
Numerical Study on Droplet Evaporation Simulation Scheme and
Evaporation Characteristics of Salt-containing Desulfurization
Wastewater
Guojun Yu
a
and Li Zhang
b
College of Energy and Mechanical Engineering, Shanghai University of Electric Power, China
Keywords: Desulfurization Wastewater, Salt-Containing Droplets, Evaporation Characteristics, Numerical Simulation.
Abstract:
In order to accurately simulate the evaporation characteristics of salt-containing desulfurization wastewater
in high temperature flue gas, five simulation schemes for droplet evaporation of desulfurization wastewater
were discussed based on DPM model of FLUENT platform and combined with the actual composition of
desulfurization wastewater. A simulation scheme for realizing the influence of soluble salts and suspended
solids on the evaporation of wastewater droplets was determined. The differences of evaporation characteristic
parameters such as evaporation time, evaporation distance, particle size change and mass change of
wastewater droplets obtained under different schemes were explored, and the evaporation characteristics of
wastewater with different salt content were studied. The results show that the evaporation characteristics of
different schemes are different. It is recommended to use the evaporation simulation scheme considering the
actual composition of desulfurized wastewater in the simulation; The greater the salt content, the shorter the
time and distance required for evaporation of waste water droplets. Under the research conditions in this paper,
when the salt content of wastewater is doubled, the particle size of remaining particles increases by 10.3%,
the relative mass of remaining particles increases by a percentage of 2.3, the total evaporation time and the
total evaporation distance decrease by 6% on average.
1
INTRODUCTION
1
Since 2015, the government has issued a series of pol-
lution control policies, which put forward the require-
ment of near zero discharge for desulfurization
wastewater from thermal power units. At the same
time, the increase in desulfurization wastewater from
large thermal power units increases the task of treat-
ment. Therefore, concentration and reduction become
necessary before the terminal treatment of desulfuri-
zation wastewater. Atomization evaporation is one of
the terminal treatment technologies of desulfurization
wastewater treatment process and has a certain appli-
cation in engineering. However, with the concentra-
tion and reduction of desulfurization wastewater, the
composition of wastewater changes. Correspond-
ingly, wastewater droplet evaporation characteristics
will also change. How to accurately master the evap-
oration characteristics of wastewater droplets is of
a
https://orcid.org/0000-0002-9039-1388
b
https://orcid.org/0000-0002-6203-6119
great engineering significance for better application
of atomization evaporation technology and improve-
ment of desulfurization wastewater treatment effect.
In previous studies, the treatment of wastewater
droplets was different. Ref. (Zhang 2011, Kang 2013,
Min 2019) considered that water accounted for the
majority of desulfurization wastewater, and treated
desulfurization wastewater as pure water which can
evaporate completely. Ref. (Chen 2016, Ran 2016,
Wang 2019) still thought that desulfurization
wastewater could be evaporated totally, the difference
was that they considered the physical property differ-
ence between waste water and pure-water. In fact,
desulfurization wastewater contains Ca, Ma, Cl and
SO
3
as well as suspended solids (Wu 2006). Espe-
cially after concentration and reduction, the propor-
tion of soluble salts in desulfurization wastewater be-
comes larger. Therefore, above research work has
shortcomings.
18
Yu, G. and Zhang, L.
Numerical Study on Droplet Evaporation Simulation Scheme and Evaporation Characteristics of Salt-containing Desulfurization Wastewater.
DOI: 10.5220/0011175800003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 18-25
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

In recent two years, researchers started paying at-
tention to the influence of soluble salts in desulfuriza-
tion wastewater on droplet evaporation. Ref. (Xiong
2020) researched the influence of soluble salts on
droplet evaporation characteristics by using a shell
formation model. Ref. (Yang 2020) regarded desulfu-
rization wastewater as homogeneous salt solution and
took into account the influence of salt crystallization
on droplet evaporation characteristics during the nu-
merical study. However, the research on atomization
evaporation of saline desulfurization wastewater is
not enough. How to consider the actual composition
of desulfurization wastewater and how to use the
droplet model provided by the numerical simulation
platform to simulate the evaporation process of desul-
furization wastewater more accurately? What are the
differences in evaporation characteristics between
different numerical simulation schemes for
wastewater droplet evaporation? These are all worthy
of in-depth study.
