
Co-composting of Wheat Straw and Food Waste with and without
Microbial Agent
Xiangdan Jin
1,2 a
, Weidang Ai
2,3 b
and Wenyi Dong
1,4 c
1
School of Civil and Environmental Engineering, Harbin Institute of Technology Shenzhen, Shenzhen, China
2
Space Science and Technology Institute (Shenzhen), Shenzhen, China
3
National key Laboratory of Human Factors Engineering, China Astronaut Research and Training Center, Beijing, China
4
Public Platform for Technological Service in Urban Waste Reuse and Energy Regeneration, Shenzhen, China
Keywords: Aerobic Composting, Microbial Agent, Wheat Straw, Maturity.
Abstract: In China, the treatment of agriculture residue and food waste is of great concern. Aerobic composting is
gaining increasing attention because it can improve both organic waste recycling and soil remediation. The
aim of this study was to evaluate the composting process of the mixture of wheat straw and food waste, and
the effect of microbial agent on the degradation process. Results showed that typical temperature variation
curves were observed with peak values at 65.8℃ and 60.5℃ with and without inoculation, respectively. VS,
DOC, NH
4
+
-N and C/N decreased over the composting process, while pH, EC, NO
3
-
-N and GI showed an
opposite trend. The microbial community diversity was analyzed and Firmicutes, Proteobacteria,
Actinobacteria, Bacteroidetes and Ascomycota enriched in the compost. At the end of the composting process,
the maturity was indicated by the finial C/N ratio and GI which reached 11.26±0.84 and 144.68±14.95% with
inoculation. Inoculation had a positive effect on composting performance but is less economic.
1 INTRODUCTION
1
As a big agricultural country, China produces
abundant of biomass wastes including agriculture
residues, forestry waste, livestock manure and food
waste annually. Among these biomass resources,
wheat straw represents a large portion in crop
residues which could be used for land application
(Zhu et al., 2020). Wheat straw can be utilized as fuel
for household cooking, silage for livestock, and
material for mulching. However, most of the straws
are burned in the open field since it is cost effective
and convenient, resulting in serious environmental
problems such as greenhouse gases and harmful
smoke generation and a waste of biomass resources.
At present, the main technologies to treat and recycle
agriculture wastes are anaerobic digestion (AD) and
aerobic composting (AC) (Li et al., 2011; Qian et al.,
2014). Since methane rich biogas can be produced
and seldom maintenance is required during the AD
process, biogas plants have been largely constructed
a
https://orcid.org/0000-0001-8812-1808
b
https://orcid.org/0000-0003-3748-227X
c
https://orcid.org/0000-0002-3055-3592
and employed for biomass conversion. Nevertheless,
crop straws which is rich in lignocellulose and
resistant to degrade is always co-digested with other
easily biodegradable substrates (Lehtomäki et al.,
2007). Problems related to crop straws in AD such as
raw material floating, low degradation rate and
difficult discharging are quite annoying. In addition,
large amount of biogas slurry and biogas residue is
produced and extra efforts on post-treatment are
necessary to eliminate the negative effects for further
land application (Wang et al., 2016).
Compared to AD, AC can convert crop straws into
organic fertilizer directly in a sustainable and
environmental friendly way which has been
recommended in recent years (Bernal et al., 2009).
AC is a process that breaks down organic wastes and
produces CO
2
, water, mineral ions and stabilized
organic matters under certain conditions. The product
is beneficial for soil amendment and plant growth.
The process is induced by the activities of various
microbial communities and influenced by factors
Jin, X., Ai, W. and Dong, W.
Co-composting of Wheat Straw and Food Waste with and without Microbial Agent.
DOI: 10.5220/0011195300003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 189-197
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
189

such as substrate properties, C/N ratio, moisture
content, temperature, pH value, bulk density, oxygen
supply and raw material size (Hubbe et al., 2010).
Nowadays, AC has been widely applied in the
treatment of various kinds of organic wastes (Wei et
al., 2017). Usually, straws are dosed as supplements
and co-composted with livestock and poultry
manures or sewage sludge (Meng et al., 2019).
Studies on AC of wheat straw as the bulking material
are still lacking. In this study, straws were used as the
main composition of the compost and food waste was
added to promote the degradation process. Wheat
straw have high C/N ratio, low moisture content and
porous structure while food waste has the opposite
physicochemical properties. The mixture of these two
kinds of materials can provide more balanced
nutrient, proper moisture content and better
ventilation condition for the microorganisms to carry
out the composting process.
Accordingly, the present study aimed to evaluate
the treatment effect of co-composting of wheat straw
and food waste, and explore the effect of selected
microbial agent on the degradation performance. The
forms and distribution characteristics of carbon and
nitrogen were determined along with other
conventional parameters. Species succession among
the microbial communities was investigated during
the composting process. Besides, the evaluation of the
compost maturity and quality was performed.
2 METHODS AND MATERIALS
2.1 Feedstocks and Microbial Agent
Wheat straw were purchased from a farm of Jiangsu
Province, China. The straw was air-dried and
smashed to 1-3 cm. Food waste was collected from
the canteen of one research institution. Bones, plastic
bags, napkins and other raffles were picked out and
leachate was drained. Collected food waste was
shredded by a food grinder into mushy mixture and
stored at -20℃ before use. The characteristics of the
feedstocks are shown in Table 1. Microbial agent
mainly containing Chelatococcus composti, Bacillus
thermoamylovorans, Aspergillus fumigatus, and
Aspergillus niger was prepared and the concentration
of each strain was about 109 cfu/mL.
Table 1: Basic physicochemical properties of the feedstock.
