However, relying on MRI
alone is insufficient in the medical field.
Mono-modal Multitask Learning. (Liu et al., 2018)
proposed a deep multi-channel multi-task convolu-
tional neural network for classification (Multi-class
classification) and regression using MRI data and BG
demographic information.
(Lopez-de Ipina et al., 2018) have developed a new
nonlinear multitasking (three tasks: animal naming
(AN), picture description (PD) and spontaneous
speech (SS)) approach based on automatic speech
analysis. They introduced linear features, perceptual
features, Castiglioni fractal dimension and Multiscale
Permutation Entropy into their analysis.
Based on the MRI data, they performed a clas-
sification, using Multilayer Perceptron (MLP) and
Deep Learning by means of Convolutional Neural
Networks (CNN) (biologically- inspired variants of
MLPs) which led to promising results.
Multi-modal Mono-task Learning. (Pan et al.,
2018) proposed a two-step deep learning framework
for the diagnosis of AD using both MRI and PET
data. in the first step, they assign the corresponding
MRI data to the missing PET data using 3D-cGAN
(3D Cycle-consistent Generative Adversarial Net-
works) to capture their underlying relationship. In the
second step, they develop LM3IL (Landmark-based
Multi-modal Multi-Instance Learning Network)
which learns and merges the characteristics necessary
for the diagnosis of AD and the prediction of MCI.
Using the ADNI dataset, (Shi et al., 2017)
proposed a method of transforming nonlinear feature
space into more linearly separable data using SVM.
They chose TPS (Thin-platespline) as a geometric
model because of its power of representation. They
also adopted a feature fusion strategy based on a deep
network by SDAE (Denoising Sparse Auto-Encoder)
to merge the transverse and longitudinal features
estimated from MRI brain images.
(Lee et al., 2019) proposed a one-task multi-
modal deep learning approach by incorporating
multi-domain longitudinal data. They applied a GRU
(Gated Recurrent Unit) for each modality (4Gru) to
produce feature vectors of fixed size which will be
concatenated to form an input for the final prediction
where regularized logistic regression is used for
the classification of MCI- C and MCI-NC (MCI
converter and MCI non-converter). The model on a
very small number of features (no addition of BG
data) has better prediction, it only optimizes binary
classification tasks.
Multi-modal Multitask Learning. (Lahmiri and
Shmuel, 2019) proposed M3T multimodal multitask
model for the prediction of AD over two years. The
latter has two main stages: (1) Selecting multitask
features using the Lasso (2) Using an SVM (Support
Vector Machine) model for separate classification and
regression.
Multi-source multitask learning (MSMT) simul-
taneously considers two types of prior knowledge. 1)
Source consistency 2) Slow temporal evolution
(Nie et al., 2016) proposed a linear MSMT model that
predicts the future disease status over 2 years (M06-
M48) of new patients, based on their health informa-
tion at the first moment (Baseline).
(Tabarestani et al., 2020) propose a distributed
multitask multimodal model to predict MMSE cog-
nitive measures of AD progression, the latter individ-
ually exploits several multitask regression coefficient
matrices for each modality, then It concatenates the
risk factors with the predicted y of each modality then
it goes through gradient boosting to group the results
of different modalities and reduce their prediction er-
ror.
(Nie et al., 2015) proposed an adaptive multi-
modal multitask linear learning model (aM2L) to reg-
ularize the modality agreement for the same task, the
temporal progression on the same modality and the
weight of the modalities.
(Nie et al., 2016) and (Nie et al., 2015) in their
predictions, don’t incorporate the follow-up observa-
tions. Example: To predict her condition at M24 (24
months after Baseline), they don’t merge the observa-
tions of Baseline, M06 AND M12.
DATA 2021 - 10th International Conference on Data Science, Technology and Applications
324

(Li et al., 2015) presented a robust deep learn-
ing system to identify the different stages of progres-
sion of patients with AD based on MRI and PET.