Based on the above analysis, combined with the
actual composition of desulfurization wastewater, this
paper discussed several research schemes on how to
simulate the droplet evaporation of desulfurization
wastewater on FLUENT platform. and obtained the
evaporation characteristics in several aspects under
different schemes. A simulation research scheme
which could realize the influence of soluble salts and
suspended solids on the droplet evaporation of
wastewater was determined. At last, the evaporation
characteristics of desulfurization wastewater with dif-
ferent salt content were studied
2 MATHEMATICAL MODEL OF
DESULFURIZATION
WASTEWATER
EVAPORATION
It is generally considered that the evaporation process
of desulfurization wastewater in flue gas is a dilute
two-phase flow.
2.1 Control Equation of Continuous
Phase
Continuous phase flue gas is considered as a mixture
of dry flue gas and water vapor. The flow and heat
exchange processes are described in a general form as
follows (Feng 2019):
𝜕
𝜌𝜙
𝜕𝑡
+𝑑𝑖𝑣𝜌𝐔
𝜙 = 𝑑𝑖𝑣𝛤
𝑔𝑟𝑎𝑑𝜙 + 𝑆
(1)
in equation: 𝜙 is a general variable for different
equations; 𝜌 is density of flue gas; 𝐔
is the veloc-
ity vector of flue gas; 𝛤
is a general diffusion coef-
ficient; 𝑆
is a general source term, which repre-
sents the droplet evaporation, the interaction force be-
tween flue gas and droplets, the heat required for
evaporation.
2.2 Trajectory Equation of Discrete
Phase
The trajectory equation of atomized droplets can be
obtained by integrating the particle forces in Laplace
coordinates (Jen 2005):
d𝐔
dt
=𝐹
𝐔
−𝐔
+
𝐠𝜌
−𝜌
𝜌
(2)
in equation: 𝐔
is the velocity of particle; 𝐹
𝐔
−
𝐔
is the drag force acting on unit mass particle.
2.3 Heat and Mass Transfer Equation
between Two Phases
During the process of evaporation, there is heat and
mass transfer phenomena between flue gas and drop-
lets. The droplets may have three different phenom-
ena: heating, evaporation and boiling. When the drop-
let temperature is lower than the "critical" evapora-
tion temperature, only heat transfer exists between the
droplet and the flue gas. When the droplet tempera-
ture is higher than the "critical" evaporation tempera-
ture and lower than the boiling point temperature, the
heat absorbed by the droplet is used for both temper-
ature rise and evaporation (i.e. unsteady evaporation)
and then enters the steady evaporation stage, the heat
is fully used for droplet evaporation. When the tem-
perature reaches the boiling point, the droplets absorb
heat and boil.
Generally, atomization evaporation of desulfuri-
zation wastewater is the above evaporation phenom-
ena. Without considering the influence of radiation,
the droplet temperature equation and the evaporation
rate formula determined by the convection mass are
as follows (Miura 1977):
𝑀
𝑐
𝑑𝑇
𝑑𝑡
=ℎ𝐴
𝑇
−𝑇
+
𝑑𝑀
𝑑𝑡
𝛾 (3)
𝑑𝑀
𝑑𝑡
=𝑘
𝐴
𝜌
𝑙𝑛
1+𝐵
(4)
in equation: 𝑀
is droplet mass; 𝑐
is the specific
heat capacity of the droplet; 𝐴
is the surface area of
the droplets; 𝑇
、
𝑇
is separately the temperature of
flue gas and the droplets. h is the convection heat co-
efficient of the droplet surface; 𝛾 is the latent heat of
vaporization; 𝑘
is the mass transfer coefficient;𝐵
Numerical Study on Droplet Evaporation Simulation Scheme and Evaporation Characteristics of Salt-containing Desulfurization Wastewater
19

is the Spalding mass transfer number.
If the droplet contains solids, the remaining parti-
cle will enter the heating process after all the evapo-
rable components evaporate. The temperature equa-
tion is as follows (Miura 1977):
𝑀
𝑐
𝑑𝑇
𝑑𝑡
=ℎ𝐴
𝑇
−𝑇
5
3 DISCUSSION ON SIMULATION
SCHEME OF DROPLET
EVAPORATION
The treatment scheme of wastewater droplet evapora-
tion simulation is related to the components of desul-
furization wastewater and the discrete phase model
provided by the numerical simulation platform.
