
Protective Effect of Gegen Hawthorn Ginseng Granules on Alcoholic
Liver Injury in Mice
Yang Li
a
, Zhenglong Li
b
, Liying Chang
c
, Shujing Zheng
d
, Maoying Yan
e
and Shumin Wang
f
College of Pharmacy, Changchun University of Chinese Medicine, Changchun, Jilin, China
wangsm@ccucm.edu.cn
Keywords: Gegen Hawthorn Ginseng Granules, Alcoholic Liver Injury, Oxidative Stress, Inflammation, Mechanism of
Action.
Abstract: Gegen hawthorn ginseng granules (GHGG) is composed of 6 Chinese medicinal materials including
pueraria lobata, hawthorn and ginseng. This study aims to explore the protective effect of GHGG on
alcoholic liver injury, and the correlation between oxidative stress, lipid metabolism, inflammation and
alcoholic liver disease. The mice were randomly divided into control group and model group, diammonium
glycyrrhizinate positive drug group, and GHGG low and high dose groups (GHGG-L and GHGG-H). Oral
alcohol was used to establish an acute alcoholic liver injury model. Determination of liver function index
ALT and AST levels after administration; Determination of superoxide dismutase (SOD), malondialdehyde
(MDA) and reduced glutathione in liver tissue (GSH), TNF-α, IL-1β, IL-6 and other biochemical indicators;
HE staining to analyze the morphological changes of tissue sections. Experimental results show that GHGG
can significantly reduce the levels of ALT, AST, and MDA in mice; increase the level of liver tissue The
activity of SOD and GSH, and GHGG can significantly inhibit the levels of biochemical indicators such as
pro-inflammatory factors TNF-α, IL-1β, IL-6. The degree of pathological changes in the liver tissue of mice
in the GHGG group was significantly reduced. In summary, GHGG can improve ALD by anti-oxidation,
inhibiting inflammation, and promoting liver cell regeneration.
1 INTRODUCTION
1
In recent years, the global incidence of alcoholic liver
disease (ALD) has been increasing year by year, and
it has become a major disease that seriously
endangers human health. The main clinical
symptoms are hepatitis and liver fibrosis, and severe
cases can lead to liver cirrhosis and liver cancer
(Kong 2019). Treatment is mainly through drug
intervention and liver transplantation (Bloom Patricia
2021). So far, there have been many researches and
developments of alcoholic liver disease drugs at
home and abroad, but they have not yet been able to
meet the clinical needs. Its pathogenesis is complex
a
https://orcid.org/0000-0003-4579-939X
b
https://orcid.org/0000-0003-4352-3023
c
https://orcid.org/0000-0001-7719-8367
d
https://orcid.org/0000-0003-3509-8362
e
https://orcid.org/0000-0001-7243-1342
f
https://orcid.org/0000-0002-0730-4475
and diverse, among which oxidative stress, abnormal
lipid metabolism, disturbance of intestinal flora, and
inflammation are currently considered to be the main
causes of ALD (Kong 2019, Natalia 2017,
Woodhouse 2018). In this experiment, by studying
the effects of GHGG on the antioxidant enzyme
activity, liver function index levels in mice with
acute alcoholic liver injury and detecting the level of
related inflammation in mice with acute alcoholic
liver injury, the GHGG can relieve alcohol The liver-
protecting effect and its mechanism of action are
preliminarily discussed to provide experimental
evidence for its clinical application.
146
Li, Y., Li, Z., Chang, L., Zheng, S., Yan, M. and Wang, S.
Protective Effect of Gegen Hawthorn Ginseng Granules on Alcoholic Liver Injury in Mice.
DOI: 10.5220/0011192300003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 146-151
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

2 MATERIALS AND METHODS
2.1 Materials
2.1.1 Medicines and Reagents
GHGG (Homemade in the laboratory); diammonium
glycyrrhizinate (China Zhengda Tianqing
Pharmaceutical Group Co., Ltd.); alanine
aminotransferase (ALT), aspartate aminotransferase
(AST), malondialdehyde (MDA), Superoxide
dismutase (SOD), reduced glutathione (GSH)
detection kit (Nanjing Jiancheng Institute of
Biological Engineering). Tumor Necrosis Factor
(TNF-α), Interleukin-6 (IL-6), IL-1β (Jiangsu
Enzyme Industry Co., Ltd.); BCA protein
concentration determination kit (Shanghai Biyuntian
Biotechnology Limited company).
