
Recent Advancements of Anti-cancer Nanomedicine in Breast Cancer
Jiachen Li
a
Indiana University Bloomington, U.S.A.
Keywords: Nanomedicine, Cancer, Anti-Cancer, Cancer Treatment.
Abstract: Nanomedicine is a novel type of cancer treatment that has been applied to clinical practice for its identified
safety and potency against cancer cells and safety. Conventional cancer treatments such as surgery,
radiation, and chemotherapy work generally well but inevitably bring severe adverse reactions and
sometimes unsatisfying results. Breast cancer is one of the most major cancer with high incidence rate and
mortality rate, characterized by high risks and a great reduction in of life quality of life with high incidence
rate and mortality rate. In the past three decades, nanotechnology emerged to giving rise to novel forms
offer anti-cancer drug delivery systems and offering greater therapeutic therapeutical advantages than
traditional cancer treatments can do. With the incorporation of nanocarriers, higher drug loading efficiency,
targeted delivery, enhanced bioavailability, and stronger cytotoxicity, and less side effects can be achieved.
This paper shows the recent developments of nanocarrier-incorporated anti-cancer drugs with specific to
breast cancer. Ways of newly synthesized drug loaded nanoparticles alleviating side effects from
conventional treatments and improving therapeutic effects are stressed in this paper.
1 INTRODUCTION
1
Cancer, the second leading cause of death, is a group
of diseases induced by uncontrollable abnormal cell
division and replication. According to the World
Health Organization’s International Agency for
Research on Cancer Global Cancer Observatory
(GLOBCAN), in 2018, there were 18 million new
cancer incidences and 9.5 million related deaths
worldwide, and by 2040 the number of new cases
will rise to 29.5 million and the number of related
deaths would be 16.4 million (Hulvat, 2020).
Conventional treatments for cancer are surgery,
radiation, and chemotherapy. For cancers in early
stage, surgery and radiation work well. Surgery is an
important approach to tumors for the ability to
remove the lower grade benign tumors. Surgery,
however, becomes extremely difficult when the
tumor is located on unreachable sites. Moreover,
side effects like headaches, fatigue, and further
damage to the brain tissue may happen after the
surgery. Radiation is a cancer therapy applying high
energy radiation to eliminate cancer cells, which is
not an ideal approach because there is an annual and
a lifetime exposure limit, and it inevitably affects
adjacent normal cells. Radiation may also bring
a
https://orcid.org/0000-0001-5389-6128
adverse symptoms such as nausea, hair loss, and
diarrhea. The other side effect is that when
metastasis occurs, the surgery and radiation become
ineffective. Metastasis refers to that the secondary
cancer develops and spreads to different sites in the
body far from its initial site. When the tumor is
metastasized, surgical removal may enhance tumor
recurrence (Tohme, Simmons, Tsung, 2017).
Similarly, radiation promotes metastasis and
increases the possibility of recurrence, suggesting by
abundant clinical data (Vilalta, Rafat, Graves, 2016).
Later, since 1940s, chemotherapy has been
utilized as a treatment of cancers. Chemotherapy is
characterized by fast killing of cancer cells by
delivering chemical agents that disturb cancer cell’s
replication. It is used to treat metastatic cancer,
which has spread to other parts of the body. The
drugs are delivered through the bloodstream and
reach cancer cells; however, chemotherapy has some
severe problems. Chemotherapy eliminates normal
cells along with cancer cells. Moreover, nausea,
vomiting, and neutropenia are commonly observed
after receiving chemotherapy.
Breast cancer is one of the major cancers with
high incidence and mortality rate (DeSantis, Ma,
Gaudet, et al 2019). Female breast cancer has
become the leading cause of global cancer incidence
152
Li, J.
Recent Advancements of Anti-cancer Nanomedicine in Breast Cancer.
DOI: 10.5220/0011192400003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 152-159
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

in 2020, in which unhealthy lifestyle plays a role in
it other than hormonal factors. The risk of breast
cancer is associated with personal lifestyle. Obesity,
insufficient physical activity, and high alcohol
consumption are risk factors of breast cancer which
are many people are currently having.
The specific cause of cancer is still under
investigation. Up until now, scientists believe that
the formation of cancer is associated with oncogenes
and tumor suppressor genes. Oncogenes are found to
be expressed at a high level in cancer cells. They
emerge when proto-oncogenes, which promotes
positive cell growth, mutate to become activated
oncogenes (Lam, Schmidt, 2012). As a result, the
cell starts to uncontrollably divide and promotes
carcinogenesis (Lam, Schmidt, 2012). On the
contrary, tumor suppressor genes, also called anti-
oncogenes, regulate normal cell division and inhibit
cell proliferation (Krasin, Davidoff, 2012). The
inactivation of tumor suppressor genes leads to their
malfunction, inducing infinite cell replication, or the
development of malignancy. (Lam, Schmidt, 2012).