They used the dropout technique to improve classical
deep learning by preventing its weight co-adaptation,
which is a typical cause of deep learning overfit-
ting. They stacked multiple RBMs (Restricted Boltz-
mann machine) to build a robust deep learning frame-
work, which integrates stability selection and multi-
task learning strategy.
(El-Sappagh et al., 2020) proposed a robust en-
semble deep learning model based on a stacked con-
volutional neural network (CNN) and a bi-directional
long-term memory network (BiLSTM). This multi-
modal multitask model jointly predicts several vari-
ables based on the fusion of five types of multimodal
time series data plus a basic knowledge set (BG). The
predicted variables include the multi-class task and
four critical cognitive score regression tasks. This
model gave equal weights for the classification and
regression tasks.
The table below 1 is a summary of some works
seen previously, it lists the advantages and limitations
of its different models.
3.3 Synthesis of Models
All the models seen previously for the study of the
prediction of the progression of AD, use the ADNI
database which can contain missing data. In order to
overcome this problem, among the methods that were
used:
DEL: We simply eliminated the subjects with either
missing sources or missing labels.
ZERO: We assigned zero value to any element that is
missing.
KNN: The k-nearest neighbor (KNN) method re-
placed the missing value in the data matrix with the
corresponding value from the nearest column ...
In recent years, machine learning algorithms (such as
SVM and random forest, SDAE) and deep learning
(CNN, GRU, LSTM, BGRU, BiLSTM,...) have been
used for the design of a predictive model of AD pro-
gression. And for the generalization of these models
to avoid overfitting ,among the regularizers that have
been used ,we find: lasso ,sparce group lasso,l2,1
norm, ridge regression ,fused group lasso(convex and
non-convex),dropout ......
(Liu et al., 2013) and (Duchesne et al., 2009) used
regular machine learning techniques to study mul-
timodal single-task classification and regression, re-
spectively.
To detect AD progression based on a multimodal
single-task deep learning model, we find; (Spasov
et al., 2019) who proposed a classification model
based on a CNN ,(Lee et al., 2019) who also proposed
a binary classification model based on GRU and used
logistic regression for regularization.
In a real medical environment, many modalities
are analyzed and multiple clinical variables must be
predicted. Multimodal single-task models will not
provide in this case sufficient information for the
study of AD progression, hence the interest of the
multimodal multitask model.
This model has been used by (Lahmiri and
Shmuel, 2019) for classification and regression using
SVM and SVR respectively. In order to generalize
their model, they used the Lasso regularizer.
It was also used by el sappagh who added to their
model the time series constraint which is consistent
with the longitudinal nature of AD because the pa-
tient’s state at a given time is not independent of his
state at an earlier time.
They used the CNN-BILTSM for multiclass clas-
sification and for regression of 4 cognitive scores. For
the regularization of their model, they opted for the
dropout.
(El-Sappagh et al., 2020) consider that the modal-
ities have the same weight of importance which is not
always true in the medical field.
4 PROPOSED MODEL
Alzheimer’s disease (AD) is a longitudinal disease,
that is to say the state of a patient at a time t depends
strongly on his state at t-1. AD cannot be triggered
spontaneously; there must be a prior appearance of
signs that predict the manifestation and evolution of
the disease. Most models studied in the previous sec-
tion do not take this notion into consideration.
Several factors can influence the diagnosis out-
come of AD, and even the most indirect symptoms
may have in some cases an effect on the detection
of AD, hence the interest of introducing Multitask
Learning. Multitask Learning will allow us to find
a relationship between the features, the instances and
the parameters involved in the detection of the dis-
ease.