2.1.2 Animals
50 SPF KM mice, provided by (Liaoning
Changsheng Biotechnology Co., Ltd., production
license number: SCXK (Liao) 2020-0001; certificate
number: 210726200100461748). The animals are
maintained under a 12h/12h light/dark cycle at
25±3℃ and a relative humidity of 50±20%. This
study was conducted in accordance with the
Declaration of Helsinki, and the protocol has been
approved by the Animal Health and Welfare
Committee of Changchun University of Chinese
Medicine (20190123). The procedures involving
animals and their care comply with the institutional
guidelines of national and international laws and
policies.
2.2 Methods
2.2.1 Design of Animal Experiment
Fifty mice were randomly divided into 5 groups,
namely control group, model group, diammonium
glycyrrhizinate positive drug group (150 mg/kg bw)
(Zhang 2020) and GHGG low and high dose groups
(800, 2000mg/kg bw), each group has 10 animals.
Gavage is given once a day for 10 consecutive days.
Four hours after the last administration, except the
control group, the mice in each group were given
56% ethanol (12mL/kg bw) to establish an acute
alcoholic liver injury model. The blank control
group mice were given an equal volume of distilled
water.
2.2.2 Measurement of Liver Index
The liver of the mice was aseptically removed, the
final body mass and organ mass of the mice were
weighed, and the organ coefficient was calculated
according to the ratio of the organ mass to the body
mass.And weighed to measured organ index
according to thefollowing formula:
Organ index (%) = organ mass/final body
mass × 100%
(1)
2.2.3 Determination of Biochemical
Indicators of Liver Function
Blood was collected from the eyeballs of mice. After
clotting at 4℃ for 1 hour, the blood was centrifuged
at 4000 r/min for 15 minutes. The supernatant was
collected as serum. The ALT and AST levels were
determined according to the kit instructions.
2.2.4 Determination of Oxidative Stress
Indicators
Take the liver, place it in a glass tissue homogenizer,
add appropriate amount of physiological saline,
prepare a 10% (m:V) tissue homogenate, centrifuge
at 4000r/min for 15 minutes, collect the supernatant,
and determine SOD, MDA and GSH according to
the kit instructions level.
2.2.5 Determination of Hepatic
Proinflammatory Cytokines
Take the liver, place it in a glass tissue homogenizer,
add an appropriate amount of physiological saline,
prepare a 10% (m:V) tissue homogenate, centrifuge
at 4000r/min for 15 minutes, collect the supernatant,
and according to the kit instructions to determine the
levels of IL-6, IL-1β and TNF-α.
2.2.6 Histological Investigation of Liver
Samples of liver were separated from each mouse
and fixed in formalin solution (10%) for 24 h, and
after that dehydrated using graded alcohol and
xylene, and implanted in paraffin. Paraffin-
embedded sections, stained with hematoxylin and
eosin (H&E) for histological investigation.
2.2.7 Statistical Analysis
The data of the animal experiment was evaluated by
using Graph Pad Prism version 5 (La Jolla, CA,
USA). One-way analysis of variance (ANOVA) was
used to compare variations between groups,
Protective Effect of Gegen Hawthorn Ginseng Granules on Alcoholic Liver Injury in Mice
147
followed by Duncan’s multiple range test.
Differences between groups were found statistically
significant at p < 0.01 or p < 0.05 and the data was
expressed as mean ± SD.
3 RESULTS
3.1 Effect of GHGG on Body Weight
and Organ Index in ALD Mice
The food intake of each group of mice was recorded,
and the calculation formula was intake g/day. The
results in Table 1 show that compared with the
normal group, the food utilization rate of the model
group was significantly reduced (p<0.05), and the
feed intake of GHGG-H was significantly improved
(P<0.05). The liver index results are shown in Table
1. The liver index of the model group was
significantly higher than that of the control group
(p<0.05). However, compared with the model group,
giving different doses of GHGG (GHGG-L and
GHGG-H) can reduce liver swelling. There was no
significant difference between GHGG-L and
GHGG-H groups (p>0.05).
Table 1: Body weight and organ index in ALD mice.