Most current anti-cancer drugs or therapies either
kill fast-growing cancer cells along with normal
cells (e.g., chemotherapy, radiation) or target
specific proteins inside or outside cancer cells. (e.g.,
small molecule drugs and monoclonal antibodies).
Target therapies can be identified through the
examination of highly expressed proteins in cancer
cells; while in normal cells, the amount of the same
kind of proteins remains low (Kampen, 2011). For
example, human epidermal growth factor receptor 2
(HER2) is a protein overexpressing on the surface of
approximately 20-30 % of breast cancer cells. (Mitri,
Constantine, O’Regan, 2012). Trastuzumab and
Pertuzumab are antibodies that target HER2. (Kunte,
Abraham, Montero, 2020). Another difference
between cancer cells and normal ones that inspire
novel drugs is that cancer cells require large supply
of oxygen to sustain cell replication and to spread.
Cancer cells often experience hypoxia, the decreased
level of oxygen (McKeown, 2014), so the average
oxygen level is lower in cancer cells. Angiogenesis,
the formation of new blood vessels, is observed a
large increase when benign tumor transforms into
malignant one (Brustmann, Riss, Naudé, 1997),
which is the induced response of hypoxia (Chen,
Endler, Shibasaki, 2009). Under such circumstances,
angiogenesis inhibitors are applied to restrain the
cancer blood vessel growth (Klagsburn, Moses,
1999). Clinically approved drugs are Trastuzumab
and Pertuzumab for advanced breast cancer, only to
name a few. Along with conventional treatments,
anti-tumor target drugs alone such as Trastuzumab
and Pertuzumab could bring serious side effects
(Bines, Clark, Barton, et al, 2021) Given such
situation, a less painful approach with higher
therapeutic efficiency is needed.
In recent years, people have gained greater
understanding of nanomedicine as a novel approach
to cancer treatment. Nanomedicine is the application
of nanotechnology for medical therapeutics by using
nano-scaled agents to treat diseases. Nanomedicine
possesses properties of targeted delivery, small-scale
size, decent permeability, and bioavailability (Patra,
Das, Fraceto, et al, 2018). Nanodrugs synthesized by
materials such as liposomes, polymers, inorganic
particles, and peptides are proved to be feasible
ways to enhance the effectiveness of cancer
treatments by the clinical data. They target specific
to prevent the damage to normal tissues and cells,
thereby exhibiting high cytotoxic concentration in
tumors. The encapsulation of drugs into nanocarriers
protects anti-cancer drugs from degradation,
improving the drug delivery efficiency (Patra, Das,
Fraceto, et al, 2018).
Previous researches have done to integrate the
advantages and clinical practices of nanocarriers
with a broad focus on several cancers. However,
within the rapid development of nanomedicine in
past ten years, new forms of nanodrugs and clinical
applications emerge; therefore, an update that
includes the analysis of past nanomedicine and how
they evolve within times with more specific focus is
needed.
This paper provides a description of recent
nanomedicine-incorporated cancer treatments with
specific to breast cancer. The specific advantages
and working mechanisms of common nanoparticles:
liposome, porous silicon, and dendrimer are
described. Disadvantages of traditional drugs such as
Tamoxifen, Doxorubicin, and Trastuzumab, and
how newly synthesized drug loaded nanoparticles
address these problems and offers therapeutic effects
are stressed in this paper.
2 COMMON NANOMEDICINE
FOR BREAST CANCER
TREATMENT
Nanomedicines has been applied to clinical practice
and they are still under intensive investigation, for
its potential and effectiveness of anti-tumor.
Liposome, porous silicon, and dendrimer are
commonly used nanocarriers to treat breast cancer.