Paramete
r
Wheat straw Food waste
Input in each
reactor (kg)
2.2±0.1 2.5±0.1
Water content
(
%
)
10.24±0.23 80.57±0.34
Volatile solid
content
(
%
)
94.83±0.13 87.25±0.91
TC (%Dry
weight)
50.16±0.21 40.24±0.31
TN (%Dry
wei
g
ht
)
1.12±0.11 2.96±0.13
C/N ratio 44.84 14.13
2.2 Experimental Apparatus and Tests
Composting was conducted in plastic bins of 30 L
valid capacity with 46.5 cm in height and 32 cm in
diameter. The compost bins were covered with
insulating cotton to retain metabolic heat. An annular
aeration pipe was placed at the bottom of the bin and
1 L/min aeration rate was set through an aerator pump
for the composting process. A perforated
polyvinylchloride tray was installed above the
aeration pipe to support the compost and distribute
the air uniformly. The structure of the composting
reactor can be referred to Zhang et al (2021). Firstly,
wheat straw was placed in a large-size plastic drum
and certain amount of distill water was added to
obtain the water content at around 65%. Then food
waste was added to adjust the C/N ratio of mixed
substrates to 32-35. Substrates were mixed
thoroughly by hand to assure the maximum
homogeneity.
For the inoculated treatments, a concentration of
0.5% (dry weight basis, w/w) microbial agent was
introduced. Treatments with identical substrate
composition but no microbial agent were served as
the control groups. About 7.2 kg mixed substrate was
placed in each compost bin occupying 85% of the
volume. The experiment lasted for 30 days and
samples were collected every 4 days for parameter
analysis. Turning and mixing was conducted
manually every 7 days to break any lumps formed and
ensure the optimal aeration in the system.
2.3 Analytical Methods
Temperature was measured daily by a thermometer
inserting at three locations in the compost bins
(surface, 10 cm depth; core, 25 cm depth; and bottom,
38 cm depth), and the average temperature was
recorded. The water content and volatile solid (VS)
content were measured according to Standard
Methods (APHA, 1998). Total carbon and total
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
190
nitrogen content of dried samples were analyzed by
an auto elemental analyzer (Vario MACRO cube,
Elementar, Germany). About 5.0 g of fresh sample
was extracted with 50 mL deionized water (1:10 w/v
ratio) using a shaker at 100 rpm for 2 h followed by
centrifugation at 10000 rpm for 10 min. Then the
supernatant was measured for pH and electrical
conductivity (EC) by a pH/EC meter (Orion VERSA
STAR Pro, Thermo Fisher Scientific, USA). The
absorbance of supernatant at wavelengths of 465 nm
(E4) and 665 nm (E6) was measured using a
spectrophotometer (UV-2600, Shimadzu, Japan). The
supernatant was then filtered through 0.45 μm filter
membranes. Dissolved organic carbon (DOC) and
nitrate (NO
3
-
-N) were measured by a TOC/TN
analyzer (TOC-L, Shimadzu, Japan) and an ion
chromatography (ICS-5000+, Thermo Fisher, USA),
respectively. Ammonium nitrogen (NH
4
+
-N) was
analyzed via a continuous flow analyzer (Syslyzer Ⅲ,
Systea, Italy).
For microbial community determination, about
0.3-0.5 g fresh samples were stored at -20℃ for
genomic DNA extraction and 16s rRNA/18S rRNA
amplification. The V3-V4 hypervariable-region was
amplified with the bacterial universal primer sets:
338F (5’-ACTCCTACGGGAGGCAGCAG-3’) and
806R (5’-GGACTACHVGGGTWTCTAAT-3’).
Primers of 18S rRNA: 528F (5’-
GCGGTAATTCCAGCTCCAA-3’) and 706R (5’-
AATCCRAGAATTTCACCTCT-3’), were used to
analyze fungal communities. All the sequencing
analysis was conducted by Shanghai Majorbio Bio-
Pharm Technology Co., Ltd (Shanghai, China) on an
Illumina Miseq platform. All the pairs of sequences
were clustered into Operational Taxonomic Units
(OTUs) based on a ≥97% identity threshold by the
SILVA database.
A phytotoxicity test was performed by seed
germination with Chinese cabbage seeds. The 1:10
aqueous extract of compost was prepared and 6 mL
of the extract was added into a sterile petri dish (90
mm in diameter) containing a filter paper. About 20
Chinese cabbage seeds were placed on the filter paper
and incubated in the dark at 25℃. In contrast, 6 mL
deionized water was used as the control experiment.
After incubation for 48-72 h, the numbers of
germinated seed and root length were measured and
recorded. Germination index (GI) was calculated
according to the reference (Zucconi, 1981).
2.4 Statistical Analysis
Statistical analyses were performed in duplicate
samples and the average values with standard
deviation were reported. The data were processed to
a one-way analysis of variance (ANOVA) using IBM
SPSS statistics ver. 22.