Control Model Positive drug GHGG-L GHGG-H
Food intake
g/day
6.30±0.25 4.94±0.25
#
5.67±044
*
5.24±0.18 5.47±0.40
*
Liver index
%
4.01±0.27 6.07±0.33
#
4.60±0.17
*
4.31±0.43
*
4.46±0.53
*
p
#
<0.05 represents compared with the control group, and p
*
<0.05represents compared with the model group.
3.2 Effects of GHGG on Liver
Function Indexes of ALD Mice
AST and ALT are biochemical markers for clinical
evaluation of liver function. When liver function is
impaired, the levels of AST and ALT increase
sharply compared with normal physiological
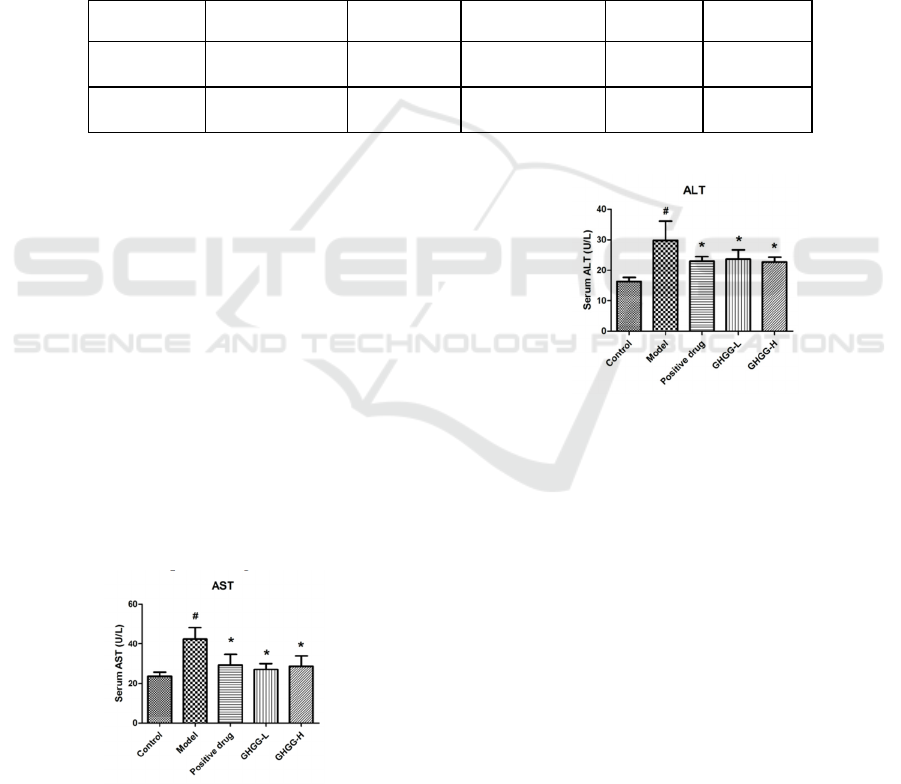
conditions (Katrine 2021, Sun 2021). Figure 1-2
shows the effect of GHGG on serum AST and ALT
in ALD mice. The levels of AST and ALT in the
model group were significantly higher than those in
the blank group (P<0.05), indicating that the liver
was damaged after a large amount of alcohol intake,
which led to a significant increase in serum ALT and
AST. GHGG in different dose groups can
significantly reduce the serum content level and
achieve the effect of protecting the liver.
Figure 1: AST levels in serum of ALD mice.
Figure 2: ALT levels in serum of ALD mice.