Their working mechanisms and advantages are
Recent Advancements of Anti-cancer Nanomedicine in Breast Cancer
153
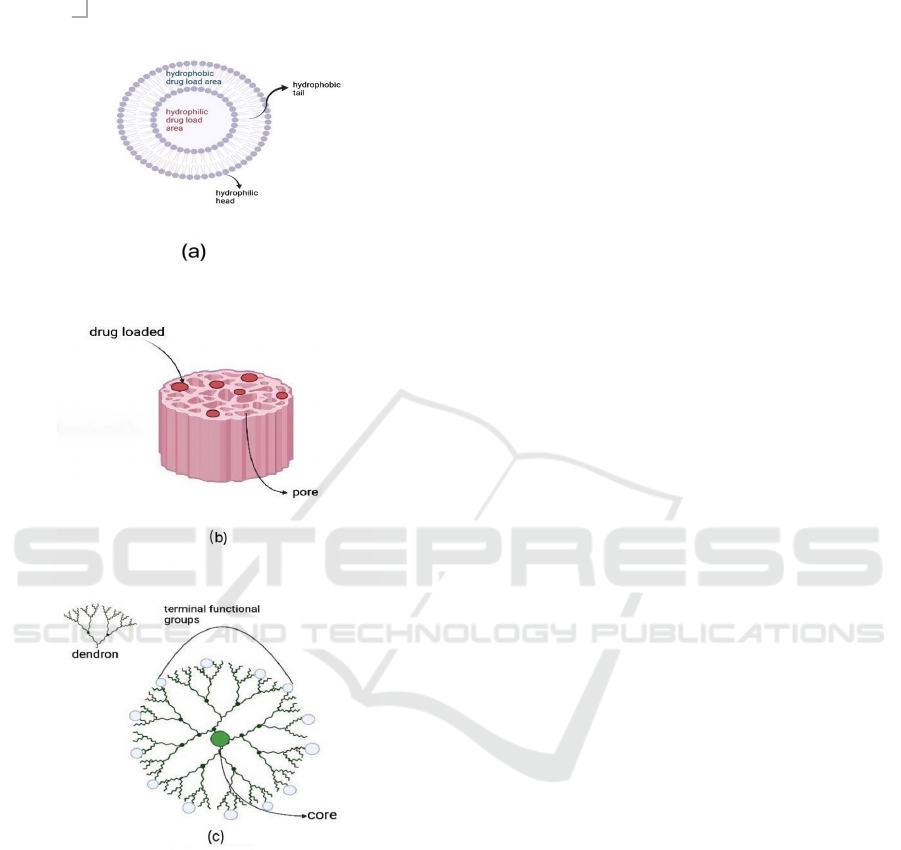
briefly described below, for which are core concepts
in understanding later introduction of nanoparticle-
incorporated drugs.
Figure 1: Scheme diagram for six nanocarriers: (a)
Liposome, (b) Porous Silicon, (c) Dendrimer.
2.1 Liposome
Liposome is a spherical nanosized vesicle that
consists of an aqueous core and phospholipid
bilayers. The bilayer is composed of hydrophilic
heads and hydrophobic tails, making it amphipathic.
As a result, liposomes can carry both hydrophilic
and hydrophobic drugs without degeneration.
Normally, drug-loaded liposome works in four
patterns: (Sharif, Fazle, Nazir, 2006)
1. Endocytosis by phagocytic cells, absorbing
substances by cell membrane’s engulfment
(Phagocytosis and Intracellular Killing,
2012). Adsorption to the surface of the cell
by interactions with components on the
surface.
2. Fusion with the plasma membrane by the
interaction between phospholipid bilayers
and plasma membrane, releasing the
contents loaded in the core of liposome.
3. Transfer of liposomal membranes to
cellular membranes.
2.2 Porous Silicon (pSi)
Porous silicon is a sponge-like nanostructure in
which microstate crystalline silicon is introduced.
The pSi has proved to possess excellent
biocompatibility and biodegradability due to its
unique porous structure and chemical properties
(Kumeria, McLinnes, Maher, Santos, 2017). Porous
silicon is identified by large surface area and internal
volume, allowing high loading capacity and
enhanced adsorption ability (Santos, Mäkilä,
Airaksinen, Bimbo, Hirvonen, 2014). Once pSi
arrives at the targeted site and releases the loaded
drug, it degrades into silicic acid which is harmless
to human body and easily removed by kidneys
(Manj, Chen, Rehman, Zhu, Luo, Yang, 2018).
2.3 Dendrimer
Dendrimers are ordered, branched three dimensional
polymetric molecules. They have symmetric and
monodisperse structure which consists of a core,
branched, symmetric dendrons, and terminal
functional groups (Abbasi, Aval, Akbarzadeh, et al,
2014) The internal cavities of dendrimers enable the
encapsulation of drugs, making excellent stability
and solubility (Santos, Veiga, Figueiras, 2020).
Dendrimers’ properties can be possibly modified
and controlled, for various terminal groups attached
are responsible for the interaction of dendrimers and
external molecules (Han YL, Kim SY, Kim T, Kim
KH, Park JW, 2020).
3 BREAST CANCER
3.1 Tamoxifen
Tamoxifen (TMX), an antiestrogen, is a traditional
clinically proved hormonal treatment for breast
cancer (Yang, Nowsheen, Aziz, Georgakilas, 2013).
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
154

It has dual mechanism of action: (1) inhibiting
estrogen action and blocking the binding of estradiol
(E
2
), (2) binding with DNA after metabolic
activation and initiating carcinogenesis (Yu, Bender,
2001, Craig Jordan, 1992). TMX is reported to
reduce the incidence of oestrogen positive breast
cancer by 38% among high-risk patients (Singh,
2021). It decreases the taker’s death rate and
recurrence rate (Gray, Rea, Handley, et al, 2013).
TMX, however, induces side effects (Osborne,
1998). Besides those common adverse reactions
such as hot flashes, sleep problems, and vaginal
dryness, it stays on estrogen receptors in tumor
tissue for several months after the treatment is
stopped and gives false negative results (Osborne,
1998). Moreover, TMX treatment promotes the
development of endometrial cancer and increases the
risk of it (Bergman, Beelen, Gallee, Hollema,
Benraadt, Van Leeuwen, 2000).