3 RESULTS AND DISCUSSION
3.1 Temperature Variation during the
Composting Process
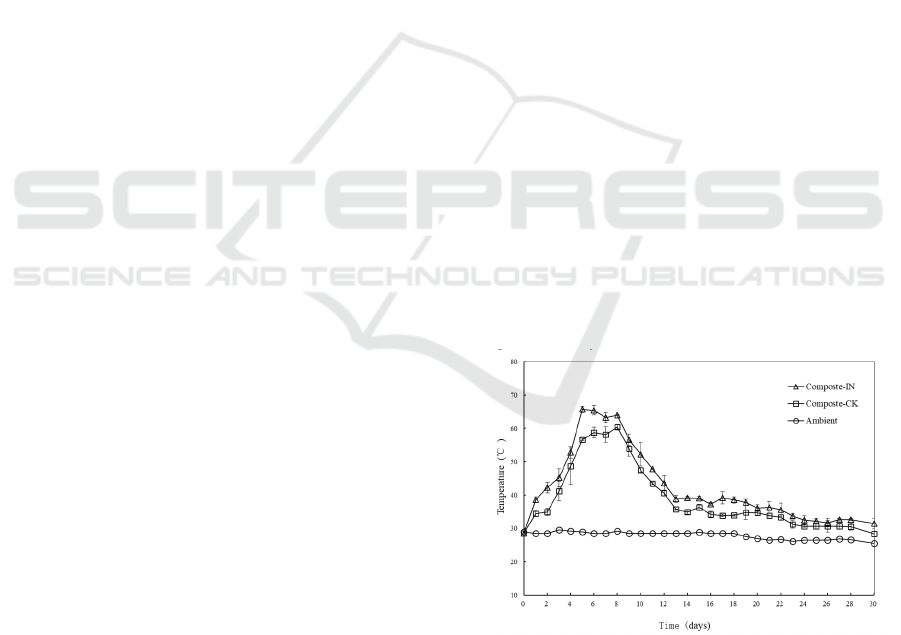
Temperature variations in all composting bins were
monitored and showed in Figure 1. The ambient
temperature varied within a narrow range from
25.5℃ to 29.0℃. In experiments with inoculation,
the temperature increased rapidly to the thermophilic
value (>50℃) after 4 days’ composting. A further
increase was observed and temperature reached its
peak at 65.8±1.0℃ on day 5. The thermophilic stage
lasted for 7 days when most potential pathogens, pets
and weed seeds were likely to be killed. Then
temperature of composts showed a downward trend
to around 40℃ followed by a maturation phase when
the temperature ranged from 39.2℃ to 31.5℃ until
the end of experiment. In the control treatments,
temperature variations experienced similar
mesophilic, thermophilic, cooling and maturation
phases. The maximum temperature reached
60.5±1.0℃ on day 8 and the duration of thermophilic
stage was 5 days.
Figure 1: Temperature variation during the composting
process. Compost IN means composting experiment with
inoculum; Compost CK means control experiment.
In this work, though the main component was
wheat straw which is rich in lignocellulose and
recalcitrant to decomposed (Yu et al., 2007), the
presence of easily degradable organic materials
Co-composting of Wheat Straw and Food Waste with and without Microbial Agent
191
supplied by food waste contributed to initiate the bio-
process successfully. Metabolic heat was released
significantly by the activity of microorganism in the
organic matter decomposition process. In the control
tests, the temperature increased rapidly during
mesophilic and well maintained in thermophilic
phase with the effect of endogenous microorganisms.
However, slightly lower temperature values were
observed during the whole experiment as well as a
shorter thermophilic duration time in the control
groups compared those in tests with exogenous
microbial agent.
3.2 Changes of Moisture, pH, EC and
E4/E6
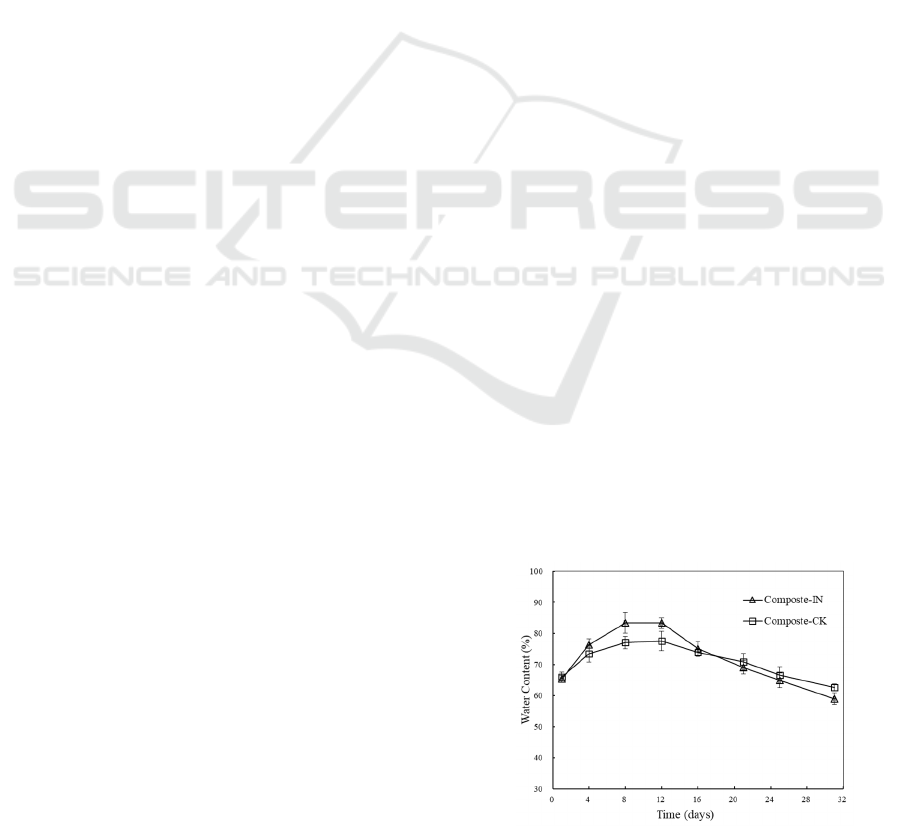
Many studies have stated that the optimal water
content for AC is 50-60% (Mohee and Mudoo, 2005).
However, the initial water content in this study was
adjusted slightly higher at 65% with lignocellulose-
rich and porous structure substrates. A rise in the
water content was observed in the early stage due to
thriving microbial metabolic activities (Figure 2a).
The water content peaked at 83.43±3.41 % on day 8
and at 77.63±3.12 % on day 12 with and without
inoculation, respectively. Though high temperature
enhanced the moisture evaporation, the generation of
metabolized water was stronger than the moisture
ventilation during the mesophilic and thermophilic
stages. Moreover, moisture condensed on the lid of
the bin and fell back to the mixture which also caused
an increase in water level and the hydrothermal
environment. Along with the constant ventilation
forced by aeration and decreasing microbial
decomposition rate, water content was reduced till the
end of the composting to around 59.07±1.84 % and
62.75±1.17% with and without inoculation,
respectively. No leachate was collected at the bottom
of the bin.
Similar changes in pH were observed in
inoculated and non-inoculated treatments (Figure 2b).