3.1 Component Analysis of
Desulfurization Wastewater
Desulfurization wastewater is the most difficult ter-
minal wastewater in thermal power plant, in which
there are many kinds of pollutants such as suspended
solids, salt content, heavy metal, fluoride and so on.
Measurement results showed that desulfurization
wastewater after pretreatment still contains Cl
-
, SO
4
2-
,
Na
+
, K
+
, Mg
2+
, Ca
2+
and suspended solids. Therefore,
desulfurization wastewater is generally considered to
consist of pure water, soluble salts and suspended sol-
ids (Liang, 2019). Because the crystallization of salts
and existence of suspended solids would affect the
droplet evaporation characteristics, the simulation
scheme of wastewater droplet considering the exist-
ence of these substances is a more reasonable scheme.
3.2 Discussions on Discrete Phase
Model of FLUENT Simulation
Platform
FLUENT platform could simulate many physical
phenomena such as flow, heat transfer, mass transfer,
combustion, radiation and so on. The platform pro-
vides several particle models for discrete phase simu-
lation. Both droplet model and multicomponent
model are suitable for simulating droplet evaporation.
Droplet model is mainly used to simulate the evapo-
ration of homogeneous droplets which the physical
parameters doesn’t change during the simulation.
Therefore, droplet model can’t simulate the crystalli-
zation of soluble salts and the presence of suspen-
sions. The multicomponent model can define several
components of the droplet, and can define the physi-
cal properties and evaporation of each component
separately. With the evaporation or crystallization of
the droplet components, the composition and physical
properties of the droplet change during the simula-
tion. Therefore, the multicomponent model could
simulate the mixture with salt crystallization and sus-
pension, and realize different simulation schemes of
wastewater droplets by defining the composition and
content.
3.3 Discussion on Droplet Evaporation
Scheme of Desulfurization
Wastewater
Based on the above analysis, combining with the
DPM model provided by FLUENT simulation plat-
form, considering the actual composition of desulfu-
rization wastewater and the treatment scheme of
desulfurization wastewater droplets in previous liter-
atures, the following five schemes which may simu-
late the evaporation of wastewater droplets are deter-
mined:
(1) Scheme 1: Disregard soluble salts and sus-
pended solids, treat wastewater as pure water, select
droplet model.
(2) Scheme 2: Disregard suspended solids, treat
wastewater as a mixture of pure water and soluble
salt, select droplet model.
(3) Scheme 3: Treat wastewater as a mixture of
pure water, soluble salt and suspended solids, select
droplet model.
(4) Scheme 4: Disregard suspended solids, treat
wastewater as a mixture of pure water and soluble
salt, select multicomponent model, define the physi-
cal properties and proportion of pure water and solu-
ble salt respectively.
(5) Scheme 5: Treat wastewater as a mixture of
pure water, soluble salt and suspended solids, select
multicomponent model, define the physical proper-
ties and proportion of pure water, soluble salt and sus-
pension respectively.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
20

4 RESEARCH OBJECT AND
CALCULATION CONDITION
SETTING
4.1 Research Object
Taking the evaporation of desulfurization wastewater
in flue as an example, above five schemes of desulfu-
rized wastewater are compared. The flue between air
preheater and dust collector of one unit is chose. Fig.1
gives the size of the flue and the plan of the nozzle.
Tab.1 gives the relevant parameters of the flue gas and
atomized wastewater.
Figure 1: Flue structure and nozzle layout.
Table 1: Related parameters of flue gas and wastewater.
Paramete
r
Unit Value
Flue gas flow rate m/s 10
Flue
g
as tem
p
erature
K
433
Mass fraction ratio of water vapor
in flue
g
as
% 0.08
Waste water temperature
K
323
Waste water
q
uantit
y
t/h 4.2
Number of nozzles 6
Dro
p
let in
j
ection rate m/s 5
Droplet size μm 60
4.2 Composition of Desulfurization
Wastewater
The main content of desulfurization wastewater is
shown in Tab.2. Refer to Ref. (Liang, 2019), it is as-
sumed that the soluble salts containing in the
wastewater are CaCl
2
, MgCl
2
, NaCl, MgSO
4
and
KCl. The contents of the soluble salts are calculated.
It is assumed that the suspension in wastewater is
mainly calcium sulfate. The content is 70mg/L ac-
cording to the national standard. Tab.3 shows the
composition and physical properties of desulfuriza-
tion wastewater.