Porous silicon (pSi) based nanomaterials have
been identified the potential to be excellent carriers
for cancer treatment. Inspired by the side effects of
TMX and the advantages of pSi, researchers
synthesized TMX-loaded pSi nanoparticle to further
improve the bioavailability of TMX (Haidary,
Mohammed, Córcoles, Ali, Ahmed, 2016). The drug
release is controlled by the rate of degradation of pSi
due to its biodegradable property (Haidary,
Mohammed, Córcoles, Ali, Ahmed, 2016).
Biocompatible, non-toxic material chitosan and
silica xerogel hybrid is used on surface coating to
prevent infection, and the hybrid coating produces
outstanding drug release results (Haidary,
Mohammed, Córcoles, Ali, Ahmed, 2016).
The price of chemicals needed for preparation,
hydrosilylation, and bioactive coating is moderate.
For instance, 2.5 L 37% hydrochloric acid is about
$112 on Sigma Aldrich, while only 0.5 mL diluted
HCI (2%) is needed for silica xerogel preparation,
similar situation to other chemicals required.
Obstacles of industrial production of TMX-loaded
pSi nanoparticle, however, still remain. The process
of preparation of pSi particle, the hydrosilylation,
and the bioactive coating, is complicated, making
the large production expensive and time-consuming.
3.2 Doxorubicin
Doxorubicin (DOX), an antibiotic derived from
bacterium Streptomyces peucetius, is another
commonly used anti-breast cancer agent with strong
effectiveness (Christowitz, Davis, Isaacs, Van
Niekerk, Hattingh, Engelbrecht, 2019). The primary
working mechanism of DOX involves intercalation
of DNA pairs, breaking the DNA strand and
inhibiting the DNA and RNA synthesis (Agrawal,
2007). DOX brings severe adverse effects like other
widely applied agents. For instance, DOX is highly
toxic and it increases the risk of potentially fatal
cardiotoxicity; therefore, its dose should be limited
strictly (Zhao, Ding, Shen, Zhang, Xu, 2017). Other
deleterious side effects include myocardial damage
and heart failure (Redfors, Shao, Råmunddal, et al,
2012).
When treating tumors, DOX alone is of rather
low drug loading efficiency due to the hamper of
abnormal, tortuous blood vessels; only 5-10% of
drugs enter the tumor tissue and take effect (Chang,
Li, Lu, Jane, Wu, 2013). To increase the higher drug
load efficiency and to achieve better therapeutic
effects, PEGylated liposomal doxorubicin (PLD), a
formulation of doxorubicin packed into liposome
with polyethylene glycol outer coating, was created
by reseachers (Green, Rose, 2006). With
nanocarrier’s encapsulation, 15,000 DOX molecules
per vesicle with over 95% drug loading efficiency is
achieved (Chang, Li, Lu, Jane, Wu, 2013, Gabizon,
2001). Small size of liposomal carrier contributes to
better tumor accumulation; the smaller the size, the
better tumor accumulation (Gabizon, 2001).
Moreover, PLD is observed to have longer half-life
and slower clearance than non-PEGylated liposome
and free DOX, which means that PLD has the ability
to achieve longer circulation time (Gabizon, 2001).
All in all, PLD has revealed great potential in
making a perfect anti-cancer practice.
The therapeutic value of DOX is further
improved by the encapsulation of nanocarriers on
which modified by other tumor target chemical
agents. For example, after the Clot-binding
pentapeptide Cys-Arg-Glu-Lys-Ala (CREKA) has
gained the recognition of the ability to recognize
fibrin-fibronectin complexes that overexpress in
tumor vessel endothelium and stroma rather than
normal cells, making CREKA a target peptide of
effectiveness and precise target delivery (Shi,
Zhang, Liu, et al, 2018, Jiang, Song, Yang, et al,
2018). In a recent study, CREKA modified
liposomal DOX (CREKA-Lipo-DOX) has been
synthesized and proved its therapeutic effects (Jiang,
Song, Yang, et al, 2018). Compared to free DOX
with rapid release, the drug release of CREKA-Lipo-
DOX is more sustained with little burst; the release
of CREKA-Lipo-DOX is slightly faster than those of
PLD.[40] Though PLD improves the anticancer
efficiency of free DOX, CREKA-Lipo-DOX can
significantly inhibit cancer cell growth and
metastasis in vivo. Furthermore, CREKA-Lipo-DOX
Recent Advancements of Anti-cancer Nanomedicine in Breast Cancer
155
is safer than PLD and DOX (Attia, Anton, Wallyn,
Omran, Vandamme, 2019). Study demonstrates that
CREKA-Lipo-DOX shows no severe cardiotoxicity
and all other organs are of normality without
obvious histopathological lesions (Jiang, Song,
Yang, et al, 2018).