Moreover, patients’ data is considered multimodal
since they’re gathered from various sources such as
MRI, PET, CSD, ASD, NPD etc... These modalities
can also interact with each other, because the infor-
mation extracted from a modality can be complemen-
tary to other modalities, which opens the possibility
of improving the performance of the model for AD
progression prediction. Additionally, it is important
to note that the data retrieved via different modalities
Toward a Multimodal Multitask Model for Neurodegenerative Diseases Diagnosis and Progression Prediction
325
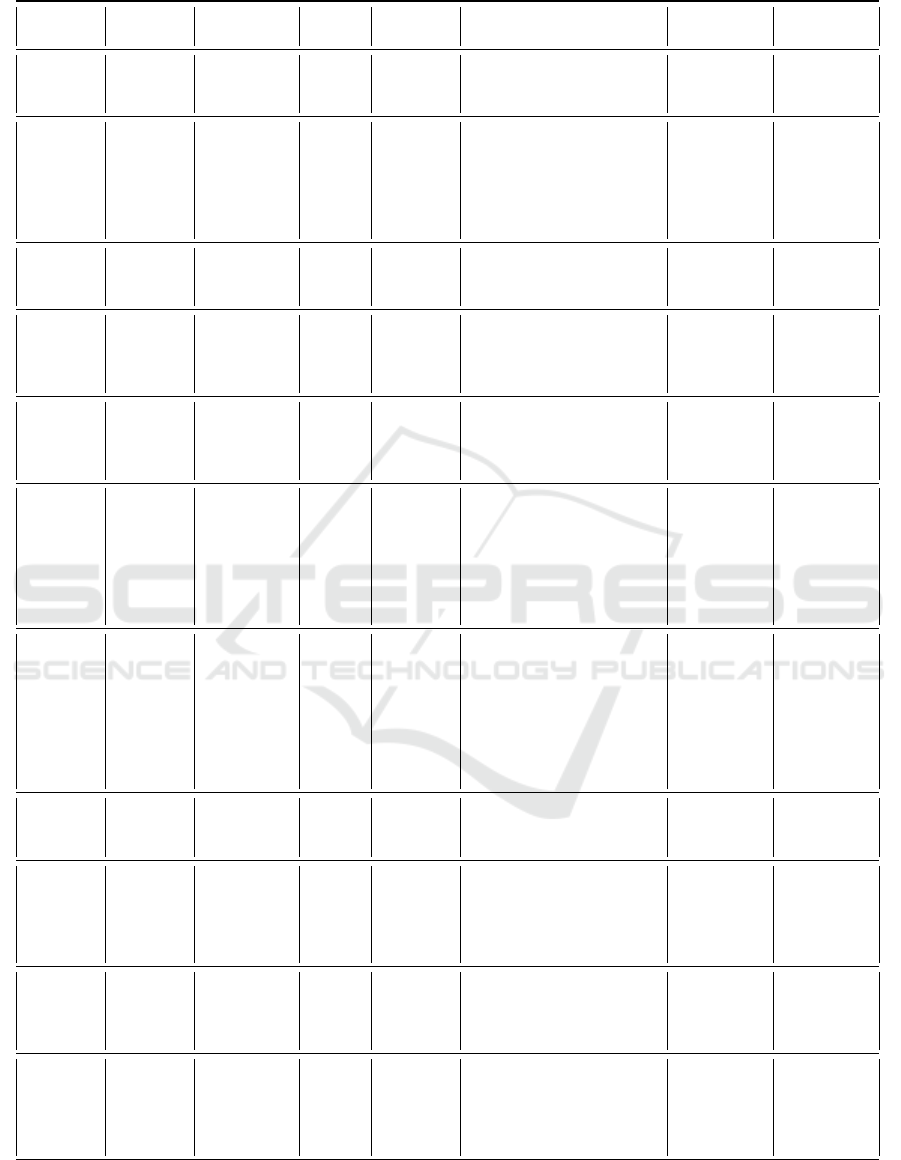
Table 1: Comparative table of models for predicting the progression of Alzheimer’s disease.