Table 2: Main ion content in wastewater.
Ions Ca
2+
Mg
2+
Na
+
K
+
Cl
-
SO
Content(mg/L) 500 3954 683.5 175.2 6594.5 9600.3
Table 3: Composition and physical parameters of wastewater.
Component Water
Soluble salts Suspended solids
CaCl
2
MgCl
2
NaCl MgSO
4
KCl
CaSO
4
Content (g/L) 978.32 1.388 6.151 1.738 12.00 0.335 0.07
Density (kg/m
3
) 1000 2152 2325 2165 2660 1984 2960
C
P
(J/kg·k) 4180 657.5 752.6 870.4 805.5 657.5 733.8
4.3 Calculation Condition Setting
Three sets of grids with total numbers of 1.17, 2.03
and 2.4 million are generated. After verification of
grid independence, the number of grids used for cal-
culation is 2.03 million.
In the simulation, it is considered that the contin-
uous phase is composed of dry flue gas and water va-
por. The component transport model is used to con-
sider the evaporative mass transfer of droplets. The
inlet of flue is set as the velocity inlet boundary, the
outlet is set as the pressure outlet boundary and the
wall is insulated without slip. Discrete phase droplets
are injected from nozzles with uniform particle
size(60μm). The droplet trajectory is calculated by
discrete random walk model. The wall collision
model adopts capture type and the flue outlet is es-
cape type. The discrete phase and the continuous
phase are coupled and the steady-state tracking
method is used with the maximum iteration step of
50000. SIMPLE algorithm is used for pressure-veloc-
ity calculation and second-order upwind mode is used
for equation discretization. The physical parameters
of waste water droplets in different schemes are set
according to Tab.3.
Numerical Study on Droplet Evaporation Simulation Scheme and Evaporation Characteristics of Salt-containing Desulfurization Wastewater
21
5 DISCUSSION OF SIMULATION
RESULTS OF DIFFERENT
DROPLET EVAPORATION
SCHEMES
5.1 Particle Motion Analysis
According to the above numerical methods, the sim-
ulation of five schemes for droplet evaporation of
wastewater was completed. The continuous phase
flow fields obtained have little difference, but the par-
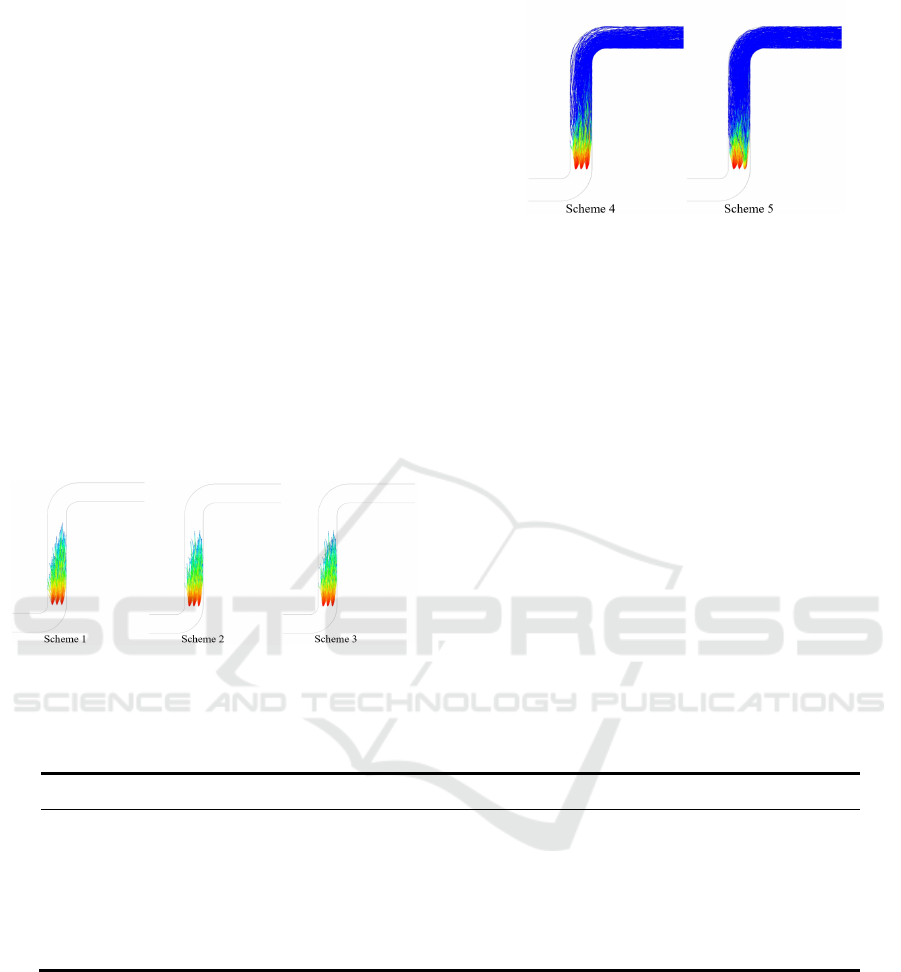
ticle motion is different. Fig.2 shows the particle mo-
tion of five schemes. It can be seen qualitatively that
the wastewater droplets of Scheme1, 2 or 3 evaporate
completely in a vertical flue, only the evaporation
processes are slightly different. However, for Scheme
4 or 5, there are residual particle after wastewater
evaporation due to the salt or suspended solids. The
residual particles are discharged with the flue gas.