3.3 Effects of GHGG on Antioxidant
Capacity of Liver Tissue in ALD
Mice
Oxidative stress is one of the main mechanisms of
the pathogenesis of alcoholic liver disease, and it has
an important impact on the initial liver fibrosis and
hepatocellular carcinoma (Zhang 2018, NWFLALD
2018, Xia 2017). MDA, SOD and GSH play an
important role in protecting alcohol-induced liver
damage and oxidative stress. Figure 3-5 shows the
effect of GHGG on liver antioxidant enzymes MDA,
SOD and GSH. Compared with the control group,
the liver MDA level of the model group was
significantly increased (p<0.05). Compared with the
model group, the MDA content of the GHGG
treatment group (GHGG-L and GHGG-H) was
significantly reduced (p<0.05). In addition,
compared with the control group, the liver SOD and
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
148
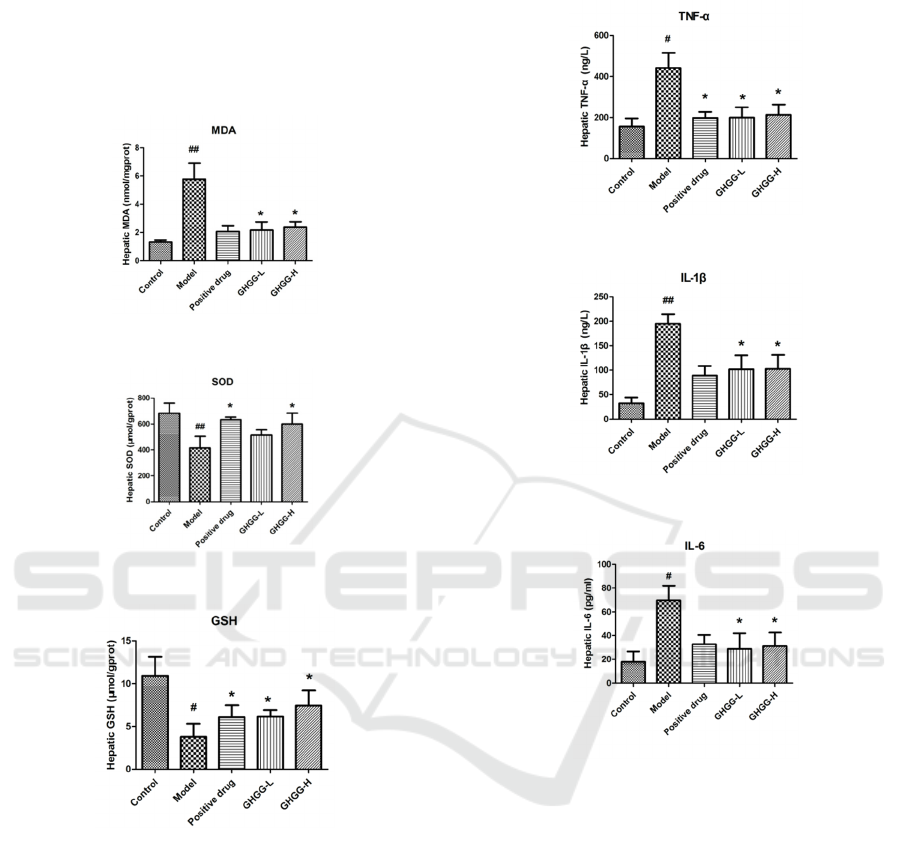
GSH levels in the model group were significantly
lower (p<0.05), and the GHGG administration group
(GHGG-L and GHGG-H) could increase their
levels. The experimental results show that GHGG
can reduce the content of MDA and increase the
activity of antioxidant enzymes SOD and GSH to
protect the liver in mice with alcoholic liver injury.
Figure 3: MDA levels in tissues of ALD mice.
Figure 4: SOD levels in tissues of ALD mice.
Figure 5: GSH levels in tissues of ALD mice.
3.4 Effects of GHGG on the Level of
Pro-inflammatory Cytokines in the
Liver of ALD Mice
The results of pro-inflammatory cytokines such as
IL-6, IL-1β and TNF-α in mouse liver tissue are
shown in Figure 6-8. Compared with the blank
group, the liver TNF-α, IL-1β and IL-6 of the mice
in the model group all increased significantly
(p<0.05). Compared with the model group, the
GHGG administration group (GHGG-L and GHGG-
H) can significantly reduce the levels of TNF-α, IL-
1β and IL-6 (p<0.05). The above results indicate that
GHGG can protect liver cells by reducing the
occurrence of alcohol-induced liver inflammation.
Figure 6: TNF-α levels in tissues of ALD mice.
Figure 7: IL-1β levels in tissues of ALD mice.
Figure 8: IL-6 levels in tissues of ALD mice.