Initially, pH was acidic and the value was around 4.5
in all bins. The low pH was attributed by amino acids
and fatty acids which were produced from the easy-
degraded organic matters from food waste such as
carbohydrates, protein and fat. Along with the
consumption of intermediate compounds (mainly
organic acids) and the release of ammonia, pH
increased immediately to 7.0-8.0 and then fluctuated
around this value until the end of composting. With
easy-degraded organic materials exhausted, complex
lignocellulosic substrates were gradually degraded
since the fiber surface has been soften in the humid
and acidic environment. The compost samples from
maturation phase were slightly alkaline (7.5-8.5)
which were suitable for the growth of microorganism
and plant seedlings (Bustamante et al., 2008).
EC indicates the salinity level of substrates and
the possible phytotoxic effects. As illustrated in
Figure 2c, EC showed a continuously increasing trend
from 1.86±0.01 mS/cm and 1.61±0.11 mS/cm with
and without inoculation, respectively, to about 3.50
mS/cm at the end of the composting process. The
increase in EC was induced by the decomposition of
complex organic matters into small molecule
dissolved organic matters as well as the release of
mineral ions. During the whole process, EC values
with inoculation were higher than those in
experiments without inoculation, suggesting that the
degradation and mineralization of organic matters
could be enhanced by the activities of both
endogenous and exogenous microorganisms. Some
researches have stated that a high EC (>4 mS/cm)
related with high salt content had adverse effects on
plant cultivation (Meng et al., 2019). In this study, EC
of processed compost was found below 4 mS/cm
which was within the prescribed limits of
phytotoxicity.
The absorbance at 465 nm (E4) and 665 nm (E6)
of aqueous extracts of compost were determined and
E4/E6 ratio was described in Figure 2d. E4/E6
underwent an increase and the highest value reached
to 8.04±0.32 and 7.23±0.71 with and without
inoculation, respectively. Positive linear relationship
between water soluble organic carbon and absorbance
at 465 nm has been approved. Therefore, the increase
in E4/E6 ratio indicated that more water soluble
organic carbon existed via decomposition of organic
compounds. Then E4/E6 ratio of the inoculation
experiment and control experiment declined to
3.36±0.11 and 4.05±0.16 at the end of the
composting, respectively. Information on
condensation degree of humus with aromatic nucleus
can be provided by E4/E6 ratio (Inbar et al., 1993).
The decline in E4/E6 ratio was likely caused by the
consumption of small molecule organic matters and
the formation of humic substances.
(a)
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
192
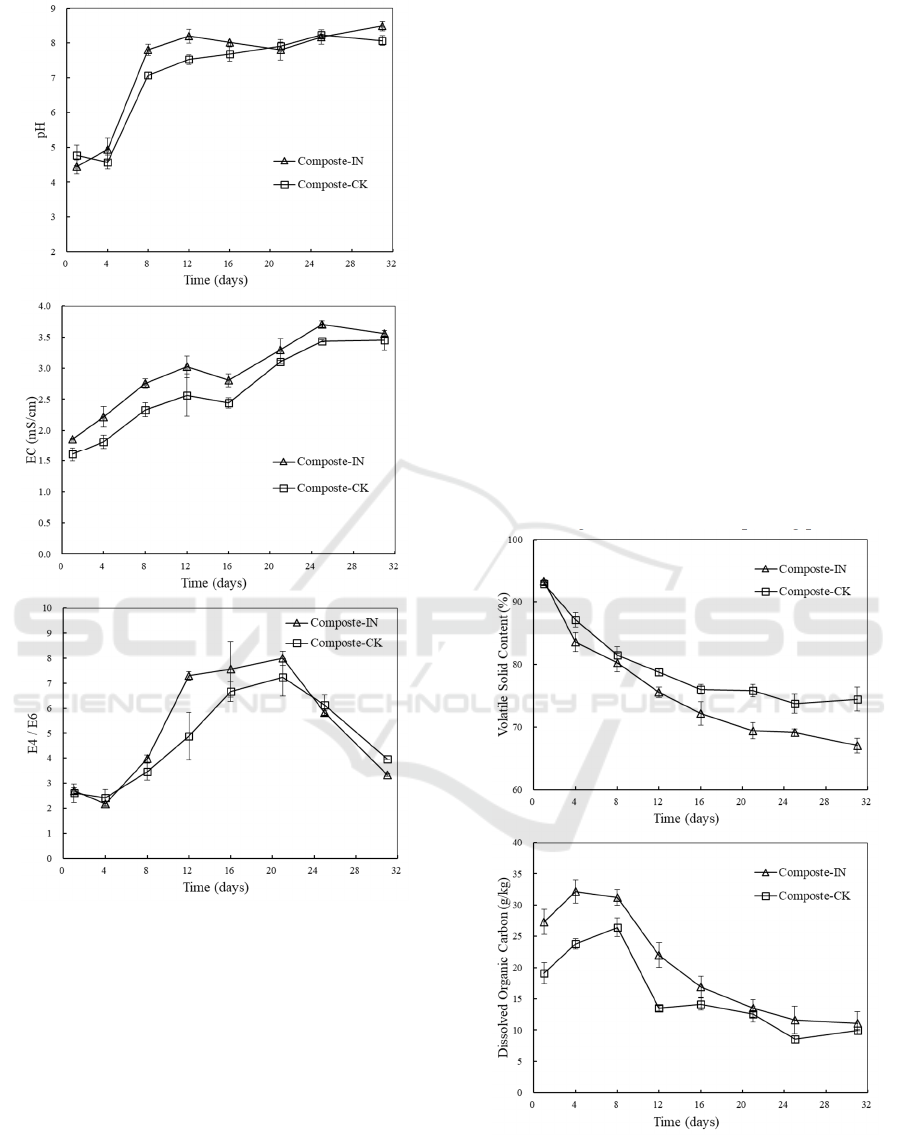
Figure 2: Changes in physicochemical parameters during
composting.