The needed chemicals and materials in this study
are of large amount and overall price of all material
is high. The researchers implemented the thin-film
hydration method to prepare liposomes for its
simplicity; however, this method may result in no
controlled size in production of liposomes and poor
encapsulation efficiency of hydrophilic drugs
(Nkanga, Bapolisi, Okafor, Krause, 2019). Also, this
study mainly focuses on anti-metastasis efficacy;
more data specifically about anti-tumor is needed for
further development.
3.3 Trastuzumab
Trastuzumab is a traditional anti-breast cancer
monoclonal antibody targeting HER2 (a gene that
activates the growth factor signal) positive cells
(Bines, Clark, Barton, et al, 2021). Trastuzumab
binds to the juxtamembrane portion of the domain of
HER2 receptor and prevents the overexpression of
HER2 (Hudis, 2007). In general, patients who
receive Trastuzumab improves all clinical outcome
parameters including overall survival rate of
patients.
Despite these benefits, Trastuzumab
induces serious cardiac and gastrointestinal side
effects after investigating the toxicity associated
with it (Huszno, Leś, Sarzyczny-Słota, Nowara,
2013). In addition, diarrhea, fever, nausea is
commonly observed after treatment.
A new form of Trastuzumab treatment has been
invented to improve its therapeutic efficiency. By
covalently attaching fluorinated dendrimer to
Trastuzumab, Trastuzumab-dendrimer-fluorine drug
delivery system targeting the HER2 receptor on
breast cancer cells is synthesized by researchers
(Bartusil-Aebisher, Chrzanowski, Bober, 2021). The
incorporation of
19
F increases the lipophilicity and
hydrophobicity of drug delivery system, while the
use of PAMAM-G5 dendrimer enhances the cellular
uptake of the drug delivery system and increases
biocompatibility (Bartusil-Aebisher, Chrzanowski,
Bober, 2021). In addition, Trastuzumab-dendrimer
drug delivery systems have shown enhanced
solubility and controlled release of Trastuzumab in
comparison to the pure drug alone (Bartusil-
Aebisher, Chrzanowski, Bober, 2021).
The Trastuzumab-dendrimer drug delivery
research was done in vitro, which means that further
study must be done to prove its safety and feasibility
before clinical use, but with long and complicated
synthesis to obtain the final product, the in vivo
stage test might face more challenges.
4 CLINICAL APPROVALS OF
NANOMEDICINE
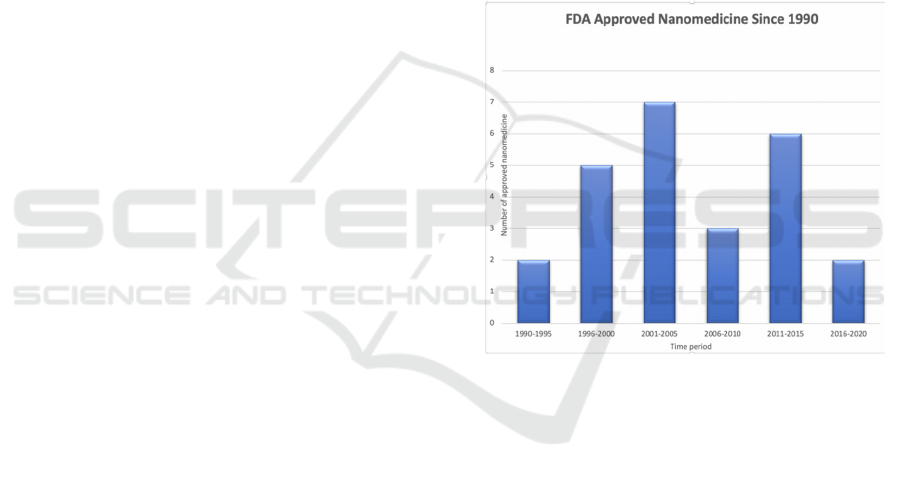
Nanomedicine has been the frontier technology in
medicine. Since 1990s, large amounts of designed
nanoparticles were created and entered clinical trial.
However, few of them were able to be approved for
clinical use. Nanoparticles being declined exhibit
extra strong cytotoxicity, unwanted adverse effects,
and low efficiency of targeted delivery (Seok, Bae,
2018).
Figure 2: FDA approved nanomedicine since1990s
(Anselmo, Mitragoti, 2019).
Since the approval of Doxil® in 1995, more
newly made nanomedicine emerge in 2000s with
significant breakthrough of drug delivery efficiency
gained approval (Seok, Bae, 2018). Although the
number of approved nanomedicines is not satisfying
compared to the fact that numerous novel
nanoparticles are ongoing for clinical trial, scientists
continue to make progresses towards improving
synthesis strategies.