Study Data Modality Merging
Time-
series
Performance Model Task
(Lee
et al.,
2019)
ADNI
(Database,
2004)
Demographic,
MRI, CSD,
CSF(LCR)
NO
YES (4
Steps)
Accuracy: 81% (MCI/AD) GRU Classification
(Lahmiri
and
Shmuel,
2019)
ADNI
MRI, FDG-
PET, LCR
NO NO
Classification Accu-
racy: 93,3%(CN/AD),
83,2%(CN/MCI), 73,9%
(sMCI/pMCI). Regression
Accuracy: 0,697(MMSE),
0,739(ADAS)
SVM
Classification
and regres-
sion
(Nie
et al.,
2016)
ADNI
MRI,PET,
CSF,PROPT,
META
NO NO
ADAS-Cog =90,94%
MMSE=87,94%
MSMT Regression
(Cui
et al.,
2019)
ADNI MRI NO
YES(6
Steps)
Classification: Accuracy:
91,33% (AD/ NC), 71,71%
(pMCI:progressive MCI /
sMCI:static MCI)
Stacked
CNN-
BGRU
Classification
(Tabarestani
et al.,
2020)
ADNI
MRI, PET,
COG, CSF
YES NO MMSE: 70.1%
distributed
multimodal
multitask
learning
Regression
(Liu
et al.,
2018)
ADNI MRI YES NO
Classification Ac-
curacy: 51,8%
(CN/sMCI/pMCI/AD).
Regression: (CDRSB,
ADAS11, ADAS13,
MMSE): 1.666, 6.2, 8.537,
2.373
CNN
Classification
and regres-
sion
(Shi et al.,
2017)
ADNI MRI, Age YES NO
Classification:
TML(Theoretic Metric
Learning)-SVM Accu-
racy:AD/NC:91.95%
MCI/NC:83,72% Multi-
modal S-DSAE Accu-
racy:80.91%(MCI/NC)
88.73%(AD/NC)
TML-SVM
SDAE
(Stacked
Denoising
Sparse AE)
Classification
(Pan
et al.,
2018)
ADNI MRI, PET YES NO
Classification Accu-
racy: 92,5%(HC/AD)
79,06%(sMCI/pMCI)
3D-
CNN+GAN
LM3IL
Classification
(Li et al.,
2015)
ADNI
MRI,PET,
CSF
NO NO
Classification Accu-
racy: 91.4%(AD /HC),
77.4%(MCI/HC),
70.1% (AD/MCI),and
57.4%(MCI.c/MCI.NC)
RBM Classification
(Nie
et al.,
2015)
ADNI
MRI,
PET, CSF,
PROPT,
META
NO NO
MMSE:89,01 %ADAS-
Cog: 91,68%
aM2L Regression
(El-
Sappagh
et al.,
2020)
ADNI
MRI, PET,
CSD, ASD,
NPD
YES
YES(15
Steps)
ACC: 92.62%, PRE:
94.02%, F1: 92.56%, REC:
98.42. MAE: 0.107, 0.076,
0.075, and 0.085, (FAQ,
ADAS, CDR, MMSE)
StackedCNN-
BiLSTM
Classification
and regres-
sion
DATA 2021 - 10th International Conference on Data Science, Technology and Applications
326

may vary in terms of importance. For instance, MRI
and PET are considered a more reliable and relevant
data sources, hence the interest in introducing the idea
of a weighted model.
According to the results of the comparative study
seen in the previous section, which follows closely
the same direction mentioned previously, we can con-
clude that the most adequate, precise and stable model
is the Multimodal Multitask Model based on time se-
ries.
The research axis that can be developed from this
study, will center around a Multimodal Multitask sys-
tem based on time series, which will be able to si-
multaneously regularize the modality weighting, the
temporal progression as well as the modality agree-
ment, i.e. the status of the patient estimated by dif-
ferent modalities must be consistent. The model will
also be capable of taking into consideration the rela-
tionship between AD progression and the patient’s co-
morbidities (cardiovascular disease, depression, gen-
itourinary renal metabolism, endocrine, etc.). Such
model has not yet been addressed in the literature.
To advance on this research axis, we propose a
Machine Learning or a Deep Learning architecture,
multitask, multimodal (using MRI, PET, CSD, ASD,
NPD), trained on the ADNI database and the patients’
demographic data. This model predicts AD progres-
sion status of a through a multiclass classification
task, and the values of two cognitive scores ADAS,
MMSE which will be implemented as two regression
tasks.