3.3 The VS Content and DOC
Evolution
The VS content decreased along with composting
time for all treatments due to the loss of organic
matters by microbial degradation (Figure 3a). The
initial VS was around 93%. Overall, experiments with
inoculation had a higher loss of VS content (27%)
than that in the control (18%). A sharper decline
during the mesophilic-thermophilic stage suggested
that easy-degraded organic matters were mainly
utilized. They were converted into small molecule
dissolved organic matters which was consistent with
the increase in DOC during the first few days (Figure
3b). The maximum values of DOC reached to
32.17±1.86 g/kg and 26.44±1.50 g/kg with and
without inoculation, respectively. Since small
molecule soluble organic matters were more available
to microbes, decreases in DOC was observed with the
depletion of soluble organic matters. Afterwards, the
relative low degree of VS loss during the cooling and
maturation stage was contributed by the
huminification of recalcitrant decomposable
compounds. The final VS contents of the composting
mixtures were 67.07±1.22% and 74.53±1.96% with
and without inoculation, respectively. At the end of
the composting, the DOC contents in all treatment
were around 10 g/kg which was identical to that in the
previous work (Zhou et al., 2014) which conducted
the co-composition of food waste and sawdust. Wider
variation ranges were observed both in VS content
and DOC with inoculation than those in the control.
This suggested a higher metabolic activity of
microorganisms with inoculation than that in the
control throughout the entire composting process.
Figure 3: VS content (a) and DOC (b) evolution during
composting process.
(
b
)
(c)
(
d
)
(a)
(
b
)
Co-composting of Wheat Straw and Food Waste with and without Microbial Agent
193
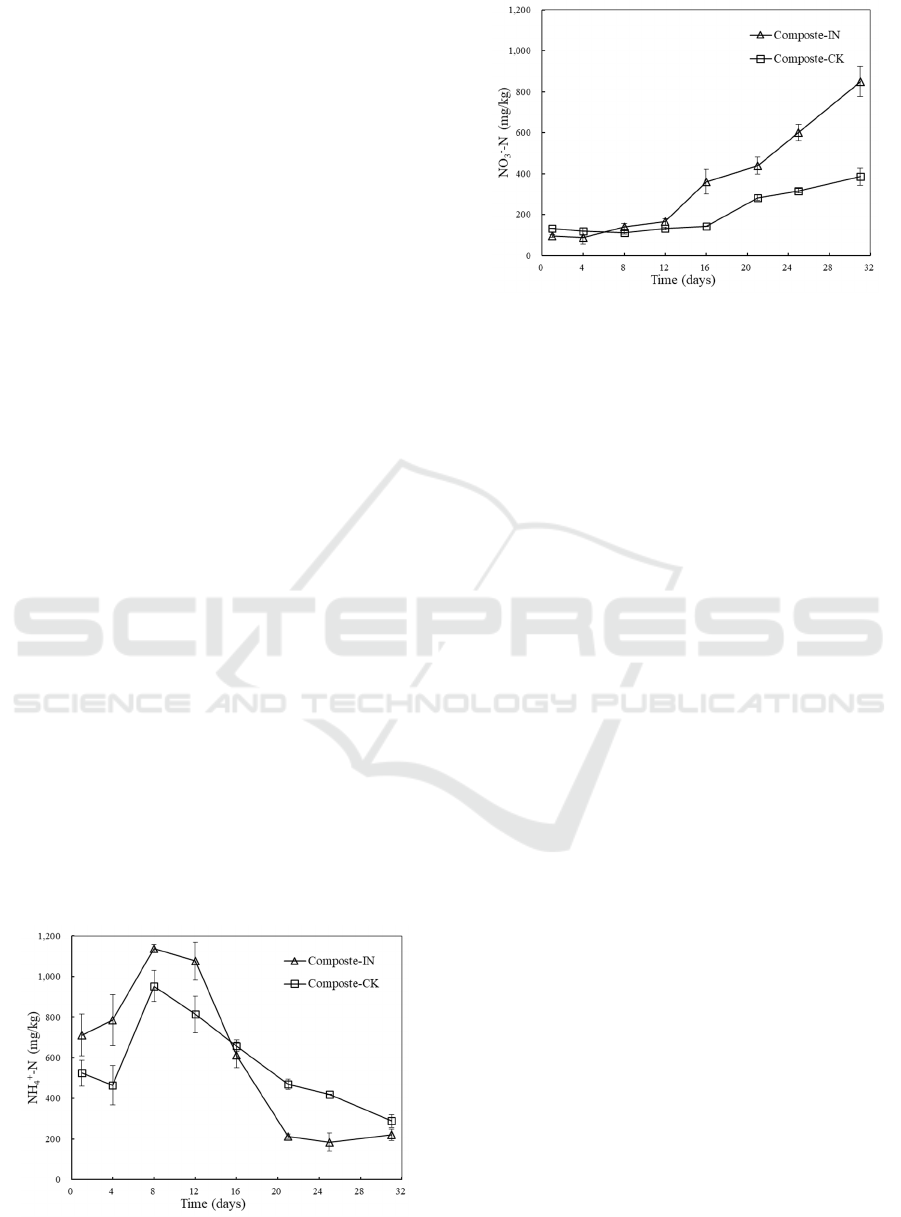
3.4 Changes in Ammonia Nitrogen and
Nitrate Nitrogen
Figure 4 shows the changes in concentrations of
NH
4
+
-N and NO
3
-
-N in extract during the composting
process. The concentration of NH
4
+
-N followed a
typical trend which NH
4
+
-N increased during the
early stage of the process, then decreased as the
process progressed and reached a low level at the end
of the composting process. The increase during the
early days was due to the conversion of organic-N
compounds into NH
4
+
-N via ammonification (Gao et
al., 2010). The amount of NH
4
+
-N reached a peak at
1138.18±18.27 mg/kg and 952.86±77.55 mg/kg with
and without inoculation, respectively, on day 8.