5 CONCLUSIONS
The presence of nanoparticles provides an
alternative way to more effective cancer treatments
other than conventional therapies. This paper
includes the working mechanisms and advantages of
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
156

partial common nanoparticles, including pSi,
liposome, polymeric materials, and peptide drugs.
They possess the characterization of small size,
high drug loading capacity and efficiency, enhanced
bioavailability, and harmlessness to human body,
making them ideal approaches to anti-cancer
practice. Nanotechnology is still evolving rapidly;
current existing nanoparticle-based drug delivery
systems will be refined, and newly designed ones
will emerge in the future. Although huge amounts of
nanocarrier incorporated drug delivery system with
excellent therapeutical effects and clinical values
have been successfully synthesized in laboratory and
entered the clinical trial, clinically approved ones are
relatively fewer. While the majority of them are
passive targeting nanoparticles, there are no active
targeting ones approved from 2007 to 2017 (Narum,
Le T, Le DP, et al, 2019). From future perspective,
active targeting nanoparticles need to be further
investigated, for they can be applied to the situation
where therapeutic drugs have difficulties crossing
the cell membrane (Attia, Anton, Wallyn, Omran,
Vandamme, 2019). Throughout the paper, we know
that the choice of nanoparticle depends on specific
cancer type, the cost of production, prior studies on
possible adverse reactions and research parameters,
and studied nanodrug-induced response in vivo, to
ensure the nanodrug’s safety and effectiveness
(Attia, Anton, Wallyn, Omran, Vandamme, 2019).
Given that, much efforts should be devoted to
investigate safe and feasible nanoparticle-based drug
delivery system for cancer treatments.
The synthesis procedures of previously described
nanomedicine are generally at high cost: expensive
chemicals, bioreactors, equipment, and instruments
are required, and more animal and clinical testing
will need to be completed before clinical
approvement. The use of organic solvents and
complicated steps to synthesize nanoparticles cannot
ensure the purity of the product. Future
investigations can focus on the alternative ways of
creating nanoparticles with fewer steps and lower
price when the yield, purity and stability can be
sustained.
All in all, this paper stresses recent advance anti-
cancer nanomedicine within a short time frame with
specific focus on breast cancer treatments. This
paper possesses potential time limitations due to the
fact that nanomedicine continues to advance. Future
investigation can cover lower cost nanomedicine
production methods, new clinical practices of
nanodrugs on different types of cancer and
treatments, and safer, more therapeutically effective
synthesis design for the time being.
REFERENCES
Abbasi E, Aval SF, Akbarzadeh A, et al. Dendrimers:
Synthesis, applications, and properties. Nanoscale Res
Lett. 2014;9(1):1-10. doi:10.1186/1556-276X-9-247
Agrawal K. Doxorubicin. In: XPharm: The
Comprehensive Pharmacology Reference. Elsevier
Inc.; 2007:1-5. doi:10.1016/B978-008055232-
3.61650-2
Anselmo AC, Mitragotri S. Nanoparticles in the clinic: An
update. Bioeng Transl Med. 2019;4(3).
doi:10.1002/BTM2.10143
Attia MF, Anton N, Wallyn J, Omran Z, Vandamme TF.
An overview of active and passive targeting strategies
to improve the nanocarriers efficiency to tumour sites.
J Pharm Pharmacol. 2019;71(8):1185-1198.
doi:10.1111/JPHP.13098
Bartusik-Aebisher D, Chrzanowski G, Bober Z, Aebisher
D. An analytical study of Trastuzumab-dendrimer-
fluorine drug delivery system in breast cancer therapy
in vitro. Biomed Pharmacother. 2021;133:111053.
doi:10.1016/j.biopha.2020.111053
Bergman L, Beelen MLR, Gallee MPW, Hollema H,
Benraadt J, Van Leeuwen FE. Risk and prognosis of
endometrial cancer after tamoxifen for breast cancer.
Lancet. 2000;356(9233):881-887. doi:10.1016/S0140-
6736(00)02677-5
Bines J, Clark E, Barton C, et al. Patient-reported function,
health-related quality of life, and symptoms in
APHINITY: pertuzumab plus trastuzumab and
chemotherapy in HER2-positive early breast cancer.
Br J Cancer 2021 1251. 2021;125(1):38-47.
doi:10.1038/s41416-021-01323-y
Brustmann H, Riss P, Naudé S. The relevance of
angiogenesis in benign and malignant epithelial
tumors of the ovary: A quantitative histologic study.
Gynecol Oncol. 1997;67(1):20-26.
doi:10.1006/gyno.1997.4815
Chang D-K, Li P-C, Lu R-M, Jane W-N, Wu H-C.
Peptide-Mediated Liposomal Doxorubicin Enhances
Drug Delivery Efficiency and Therapeutic Efficacy in
Animal Models. PLoS One. 2013;8(12):e83239.
doi:10.1371/JOURNAL.PONE.0083239
Chen L, Endler A, Shibasaki F. Hypoxia and
angiogenesis: Regulation of hypoxia-inducible factors
via novel binding factors. Exp Mol Med.