Figure 2: Droplet trajectory of each scheme.
5.2 Comparison of Evaporation
Characteristics
Table 4 shows the evaporation time and distance of
the wastewater droplets under each scheme. It can be
seen that the time and distance required for droplet
evaporation in Scheme 1, 2 or 3 are not significantly
different, and the minor differences are mainly caused
by the slight changes of physical parameters of
wastewater, such as density and specific heat capac-
ity. However, compared with the first three schemes,
the evaporation time and distance are longer in
Scheme 4 or 5 due to considering the presence of
crystalline salts and suspended solids, because these
have adverse effects on droplet evaporation. The re-
search object in this paper has a vertical flue of 12.5m.
The simulation results in Tab.4 shows that the drop-
lets have evaporated completely before leaving the
bend and do not impact on downstream equipment.
Table 4: Evaporation time and distance of each scheme.
Scheme 1 2 3 4 5
Average evaporation time (s) 0.603 0.586 0.586 0.621 0.624
Complete evaporation time
(
s
)
0.975 0.978 0.978 1.311 1.307
Average evaporation distance
(
m
)
5.900 5.680 5.680 5.930 5.993
Complete evaporation dis-
tance (m)
9.197 9.201 9.201 12.08 12.03
Fig.3 shows the variation of droplet temperature
with evaporation time. It can be seen that the evapo-
ration first experienced the unsteady stage since the
droplets of Scheme 1, 2 and 3 are all pure solutions.
Then the evaporation rapidly enters a steady stage
with a temperature of about 331.8K until the droplets
completely evaporate. For Schemes 4 and 5, the un-
steady evaporation stage is relatively slow, because of
the salt or suspended solids in Schemes 4 and 5,
which may need additional heat to rise temperature.
Then the evaporation also enters a steady stage with
almost the same evaporation temperature as the first
three schemes. After the steady evaporation lasts for
a short time, all the water evaporates, the remaining
crystalline salts or solids begin to absorb heat and rise
temperature. In addition, duo to discard the suspended
solids in Scheme 4, the temperature of wastewater
droplet is slightly different from that in Scheme 5.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
22

Figure 3: Variation of average temperature of droplet with
time of each scheme.
Fig.4 compares the change of droplet mean diam-
eter with evaporation time. It shows that the average
droplet size of the five schemes decreases rapidly
with evaporation time in the early stage of droplet
evaporation, and the change rate is basically the same.
However, during the later stage of evaporation, the
change of average droplet size is different. The results
of Scheme 1, 2 and 3 show that the droplets evaporate
completely and rapidly, while the average particle
size of Scheme 4 and 5 tend to a fixed value due to
the retention of crystalline salts and suspended solid.
Figure 4: Variation of average size of droplet with time of
each scheme.
Fig.5 is a graph of the change of the relative mass
of droplets with evaporation time. It can be seen from
the figure that the change trend of droplet mass ob-
tained by each scheme is basically the same. In the
early stage of evaporation, the droplets are evaporated
quickly by about 50% due to the large temperature
difference between droplets and flue gas. For the re-
maining 50% mass, the heat transfer between droplets
and flue gas is reduced due to the reduction of tem-
perature difference between droplets and flue gas and
the reduction of droplet surface area. Therefore, it
takes a long time to completely evaporate.