3.5 Effects of GHGG on
Histopathological Characteristics of
Liver in ALD Mice
The H&E staining method was used to study the
pathological changes of liver tissue in ALD mice. As
shown in Figure 9, the structure of the liver lobules
in the control group was complete and clear, the
liver cells were arranged in an orderly manner, and
the nuclei were complete and clear. The liver tissue
sections of mice in the model group showed obvious
inflammatory cell infiltration, fatty vacuoles and
hepatocyte enlargement. Compared with the model
group, the GHGG administration group (GHGG-L
and GHGG-H) had less edema, fatty vacuoles and
inflammatory cell infiltration.
Protective Effect of Gegen Hawthorn Ginseng Granules on Alcoholic Liver Injury in Mice
149
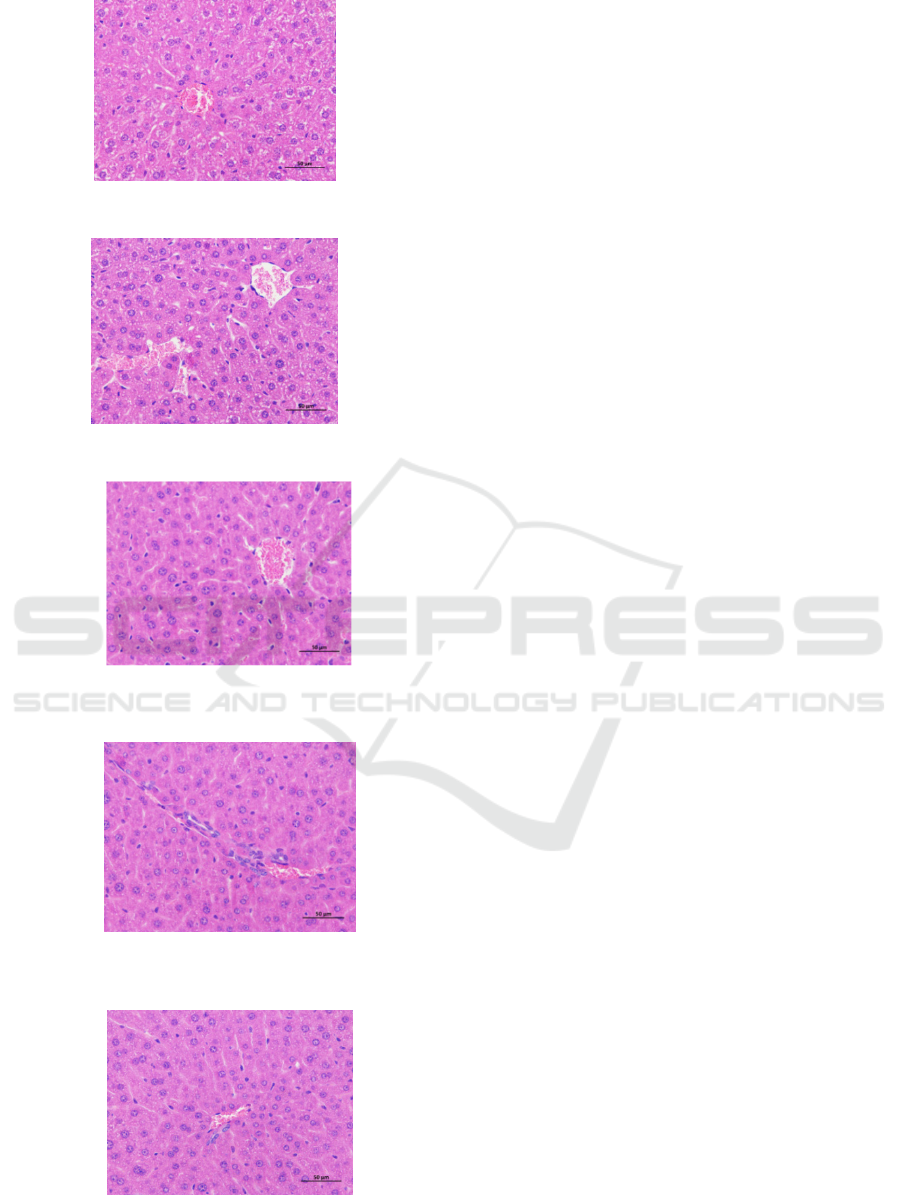
Figure 9: Liver tissue slices of mice in the control group
Figure 10: Liver tissue slices of mice in the model group
Figure 11: Liver tissue slices of mice in the positive drug
group
Figure 12: Liver tissue slices of mice in the GHGG-L
group
Figure 13: Liver tissue slices of mice in the GHGG-H
group
4 DISCUSSION
The liver is one of the main organs for alcohol
metabolism and the largest gland in the human body.