2009;41(12):849-857.
doi:10.3858/emm.2009.41.12.103
Christowitz C, Davis T, Isaacs A, Van Niekerk G,
Hattingh S, Engelbrecht AM. Mechanisms of
doxorubicin-induced drug resistance and drug resistant
tumour growth in a murine breast tumour model. BMC
Cancer. 2019;19(1):1-10. doi:10.1186/s12885-019-
5939-z
Craig Jordan V. The role of tamoxifen in the treatment and
prevention of breast cancer. Curr Probl Cancer.
1992;16(3):134-176. doi:10.1016/0147-
0272(92)90002-6
Recent Advancements of Anti-cancer Nanomedicine in Breast Cancer
157

DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer
statistics, 2019. CA Cancer J Clin. 2019;69(6):438-
451. doi:10.3322/CAAC.21583
Gabizon AA. Pegylated liposomal doxorubicin:
Metamorphosis of an old drug into a new form of
chemotherapy. Cancer Invest. 2001;19(4):424-436.
doi:10.1081/CNV-100103136
Gray RG, Rea D, Handley K, et al. aTTom: Long-term
effects of continuing adjuvant tamoxifen to 10 years
versus stopping at 5 years in 6,953 women with early
breast cancer.
https://doi.org/101200/jco20133118_suppl5.
2013;31(18_suppl):5-5.
doi:10.1200/JCO.2013.31.18_SUPPL.5
Green AE, Rose PG. Pegylated liposomal doxorubicin in
ovarian cancer. Int J Nanomedicine. 2006;1(3):229.
Accessed July 19, 2021. /pmc/articles/PMC2426807/
Haidary SM, Mohammed AB, Córcoles EP, Ali NK,
Ahmad MR. Effect of coatings and surface
modification on porous silicon nanoparticles for
delivery of the anticancer drug tamoxifen.
Microelectron Eng. 2016;161:1-6.
doi:10.1016/j.mee.2016.03.051
Han YL, Kim SY, Kim T, Kim KH, Park JW. The role of
terminal groups in dendrimer systems for the treatment
of organic contaminants in aqueous environments. J
Clean Prod. 2020; 250:119494.
doi:10.1016/j.jclepro.2019.119494
Hudis CA. Trastuzumab — Mechanism of Action and Use
in Clinical Practice. N Engl J Med. 2007;357(1):39-
51. doi:10.1056/nejmra043186
Hulvat MC. Cancer Incidence and Trends. Surg Clin
North Am. 2020;100(3):469-481.
doi:10.1016/j.suc.2020.01.002
Huszno J, Leś D, Sarzyczny-Słota D, Nowara E. Cardiac
side effects of trastuzumab in breast cancer patients -
Single centere experiences. Wspolczesna Onkol.
2013;17(2):190-195. doi:10.5114/wo.2013.34624
Jiang K, Song X, Yang L, et al. Enhanced antitumor and
anti-metastasis efficacy against aggressive breast
cancer with a fibronectin-targeting liposomal
doxorubicin. J Control Release. 2018; 271:21-30.
doi:10.1016/j.jconrel.2017.12.026
Kampen K. Membrane Proteins: The Key Players of a
Cancer Cell. Artic J Membr Biol. Published online
2011. doi:10.1007/s00232-011-9381-7
Klagsbrun M, Moses MA. Molecular angiogenesis. Chem
Biol. 1999;6(8): R217-R224. doi:10.1016/S1074-
5521(99) 80081-7
Krasin MJ, Davidoff AM. Principles of Pediatric
Oncology, Genetics of Cancer, and Radiation Therapy.
Pediatr Surg. Published online January 1, 2012:397-
416. doi:10.1016/B978-0-323-07255-7.00028-3
Kumeria T, McInnes SJP, Maher S, Santos A. Porous
silicon for drug delivery applications and theranostics:
recent advances, critical review and perspectives.
Expert Opin Drug Deliv. 2017;14(12):1407-1422.
doi:10.1080/17425247.2017.1317245
Kunte S, Abraham J, Montero AJ. Novel HER2–targeted
therapies for HER2–positive metastatic breast cancer.
Cancer. 2020;126(19):4278-4288.
doi:10.1002/cncr.33102
Lam DK, Schmidt BL. Molecular Biology of Head and
Neck Cancer: Therapeutic Implications. In: Current
Therapy in Oral and Maxillofacial Surgery. Elsevier
Inc.; 2012:92-101. doi:10.1016/B978-1-4160-2527-
6.00010-4
Manj RZA, Chen X, Rehman WU, Zhu G, Luo W, Yang
J. Big potential from silicon-based porous
nanomaterials: In field of energy storage and sensors.