Simultaneously, the volatilization loss of NH
4
+
-N was
enhanced by the high temperature. Then the NH
4
+
-N
concentrations declined and the final values were
220.24±28.26 mg/kg and 288.94±32.45 mg/kg in the
inoculated and non-inoculated treatments,
respectively. Values below the maximum limit of 400
mg/kg for NH
4
+
-N content were recommended for
mature compost in many researches (Luo et al.,
2018). In this work, values blow 330 mg/kg in NH
4
+
-
N concentrations of finished compost were obtained.
The concentration of NO
3
-
-N was relatively low
and steady during of first 12 days of the composting
process. Less NO
3
-
-N was generated when little
nitrification happened during thermophilic stage
since the activity and growth of nitrifying bacteria
was inhibited by high temperature and excessive
amount of ammonia. Afterwards, the amount of NO
3
-
-N increased gradually when nitrifying bacterial
turned from dormant to active physiological state.
The final content of NO
3
-
-N reached 849.58±72.94
mg/kg and 385.63±41.71 mg/kg with and without
inoculation, respectively. Usually, the production of
nitrate rich compost is desired since NO
3
-
-N is a more
favorable source of N to be absorbed than NH
4
+
-N for
plant cultivation (Sun et al., 2016).
Figure 4: Changes in concentrations of NH
4
+
-N (a) and
NO
3
-
-N (b) during the composting process.
3.5 Changes in Microbial Community
Structure
To evaluate microbial community diversity and
dynamic changes during the AC process, samples
were collected from original, thermophilic, cooling
and maturation phases. Five phyla were dominant and
accounted for more than 97% of the sequences which
were Firmicutes, Proteobacteria, Actinobacteria,
Bacteroidetes and Ascomycota. As composting
processed, succession of the microbial community
emerged induced by differences in environmental
conditions and substrate composition.
Microbial relative abundance at the genus levels
is shown in Figure 5. At beginning, as described in
Figure 5a, the top 5 bacterial genera included
Leuconostoc, Weissella, Lactococcus, Lactobacillu
and Pediococcus whose relative abundance was
96.78%. On day 4, dominant bacteria switched to
Lactobacillus, Paenibacillus, Brevibacillus, Bacillus
and Acinetobacter. Their relative abundance were
74.83% and 75.20% in the control and inoculated
experiments, respectively. Lactobacillus bacteria is
always found in plant-derived raw material
decomposing systems and can accelerate compost
ripening (Li et al., 2020). Bacillus is thermotolerant
and able to secrete various extracellular enzymes such
as proteases, amylase and cellulases which
contributes a lot in lignocellulosic degradation
(Jurado et al., 2014). Afterwards, the relative
abundance of the top 5 bacterial genera changed and
their relative abundance decreased from 55.85% on
day 12 to 27.04% on day 22 in the control groups, and
from 47.21% to 18.36% in the inoculated groups.
Along with composting processed, the bacterial
community composition seemed more complicated
and diverse which had a positive effect on the
lignocellulosic substrate degradation.
(a)
(
b
)
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
194
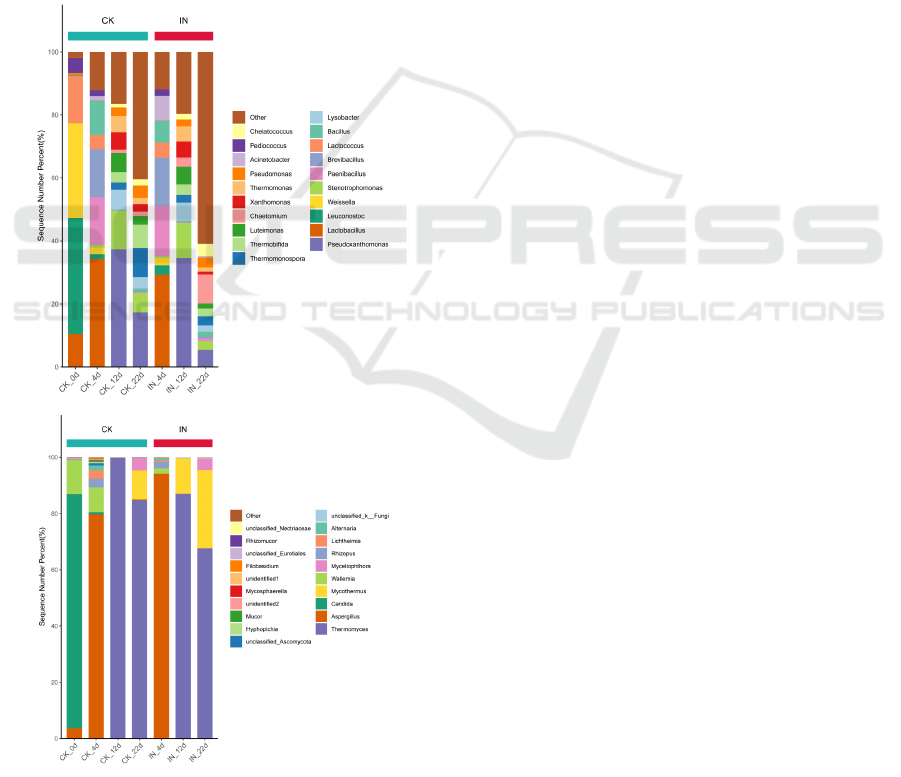
Specially, fungi are actively involved in the
degradation of lignocellullosic compounds via
producing a broad variety of functional enzymes and
physical destruction by the fungal hyphae (Yu et al.,
2007). Fungal relative abundance at the genus levels
is shown in Figure 5b. At day 0, the dominant fungal
genera were Candida (83.35%), Wallemia (11.99%)
and Aspergillus (3.64%). At day 4 in the inoculation
and control experiment, Aspergillus as a
thermotolerant fungus proliferated to 94.27% and
79.57, respectively. Later, the relative abundance of
Thermomyces showed a dominant value. During the
maturation phase, the major genera evolved to
Thermomyces (84.94%), Mycothermus (10.30%), and
Myceliophthora (4.25%) in the control groups while
those values were 67.81%, 27.66% and 3.96% with
inoculation, separately.