Figure 5: Variation of relative mass of droplet with evapo-
ration time of each scheme.
By comparing the simulation results of the above
five schemes, it is easy to find that the characteristics
of droplets simulated by the first three schemes using
droplet model have the same trend, and there are
slight differences among them, mainly due to the dif-
ferent settings of physical properties of wastewater
droplets. Similarly, the evaporation characteristics
obtained by the multicomponent model have the same
trend. The difference between Scheme 4 and Scheme
5 is mainly due to whether the presence of suspended
solids is considered and is especially in the later stage
of droplet evaporation. Due to better consideration of
the actual composition of wastewater, Scheme 5 is
recommended in this paper.
6 EVAPORATION
CHARACTERISTICS OF
WASTEWATER WITH
DIFFERENT SALT CONTENT
On the basis of the above wastewater components, it
is assumed that the wastewater is concentrated to dif-
ferent degrees. Using Scheme 5, the evaporation char-
acteristics of droplets with different salt content are
further studied.
Figure 6: Variation of average temperature of droplet with
different salt content with evaporation time.
0.00.20.40.60.81.01.21.4
320
330
340
350
360
370
380
390
400
Average temperature of waste water droplets/K
Evaporation time/s
Scheme1
Scheme2
Scheme3
Scheme4
Scheme5
0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4
0
10
20
30
40
50
60
Average particle size of waste water droplets/μm
Evaporation time
/s
Scheme1
Scheme2
Scheme3
Scheme4
Scheme5
0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4
0
20
40
60
80
100
Relative quality of waste water droplets/%
Evaporation time
/s
Scheme1
Scheme2
Scheme3
Scheme4
Scheme5
0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4
320
330
340
350
360
370
380
390
400
Average temperature of waste water droplets/K
Evaporation time/s
Salt content 4.34%
Salt content 6.50%
Salt content 8.67%
Salt content 10.84%
Salt content 13.01%
Numerical Study on Droplet Evaporation Simulation Scheme and Evaporation Characteristics of Salt-containing Desulfurization Wastewater
23

Figure 7: Variation of average particle size of droplet with
different salt content with evaporation time.
Figure 8: Variation of relative mass of droplet with different
salt content with evaporation time.
Fig.6-8 show the variation of average tempera-
ture, average particle size and relative mass with
evaporation time when droplets of desulfurization
wastewater with different salt content evaporate.
Fig.6 shows that the less the salt content, the later the
wastewater droplets enter the steady evaporation, the
longer the steady evaporation duration, the lower the
steady evaporation temperature, and the lower the
temperature of the final particles. It can be seen from
Fig.7 and Fig.8 that with the increase of salt content,
the earlier the particles enter the stage of constant par-
ticle size and mass, indicating that the complete evap-
oration time of droplets becomes shorter. At the same
time, it can be seen that the larger the salt content, the
larger the diameter and mass of the final remaining
particle.
Finally, the evaporation time, distance and resid-
ual relative mass of wastewater with different salt
content are extracted from the simulation results as
shown in Tab.5. It can be seen that the relative mass
of the remaining particles obtained by the numerical
simulation is the same as the mass fraction of salt in
wastewater before evaporation, which also shows the
accuracy of the calculation to a certain extent. In ad-
dition, the table shows that the higher the salt content,
the shorter the time and distance required for the
evaporation of wastewater droplets, and the greater
the influence on the evaporation characteristics.
Therefore, for the atomization evaporation simulation
of desulfurization wastewater after concentration, it is
more necessary to adopt wastewater droplet simula-
tion scheme considering the existence of crystalline
salt precipitation and suspended solids.
Table 5: Evaporation time and distance of droplets of wastewater with different salt content.