Front Chem. 2018;6(NOV).
doi:10.3389/fchem.2018.00539
McKeown SR. Defining normoxia, physoxia and hypoxia
in tumours Implications for treatment response. Br J
Radiol. 2014;87(1035). doi:10.1259/bjr.20130676
Mitri Z, Constantine T, O’Regan R. The HER2 Receptor
in Breast Cancer: Pathophysiology, Clinical Use, and
New Advances in Therapy. Chemother Res Pract.
2012;2012:1-7. doi:10.1155/2012/743193
Narum SM, Le T, Le DP, et al. Passive Targeting in
Nanomedicine: Fundamental Concepts, Body
Interactions, and Clinical Potential. Elsevier Inc.;
2019. doi:10.1016/B978-0-12-816662-8.00004-7
Nkanga CI, Bapolisi AM, Okafor NI, Krause RWM.
General Perception of Liposomes: Formation,
Manufacturing and Applications. Liposomes - Adv
Perspect. Published online March 26, 2019.
doi:10.5772/INTECHOPEN.84255
Osborne CK. Tamoxifen in the Treatment of Breast
Cancer. Wood AJJ, ed. N Engl J Med.
1998;339(22):1609-1618.
doi:10.1056/NEJM199811263392207
Patra JK, Das G, Fraceto LF, et al. Nano based drug
delivery systems: Recent developments and future
prospects 10 Technology 1007 Nanotechnology 03
Chemical Sciences 0306 Physical Chemistry (incl.
Structural) 03 Chemical Sciences 0303
Macromolecular and Materials Chemistry 11 Medical
and Health Sciences 1115 Pharmacology and
Pharmaceutical Sciences 09 Engineering 0903
Biomedical Engineering Prof Ueli Aebi, Prof Peter
Gehr. J Nanobiotechnology. 2018;16(1).
doi:10.1186/s12951-018-0392-8
Phagocytosis and Intracellular Killing. Immunol Pharm.
Published online January 1, 2012:97-101.
doi:10.1016/B978-0-323-06947-2.10012-4
Redfors B, Shao Y, Råmunddal T, et al. Effects of
doxorubicin on myocardial expression of
apolipoprotein-B. Scand Cardiovasc J. 2012;46(2):93-
98. doi:10.3109/14017431.2012.653825
Santos A, Veiga F, Figueiras A. Dendrimers as
Pharmaceutical Excipients: Synthesis, Properties,
Toxicity and Biomedical Applications. Vol 13.; 2020.
doi:10.3390/ma13010065
Santos HA, Mäkilä E, Airaksinen AJ, Bimbo LM,
Hirvonen J. Porous silicon nanoparticles for
nanomedicine: Preparation and biomedical
applications. Nanomedicine. 2014;9(4):535-554.
doi:10.2217/nnm.13.223
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
158

Seok Youn Y, Bae H. Perspectives on the past, present,
and future of cancer nanomedicine ☆. Published
online 2018. doi:10.1016/j.addr.2018.05.008
Sharif Mohammad Shaheen, Fazle Rabbi Shakil Ahmed,
Md. Nazir Hossen, Maruf Ahmed MSA and MA-U-I.
Liposome as a Carrier for Advanced Drug Delivery.
Pdf. Published online 2006: Pakistan Journal of
Biological Sciences, 9: 1181-1.
Shi Q, Zhang Y, Liu S, et al. Specific tissue factor
delivery using a tumor-homing peptide for inducing
tumor infarction. Biochem Pharmacol. 2018; 156:
501-510. doi:10.1016/j.bcp.2018.09.020
Singh D. Tamoxifen reduces risk of breast cancer in high
risk patients. BMJ Br Med J. 2003;326(7383):244.
Accessed June 24, 2021. /pmc/articles/PMC1169220/
Tohme S, Simmons RL, Tsung A. Surgery for cancer: A
trigger for metastases. Cancer Res. 2017;77(7):1548-
1552. doi:10.1158/0008-5472.CAN-16-1536
Vilalta M, Rafat M, Graves EE. Effects of radiation on
metastasis and tumor cell migration. Cell Mol Life
Sci. 2016;73(16):2999-3007. doi:10.1007/s00018-016-
2210-5
Yang G, Nowsheen S, Aziz K, Georgakilas AG. Toxicity
and adverse effects of Tamoxifen and other anti-
estrogen drugs. Pharmacol Ther. 2013;139(3):392-
404. doi:10.1016/j.pharmthera.2013.05.005
Yu F, Bender W. The mechanism of tamoxifen in breast
cancer prevention. Breast Cancer Res. 2001;3(S1):
A74. doi:10.1186/bcr404
Zhao M, Ding X feng, Shen J yu, Zhang X ping, Ding X
wen, Xu B. Use of liposomal doxorubicin for adjuvant
chemotherapy of breast cancer in clinical practice. J
Zhejiang Univ Sci B. 2017;18(1):15-26.
doi:10.1631/jzus.B1600303
Recent Advancements of Anti-cancer Nanomedicine in Breast Cancer
159