Figure 5: The genus composition of the bacterial
community (a) and fungal community (b) at different stages
of AC.
More diversity appeared during the maturation
phase in the inoculation groups compared those in the
control groups. Though the inoculated strains did not
proliferated during the whole period, the richness and
diversity of the microbial community increased with
more metabolism pathways of substrates which led to
the better composting efficiency.
3.6 Compost Maturity Evaluation with
C/N and Germination Experiment
Organic carbon and nitrogen are commonly utilized
by microorganisms for cell growth and metabolic
activity during AC, which leads to variations in the
C/N ratio. The loss of carbon and nitrogen is mainly
in the form of CO
2
and NH
3
stripping. Since the
degradation of carbon is faster than the release of
nitrogen, the decline in the C/N ratio during the
composting period is always observed (Zhou et al.,
2014). In our work, the initial C/N ratio of the
composting mixtures was around 33 which located
within the appropriate levels for composting
microbes (Figure 6a). The C/N ratio showed a
downward trend and the final values dropped to
11.26±0.84 and 13.95±0.93 with and without
inoculation, respectively. The final C/N ratio can be
used to assess the compost maturity. Some studies
stated a value equal to or less than 20 indicates a
satisfactory maturation (Fourti, 2013).
Phytotoxicity determined by the germination
experiment is also used to test compost safety and
maturity (Yang et al., 2013). The response of Chinese
cabbage to the toxicity of the compost water extract
in term of GI is illustrated in Figure 6b. The GI values
of all experiments dropped during the early stage of
composting process. The lowest values reached about
10% which suggested a very toxic extract of the
compost product. The seed germination was inhibited
by the excessive toxic materials such as short chain
volatile fatty acids and ammonia which was proved
by the low pH (Figure 2b) and high NH
4
+
-N content
(Figure 4a). With the depletion of the toxic materials,
the GI values rose significantly and reached to
144.68±14.95% and 92.64±12.27% at the end of
composting with and without inoculation,
respectively. Many reports have claimed that a more
than 50% GI indicated the compost was phytotoxic-
free while a more than 80% value indicated mature
product (Luo et al., 2018). Values above 1 indicated
a positive effect of finished compost on seed
germination. Clearly, inoculation was helpful in
enhancing the maturity and releasing of nutrients in
accordance with the higher NO
3
-
-N concentration in
compost extract.
(a)
(
b
)
Co-composting of Wheat Straw and Food Waste with and without Microbial Agent
195
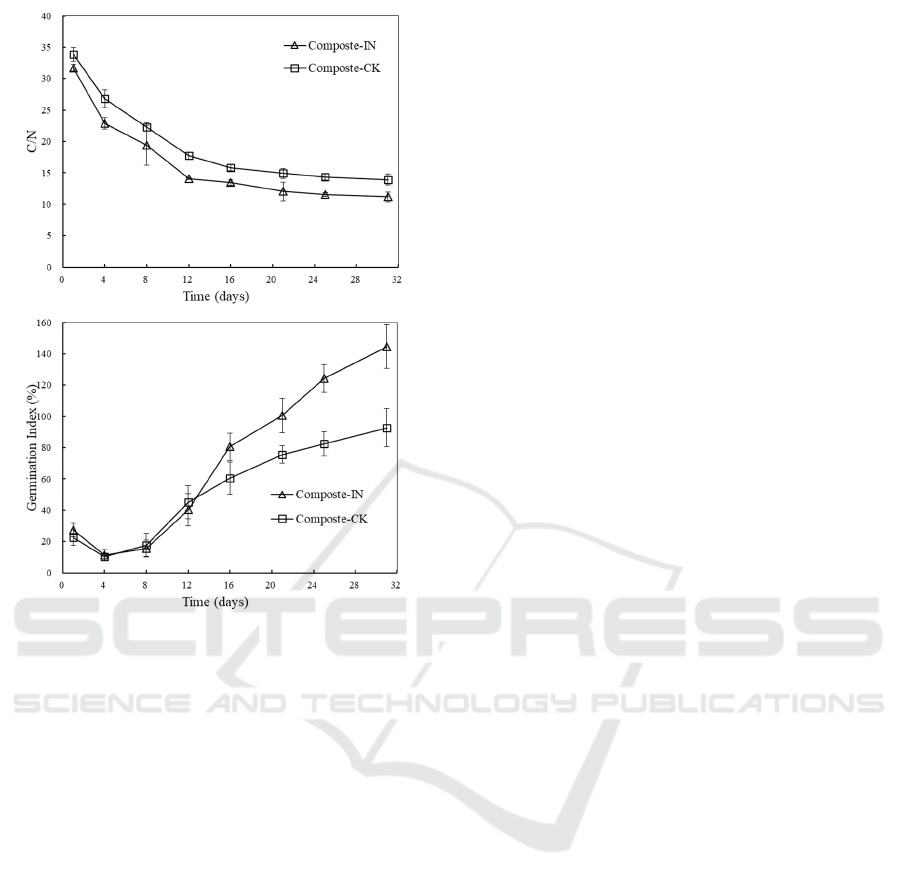
Figure 6: Changes in C/N (a) and germination index (GI)
(b) during the composting process.
4 CONCLUSIONS
In all treatments, typical variation curves in
parameters such as temperature, EC, DOC, C/N and
the succession of microbial community were
observed. Produced composts were found to be
phytotoxic-free convinced by the germination
experiment. When microbial agent was applied,
better performance was obtained during co-
composting of wheat straw and food waste proved by
the relatively higher thermophilic temperatures,
lower C/N ratio and higher GI value. Inoculation
contributed to a more diverse microbial community
and had a clear advantage in acceleration of the
compost degradation, sanitation and maturation
process. However, application of microbial agent is
less economic. Without microbial agent, satisfied
results could still be achieved because of the
appropriate composting conditions such as nutrient
adjustment, forced aeration and active endogenous
microorganisms.