Salt content (%) 4.336 6.505 8.673 10.841 13.009
Relative mass of remaining
p
articles
(
%
)
4.336 6.505 8.673 10.841 13.009
Residual
p
article size
(
μ
m
)
15.74 18.10 20.01 21.66 23.12
Average evaporation time
(
s
)
0.602 0.587 0.566 0.503 0.489
Maximum evaporation time
(
s
)
1.252 1.134 1.093 1.034 0.971
Average evaporation dis-
tance
(
m
)
5.820 5.717 5.400 4.907 4.744
Maximum evaporation
distance
(
m
)
11.71 10.23 10.48 9.25 9.25
7 CONCLUSION
The variation of evaporation characteristics of
wastewater droplets needs further consideration un-
der the background of concentration and reduction,
which needs comprehensive analysis combined with
wastewater composition and droplet treatment
scheme. By comparing evaporation simulation
schemes of wastewater droplet and exploring the in-
fluence of salt content on droplet evaporation charac-
teristics, the following conclusions are obtained:
(1) The evaporation characteristics of wastewater
droplets obtained by different simulation schemes are
different. For the simulation scheme considering salt
or suspended solids, the unsteady evaporation process
is slow, and the temperature rise of residual particle
0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4
0
20
40
60
80
100
Relative quality of waste water droplets/%
Evaporation time/s
Salt content 4.34%
Salt content 6.50%
Salt content 8.67%
Salt content 10.84%
Salt content 13.01%
0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4
10
20
30
40
50
60
Average particle size of waste water droplets/μm
Evaporation time
/s
Salt content 4.34%
Salt content 6.50%
Salt content 8.67%
Salt content 10.84%
Salt content 13.01%
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
24

after evaporation of wastewater droplets can be sim-
ulated, and the evaporation time and evaporation dis-
tance are longer.
(2) Scheme 5 takes into account the actual com-
position of desulfurization wastewater. The simula-
tion scheme is recommended when simulating atom-
ization evaporation of wastewater.
(3) Under the research conditions in this paper,
when the salt content of wastewater is doubled, the
particle size of remaining particles is increased by
10.3%, the residual relative mass is increased by a
percentage of 2.3, and the total evaporation time and
distance are reduced by 6% on average.
REFERENCES
B. Yang, L. Zhang, Bb. Zuo, et al.. Numerical Simulation
of Evaporation characteristics for Salt-containing
Desulfurization Wastewater Droplets in Low Tempera-
ture Flue [J]. Journal of Engineering Thermo-physics,
41(04): 925-932, 2020.
CHEN Q, THU K, BUI T D, et al. Development of a model
for spray evaporation based on droplet analysis[J]. De-
salination, 399: 69-77,2016.
Gl. Xiong, Hs. Wu, Sq. Li, et al. Numerical Simulation of
the Influence of Soluble Salt on Evaporation Character-
istics of Desulfurization Wastewater Droplet in High
Temperature Flue Gas [J]. Proceedings of the CSEE,
40(16): 5239-5247, 2020.
Gt. Wang. Numerical simulation of flue gas desulfurization
wastewater [D]. North China Electric Power Univer-
sity, 2019.
Hl. Min. Study on Evaporation Process of Droplet in Boiler
Tail gas [D]. Southeast University, 2019.
JEN T C, LI L, CUI W, et al. Numerical investigations on
cold gas dynamic spray process with nano- and micro-
size particles[J]. International Journal of Heat and Mass
Transfer,48(21-22): 4384-4396, 2005.
Jy. Ran, Zr. Zhang. Numerical Study on Evaporation Char-
acteristics of Different Substance Droplet in Low Tem-
perature Flue Gas [J]. Proceedings of the CSEE, 30(26):
62-68, 2016.
K. Miura, T. Miura, S. Ohtani. Heat and mass transfer to
and from droplets[J]. Chemical Engineering Progress
Symposium Series,163(73): 95-102, 1977.
Mq. Kang. Study on flue gas desulfurization wastewater
duct evaporation treatment system design and experi-
ment [D]. Chongqing University, 2013.
Sq, Feng. Numerical simulation on Desulfurization
Wastewater Evaporation Characteristics in Flue Gas
and Process Design of in thermal power plant [D].
North China Electric Power University (Beijing), 2019.
Yw. Wu. Study on the wastewater treatment in limestone-
gypsum wet FGD process [J]. Electric Power, 04: 75-
78, 2006.
Zr. Zhang. Study on Key Problems of the Thermal Power
Plant FGD Waste water with Evaporation treatment
[D]. Chongqing University, 2011.
Zx. Liang. Study on the Characteristics of the Spray Struc-
ture and the Unsteady Evaporation of the Droplets for
the Desulfurization Wastewater [D]. Chongqing Uni-
versity, 2019.
Numerical Study on Droplet Evaporation Simulation Scheme and Evaporation Characteristics of Salt-containing Desulfurization Wastewater
25