In order to meet the different expectations as well
as possible, two possible scenarios seem to open up at
the moment:
- The first approach consists in extracting separately
the temporal characteristics of each modality (MRI,
PET, CSD, ASD, NPD), then merged with the de-
mographic data to extract the common characteris-
tics that respond to each task by applying a multitask
learning.
- Or by applying multitasking to each modality and
merging the initial results with demographic data,
which is assumed to be a time-invariant information.
This model has the ability to stop the propagation of
an error from one modality to another.
The loss function in both approaches will include
a first term for classification, a second term for regres-
sion and 2 last terms to regularize the weight and the
agreement of the modalities.
5 CONCLUSION
Alzheimer’s disease (Thung et al., 2017) is currently
calling the attention of many researchers. A consid-
erable amount of effort is being put to understand
the biological and physiological mechanisms of AD
as well as its monitoring and early detection. The
presented works in this paper have raised several ap-
proaches for the detection of AD, namely Monomodal
monotask, Monomodal multitask Multimodal mono-
task and Multimodal multitasking. Following our
comparative study of different works and papers, we
believe that a major research axis has been revealed
in which the Multimodal Multitask based on time se-
ries could at the same time to regularize the modal-
ity weighting, the temporal progression, the modality
agreement and which will even take into considera-
tion the relationship between the progression of AD
and the patient’s comorbidities. This model could
bring a considerable advance in the field of medical
research.
REFERENCES
Baheti, P. (2020). Introduction to Multimodal Deep
Learning. https://heartbeat.fritz.ai/introduction-to-
multimodal-deep-learning-630b259f9291.
Cui, R., Liu, M., Initiative, A. D. N., et al. (2019).
Rnn-based longitudinal analysis for diagnosis of
alzheimer’s disease. Computerized Medical Imaging
and Graphics, 73:1–10.
Database (2004). Introduction to Multimodal Deep Learn-
ing. http://adni.loni.usc.edu.
Duchesne, S., Caroli, A., Geroldi, C., Collins, D. L., and
Frisoni, G. B. (2009). Relating one-year cognitive
change in mild cognitive impairment to baseline mri
features. Neuroimage, 47(4):1363–1370.
El-Sappagh, S., Abuhmed, T., Islam, S. R., and Kwak, K. S.
(2020). Multimodal multitask deep learning model
for alzheimer’s disease progression detection based on
time series data. Neurocomputing, 412:197–215.
Jack Jr, C. R., Bernstein, M. A., Fox, N. C., Thompson,
P., Alexander, G., Harvey, D., Borowski, B., Britson,
P. J., L. Whitwell, J., Ward, C., et al. (2008). The
alzheimer’s disease neuroimaging initiative (adni):
Mri methods. Journal of Magnetic Resonance Imag-
ing: An Official Journal of the International Society
for Magnetic Resonance in Medicine, 27(4):685–691.
Jo, T., Nho, K., and Saykin, A. J. (2019). Deep learning
in alzheimer’s disease: diagnostic classification and
prognostic prediction using neuroimaging data. Fron-
tiers in aging neuroscience, 11:220.
Lahmiri, S. and Shmuel, A. (2019). Performance of ma-
chine learning methods applied to structural mri and
adas cognitive scores in diagnosing alzheimer’s dis-
Toward a Multimodal Multitask Model for Neurodegenerative Diseases Diagnosis and Progression Prediction
327

ease. Biomedical Signal Processing and Control,
52:414–419.
Lee, G., Nho, K., Kang, B., Sohn, K.-A., and Kim, D.
(2019). Predicting alzheimer’s disease progression us-
ing multi-modal deep learning approach. Scientific re-
ports, 9(1):1–12.
Li, F., Tran, L., Thung, K.-H., Ji, S., Shen, D., and Li, J.
(2015). A robust deep model for improved classifica-
tion of ad/mci patients. IEEE journal of biomedical
and health informatics, 19(5):1610–1616.
Liu, F., Zhou, L., Shen, C., and Yin, J. (2013). Multiple ker-
nel learning in the primal for multimodal alzheimer’s
disease classification. IEEE journal of biomedical and
health informatics, 18(3):984–990.
Liu, M., Zhang, J., Adeli, E., and Shen, D. (2018).