ACKNOWLEDGEMENTS
This work was supported by The Open Funding
Project of National Key Laboratory of Human Factors
Engineering (grant number 6142222190714), and
Key Laboratory of Shenzhen Longgang District
(grant number ZSYS2017001).
REFERENCES
APHA. (1998) Standard Methods for the Examination of
water and Wastewater, 20th ed. American PublicHeslth
Association, Washington, DC. pp. 481-486.
Bernal, M.P., Alburquerque, J., Moral, R. (2009)
Composting of animal manures and chemical criteria
for compost maturity assessment. A review. Bioresour.
Technol. 100(22): 5444-5453.
Bustamante, M.A., Paredes, C., Moral, R., Agulló, E.,
Pérez-Murcia, M.D. Abad, M. (2008) Composts from
distillery wastes as peat substitutes for transplant
production. Resour. Conserv. Recy. 52(5): 792-799.
Fourti, O. (2013) The maturity tests during the composting
of municipal solid wastes. Resour. Conserv. Recy. 72:
43-49.
Gao, M., Liang, F., Yu, A., Li, B., Yang, L. (2010)
Evaluation of stability and maturity during forced-
aeration composting of chicken manure and sawdust at
different C/N ratios. Chemosphere 78(5): 614-619.
Hubbe, M.A., Nazhad, M., Sánchez, C. (2010) Composting
as a way to convert cellulosic biomass and organic
waste into high-value soil amendments: A review.
Bioresources 5(4): 2808-2854.
Inbar, Y., Hadar, Y., Chen, Y. (1993) Recycling of cattle
manure: the composting process and characterization of
maturity. J. Environ. Qual. 22(4): 857-863.
Jurado, M., López, M., Suárez-Estrella, F., Vargas-García,
M.C., López-González, J.A., Moreno, J. (2014)
Exploiting composting biodiversity: study of the
persistent and biotechnologically relevant
microorganisms from lignocellulose-based
composting. Bioresour. Technol. 162: 283-293.
Lehtomäki, A., Huttunen, S., Rintala, J. (2007) Laboratory
investigations on co-digestion of energy crops and crop
residues with cow manure for methane production:
effect of crop to manure ratio. Resour. Conserv. Recy.
51(3): 591-609.
Li, Y., Park, S.Y., Zhu, J. (2011) Solid-state anaerobic
digestion for methane production from organic waste.
Renew. Sust. Energ. Rev. 15(1): 821-826.
Li, W., Liu, Y., Hou, Q., Huang, W., Zheng, H., Gao, X.,
Yu, J., Kwok, L., Zhang, H., Sun, Z. (2020)
Lactobacillus plantarum improves the efficiency of
sheep manure composting and the quality of the final
product. Bioresour. Technol. 297: 122456.
Luo, Y., Liang, J., Zeng, G., Chen, M., Mo, Dan., Li, G.,
Zhang, D. (2018) Seed germination test for toxicity
(
b
)
(a)
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
196

evaluation of compost: Its roles, problems and
prospects. Waste Manage. 71: 109-114.
Meng, X., Liu, B., Zhang, H., Wu, J., Yuan, X., Cui, Z.
(2019) Co-composting of the biogas residues and spent
mushroom substrate: Physicochemical properties and
maturity assessment. Bioresour. Technol. 276: 281-
287.
Mohee, R., Mudhoo, A. (2005) Analysis of the physical
properties of an in-vessel composting matrix. Powder
Technol. 155(1): 92-99.
Qian, X., Shen, G., Wang, Z., Guo, C., Liu, Y., Lei, Z.,
Zhang, Z. (2014) Co-composting of livestock manure
with rice straw: characterization and establishment of
maturity evaluation system. Waste Manage. 34(2): 530-
535.
Sun, Z.Y., Zhang, J., Zhong, X.Z., Tan, L., Tang, Y.Q.,
Kida, K. (2016) Production of nitrate-rich compost
from the solid fraction of dairy manure by a lab-scale
composting system. Waste Manage. 51: 55-64.
Wang, Z., Liang, Y., Sheng, J., Guan, Y., Wu, H., Chen, L.,
Zheng, J. (2016) Analysis of water environment risk on
biogas slurry disposal in paddy field. Trans. Chin. Soc.
Agric. Eng. 32(5): 213-220.
Wei, Y., Li, J., Shi, D., Liu, G., Zhao, Y., Shimoka, T.
(2017) Environmental challenges impeding the
composting of biodegradable municipal solid waste: A
critical review. Resour. Conserv. Recy. 122: 51-65.
Yu, H., Zeng, G., Huang, H., Xi, X., Wang, R., Huang, D.,
Huang, G., Li, J. (2007) Microbial community
succession and lignocellulose degradation during
agricultural waste composting. Biodegradation 18(6):
793-802.
Yang, F., Li, G.X., Yang, Q.Y., Luo, W.H. (2013) Effect of
bulking agents on maturity and gaseous emissions
during kitchen waste composting. Chemosphere 93(7):
1393-1399.
Zhang, Y., Ai, W., Jin, X., Feng, H., Zhang, L., Wu, C.
(2021) Effects of three microbial agents on wheat straw
aerobic composting. Chin. J. Environ. Eng. 15(2): 709-
716.
Zhou, Y., Selvam, A., Wong, J.W. (2014) Evaluation of
humic substances during co-composting of food waste,
sawdust and Chinese medicinal herbal residues.
Bioresour. Technol. 168: 229-234.
Zhu, Q., Li, X., Li, G., Li, J., Li, C., Che, L., Zhang, L.
(2020) Enhanced bioenergy production in rural areas:
synthetic urine as a pre-treatment for dry anaerobic
fermentation of wheat straw. J. Clean Prod. 260,
121164.
Zucconi, F. (1981) Evaluating toxicity of immature
compost. Biocycle 22(2): 54-57.
Co-composting of Wheat Straw and Food Waste with and without Microbial Agent
197