It has very important physiological functions (Li
2019). When the human body consumes excessive
alcohol, a large amount of metabolic waste cannot
be excreted in time, which will cause degeneration
and necrosis of liver cells, which will cause
disorders of related metabolic pathways in the cells,
cause liver cells to produce inflammation and
oxidative stress, fibrosis and lead to liver lipids. and
its can induce liver cell damage (Xie 2021, Tu 2019,
TESCHKE 2018). For oxidative stress, excessive
alcohol intake leads to the weakening of SOD's
antioxidant capacity, and the body's oxidation and
antioxidant balance is disrupted, causing oxidative
stress in the body, leading to a chain reaction of lipid
peroxidation, and damage to mitochondrial function.
Liver damage caused by endoplasmic reticulum
stress and immune inflammatory response (Zhang
2021). MDA is an important product of lipid
peroxidation, and its content can reflect the degree
of lipid peroxidation in the body (Rani 2016), GSH
is an important antioxidant substance in the body. It
is a substrate of two enzymes, GSH-Px and GSH-
ST. It is a low-molecular scavenger that can remove
O2, H2O2, so the level of GSH content is a measure
of the body’s antioxidant capacity (Wu 2021). The
balance of pro-inflammatory cytokines and anti-
inflammatory cell levels is essential for maintaining
human health (Sun 2021). In this experiment, we
tested the levels of three inflammatory cytokines,
TNF-α, IL-1β and IL-6. TNF-α is significantly
increased during alcoholic liver injury compared to
normal physiological levels (Shen 2018). It can be
activated by binding to caspase3 to induce apoptosis
of hepatocytes (Dalia 2019). At the same time, it
also activates the NF-κB signaling pathway, triggers
the secretion of inflammatory factors such as IL-1β
or IL-6, further aggravates liver inflammation and
induces the occurrence of alcoholic liver disease
(Liu 2017).
5 CONCLUSIONS
In summary, GHGG can effectively reduce liver
lipid peroxidation levels by inhibiting the increase of
liver function indicators in mice, regulate liver
metabolic disorders, and improve the antioxidant
capacity of mice, thus playing a protective role on
alcohol-induced acute liver injury in mice. This
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
150

research lays an experimental foundation for the in-
depth development of drugs for the treatment of
alcoholic liver injury, provides a basis for clinical
medication, and also broadens the use of medicinal
materials for both food and medicine.
REFERENCES
Bloom Patricia P,and Fontana Robert J. (2021) "With
Alcohol as the fuel, COVID is the match: Liver
Transplantation for Alcoholic liver disease is
increasing in the United States." J. Hepatology
(Baltimore, Md.). doi:10.1002/HEP.32141.
Dalia, F., Amira, B., Hala A, A. (2019) Hepatoprotective
activity of raspberry ketone is mediated via inhibition
of the NF-κB/TNF-α/caspase axis and mitochondrial
apoptosis in chemically induced acute liver injury. J.
Toxicology research. 8:663-676.
Kong, L.Z., Chandimali, N., Han, Y,H., Lee, D,H., Kim,
J,S., Kim, S,U., Kim, T,D., Jeong, D,K., Sun, H,N.,
Lee, D,S., Kwon, T. (2019) Pathogenesis, Early
Diagnosis, and Therapeutic Management of Alcoholic
Liver Disease. J. International Journal of Molecular
Sciences, 20:2712.
Katrine P, L., Thor L, H., Bjørn S, M., Maria, K., Linda,
M., Sönke, D., Aleksander, D., Maja, T. (2021)
Diagnostic accuracy of routine liver function tests to
identify patients with significant and advanced
alcohol-related liver fibrosis. J. Scandinavian Journal
of Gastroenterology. 56:1088-1095.
Li, T. (2019) Classification and research status of liver
function detection methods. J. Liver. 24:952-955.
Liu, Z.F., Huang, W., Li, Q., (2017) The clinical
significance of dynamic detection of cytokines in
patients with chronic acute (subacute) liver failure. J.
Anhui Medicine. 21:263-266.