Joint classification and regression via deep multi-task
multi-channel learning for alzheimer’s disease diag-
nosis. IEEE Transactions on Biomedical Engineering,
66(5):1195–1206.
Lopez-de Ipina, K., Martinez-de Lizarduy, U., Calvo, P. M.,
Mekyska, J., Beitia, B., Barroso, N., Estanga, A.,
Tainta, M., and Ecay-Torres, M. (2018). Advances
on automatic speech analysis for early detection of
alzheimer disease: a non-linear multi-task approach.
Current Alzheimer Research, 15(2):139–148.
Nie, L., Zhang, L., Meng, L., Song, X., Chang, X., and
Li, X. (2016). Modeling disease progression via
multisource multitask learners: A case study with
alzheimer’s disease. IEEE transactions on neural net-
works and learning systems, 28(7):1508–1519.
Nie, L., Zhang, L., Yang, Y., Wang, M., Hong, R., and
Chua, T.-S. (2015). Beyond doctors: Future health
prediction from multimedia and multimodal observa-
tions. In Proceedings of the 23rd ACM international
conference on Multimedia, pages 591–600.
Pan, Y., Liu, M., Lian, C., Zhou, T., Xia, Y., and Shen,
D. (2018). Synthesizing missing pet from mri with
cycle-consistent generative adversarial networks for
alzheimer’s disease diagnosis. In International Con-
ference on Medical Image Computing and Computer-
Assisted Intervention, pages 455–463. Springer.
Pironkov, G., Dupont, S., and Dutoit, T. (2016). Multi-
task learning for speech recognition: an overview. In
ESANN.
Shi, B., Chen, Y., Zhang, P., Smith, C. D., Liu, J., Initiative,
A. D. N., et al. (2017). Nonlinear feature transforma-
tion and deep fusion for alzheimer’s disease staging
analysis. Pattern recognition, 63:487–498.
Spasov, S., Passamonti, L., Duggento, A., Li
`
o, P., Toschi,
N., Initiative, A. D. N., et al. (2019). A parameter-
efficient deep learning approach to predict conversion
from mild cognitive impairment to alzheimer’s dis-
ease. Neuroimage, 189:276–287.
Tabarestani, S., Aghili, M., Eslami, M., Cabrerizo, M., Bar-
reto, A., Rishe, N., Curiel, R. E., Loewenstein, D.,
Duara, R., and Adjouadi, M. (2020). A distributed
multitask multimodal approach for the prediction of
alzheimer’s disease in a longitudinal study. NeuroIm-
age, 206:116317.
Thung, K.-H., Yap, P.-T., and Shen, D. (2017). Multi-stage
diagnosis of alzheimer’s disease with incomplete mul-
timodal data via multi-task deep learning. In Deep
learning in medical image analysis and multimodal
learning for clinical decision support, pages 160–168.
Springer.
Weiner, M. W., Veitch, D. P., Aisen, P. S., Beckett, L. A.,
Cairns, N. J., Green, R. C., Harvey, D., Jack, C. R.,
Jagust, W., Liu, E., et al. (2013). The alzheimer’s dis-
ease neuroimaging initiative: a review of papers pub-
lished since its inception. Alzheimer’s & Dementia,
9(5):e111–e194.
Worsham, J. and Kalita, J. (2020). Multi-task learning for
natural language processing in the 2020s: Where are
we going? Pattern Recognition Letters, 136:120–126.
Zhang, D., Shen, D., Initiative, A. D. N., et al. (2012).
Multi-modal multi-task learning for joint prediction
of multiple regression and classification variables in
alzheimer’s disease. NeuroImage, 59(2):895–907.
Zhang, L., Wang, M., Liu, M., and Zhang, D. (2020). A
survey on deep learning for neuroimaging-based brain
disorder analysis. Frontiers in neuroscience, 14.
Zhang, Y. and Yang, Q. (2018). An overview of multi-task
learning. National Science Review, 5(1):30–43.
DATA 2021 - 10th International Conference on Data Science, Technology and Applications
328