Natalia A, O., Terrence M, D., Kusum K, K. (2017).
Alcoholic Liver Disease: Pathogenesis and Current
Management. J. Alcohol research: current reviews,
38:147-161.
National Workshop on Fatty Liver and Alcoholic Liver
Disease, Chinese Society of Hepatology, Chinese
Medical Association; Fatty Liver Expert Committee,
Chinese Medical Doctor Association. (2018)
Guidelines for Prevention and Treatment of Alcoholic
Liver Disease (Updated in 2018). J. Journal of Clinical
Hepatobiliary Diseases. 34:939-946.
Rani, V., Deep, G., Singh, R.K., Palle, K., Yadav, U.C.S.
(2016) Oxidative stress and metabolic disorders:
Pathogenesis and therapeutic strategies. J. Life
Sciences.148:183-193.
Sun, Y.D., Tian, Z.Z., Zhou, W., Li, M., Huai, H., He, L.,
Qin, S.Y., (2021) Genome-wide association study on
liver function tests in Chinese. J. Yi chuan =
Hereditas.43:249-260.
Sun, L.L., Wen, S., Li, Q.H., Lai, X.F., Chen, R.H.,
Zhang, Z.B., Li, D.L., Sun, S.L. (2021) L-theanine
relieves acute alcoholic liver injury by regulating the
TNF-α/NF-κB signaling pathway in C57BL/6J mice.
J. Journal of Functional Foods. 86:104699.
Shen, F., Wang, Z.H., Liu, W., Liang, Y.J. (2018) Ethyl
pyruvate can alleviate alcoholic liver disease through
inhibitingNrf2 signaling pathway. J. Experimental and
Therapeutic Medicine.15:4223-4228.
Tu, Y.F., Zhu, S., Wang, J., Ezra, B., Da, J. (2019) Natural
compounds in the chemoprevention of alcoholic liver
disease. J. Phytotherapy research: PTR. 33:2192-2212.
TESCHKE, R. (2018) Alcoholic liver disease: alcohol
metabolism, cascade of molecular mechanisms,
cellular targets, and clinical aspects. J. Biomedicines.
6:106.
Woodhouse CA, Patel VC, Singanayagam A, Shawcross
DL. (2018) The gut microbiome as a therapeutic target
in the pathogenesis and treatment of chronic liver
disease. J. Aliment Pharmacol Ther.47:192–202.
Wu, X.H., Li, H., Wan, Z.J., Wang, R., Liu, J., Liu, Q.F.,
Zhao, H.Y., Wang, Z.H., Zhang, H.R., Guo, H., ,Qi,
C.H., ,Jiao, X.Y., Li, X.T. (2021)The combination of
ursolic acid and empagliflozin relieves diabetic
nephropathy by reducing inflammation, oxidative
stress and renal fibrosis. J. Biomedicine &
Pharmacotherapy. 144:112267-112267.
Xia, T., Zhang, J., Yao, J.H., Zheng, Y., Song, J., Wang,
M. (2017) Research progress on the mechanism of
oxidative stress in alcoholic liver disease. J. Chinese
Pharmacological Bulletin. 33:1353-1356.
Xie, Y.D. (2021) There are many alcoholic drinkers in
patients with liver disease, and the harm is great. J. Dr.
Liver.1:47-48.
Zhang, Y., Zhao T.Y., Wang, H., Wang, S.M. (2020) The
effect of ergosterone on acute alcoholic liver injury
and intestinal flora in mice. J. China Modern Applied
Pharmacy.37:2561-2569.
Zhang, C., Wang, N., Xu, Y., Tan, H.Y., Li, S., Feng, Y.B.
(2018) Molecular Mechanisms Involved in Oxidative
Stress-Associated Liver Injury Induced by Chinese
Herbal Medicine: An Experimental Evidence-Based
Literature Review and Network Pharmacology Study.
J. International journal of molecular sciences.19:2745.
Zhang, F.F., Cheng, M.D., Zhou, S.S., Liu, J.K., Wang, R.
(2021) Protective effects of glycyrrhizin on liver
injury in mice with acute alcohol infusion. J. Journal
of Fuyang Normal University (Natural Science
Edition). 38:69-72+84.
Protective Effect of Gegen Hawthorn Ginseng Granules on Alcoholic Liver Injury in Mice
151
