
Effects of Phosphate-solubilizing Bacteria on Micro-Tom and Soil of
Micro-Tom Rhizosphere
Wanying Zhang
1,2,a
, Shuying Wu
1,b
, Hanshi Guo
1,c
, Wanying Wang
1,d
, Yixin Li
1,e
, Jingnan Zhang
1,f,*
and Shuiqing Zhang
3,g,*
1
School of Life Sciences, Zhengzhou University, Zhengzhou, 450001, China
2
School of Agricultural Sciences, Zhengzhou University, Zhengzhou, 450001, China
3
Institute of Plant Nutrition and Environmental Resources Science, Henan Academy of Agricultural Sciences, Zhengzhou,
450002, China
National Natural Science Foundation of China, Grant/Award Number: 31800440
f,*
zhangjn@zzu.edu.cn,
g,*
zsq510@163.com
*
Corresponding author
Keywords: Phosphate-Solubilizing Bacteria, Growth-Promoting Effect, ,Inorganic Phosphorus.
Abstract: Phosphorus bacteria fertilizer can increase the utilization of soil phosphorus and promote plant growth. In
order to provide basic information for the composite engineering strains, we selected high-efficiency
phosphorus-solubilizing bacteria (PSB) from the rhizosphere of mature corn soil. In this study, a total of six
organic phosphorus strains were obtained by NBRIP medium. Further experiments were performed on
inorganic PSBs. We measured the dissolved phosphorus ratio (D/d values), NK2 and NK3 had the best
phosphate-solubilizing effects. The D/d values of NK2 and NK3 were 2.13 and 4.35, respectively. The
results of 16S rRNA amplification and sequencing showed that the NK2, NK3 were identified as
Acinetobacter sp., Pseudomonas sp., respectively. According to the data of shaker experiment for 7 days,
the maximum phosphate solubilizing contents of NK2 and NK3 were 183.10 mg·L
-1
and 79.87 mg·L
-1
,
respectively, and NK2 had genetic stability. The result of pot experiment indicated that the growth attributes
and the root indexes of the Micro-Tom, as well as the content of soil available phosphorus treated by NK2
(TCP) and NK3 (TCP) were both significantly higher than CK (P<0.05). These results imply that above two
strains could promote plant growth.
1 INTRODUCTION
Deficiency of phosphorus (P) is an important
limiting factor in agriculture production. Fertilizers
or inoculants made by P-solubilizing microbes are
applied to the soil with less available phosphorus
(Oliveira, 2009), which not only effectively avoids
the excessive application of phosphate chemical
fertilizers in agriculture, but also solves the problem
of the lack of available phosphorus in the soil
(Bojinova, 2008). So, environmentally friendly
substitutes for P fertilizers are urgently needed to
avoid adverse effects on agriculture production.
To circumvent phosphorus deficiency, the
phosphate -solubilizing bacteria (PSB) could play
an important role in supplying phosphate to plants
in a more environmentally-friendly and sustainable
manner (KHAN, 2007). A massive number of
research results have proved that the soil is the main
source of PSBs. PSBs in soil generally affect the
fertility of soils through biogeochemical cycles
(Wang, 2020). It has been reported that numerous
rhizosphere microorganisms have capability of
dissolving insoluble P (Hameeda, 2008, Henri,
2008). Due to the activity of P⁃solubilizing in
rhizosphere, PSBs supply P for plants in an
environmentally friendly and sustainable manner.
Several studies under greenhouse and field
indicated that PSBs have direct impacts on soil
conditions, nutrient availability and plant growth
(Fitriatin, 2014, Hussain, 2013). Understanding the
interaction of rhizosphere and microbial community
will assist the development of inoculants with
potentially greater consistency in performance and
survival for agroforestry ecosystems, especially
using indigenous microorganisms.
254
Zhang, W., Wu, S., Guo, H., Wang, W., Li, Y., Zhang, J. and Zhang, S.
Effects of Phosphate-solubilizing Bacteria on Micro-Tom and Soil of Micro-Tom Rhizosphere.
DOI: 10.5220/0011198500003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 254-259
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

2 MATERIALS AND METHODS
2.1 Sample Collection
Soil tightly adhering to corn roots was collected from
the national long-term monitoring station for soil
fertility. Using the five-point sampling method, three
vigorous and disease-free plants in the plot were
selected. Taken about 1 kg near-root corn soil
samples from the 15 cm of depth, and they were
collected in sterile bags and transported to the
research laboratory and stored at 4℃ until further
use.
2.2 Cultivation of PSBs
Serial dilution from 10
-5
to 10
-2
was achieved by
transferring 5.0 g of soil residue solution from each
preceding attenuation stage to the next. Extracted 0.1
mL samples from the 10
-3
, 10
-4
and 10
-5
dilutions,
and placed on NBRIP (Shekhar, 2003) in triplicate
and kept at 28℃ for 72 h. An isolate forming a clear
halo zone was selected as a PSB. Then a single
colony was pick-transferred to Luria-Bertani (LB)
for further purification.
The isolated bacteria were inoculated in NBRIP
liquid medium at 28℃ and 180 r/min for 7 days, and
their phosphate solubilizing activities were
quantitatively determined and compared. The
soluble P in the mediums was measured per day. The
amount of soluble P was determined through Mo-Sb
anti-spectrophotometry method (Sundararao, 1963).
2.3 Molecular Identification of
Bacterial Strains
The DNA was extracted using a Bacterial DNA Kit
(Biomed, Beijing, China) following the
manufacturer’s instructions. The 16S rDNA genes
were amplified by PCR using the universal primers:
27F (5-AGAGTTTGATCNTGGCTCAG-3) and
1492R (5-TACGGYTACCTTGTTACGACTT-3),
and sequenced as described. The amplification
reaction mixture contained 12.5 μL of Prime STAR
Max, 1 μL of 10 μmol primer (F and R), 1 μL of
template DNA and ddH
2
O to make up to 25.0 μL.
The resulting products were analyzed by
electrophoresis in 2.0% agarose gel and sent to the
Sangon Biotech Company (Zhengzhou, China) for
sequencing. The 16S rDNA sequences of strain NK2
and NK3 were analyzed initially by BLASTn to
acquire the closest reference sequences.
2.4 Growth Curve of Bacteria
In microbial transformation research and industrial
production applications, the physiological activity
and transformation activity of transformed bacteria
are necessary. To quantitatively determine the
transformation rate of bacteria, the concentration and
biomass of the bacterial solution should be
accurately determined to ensure the continuous and
efficient conversion process (Li, 2003). In this
section, through the optic density (OD) value assay
of culture with vary vaccination time, a chart
between OD600 and time was established.
Configured the NBRIP liquid medium with NaCl
concentration gradients of 0, 2.5%, 5%, 10%, 15%,
and 20%, respectively, the filling volume was 50
ml/250 ml. Each concentration was set to 3 parallel.
Inoculation 1 ml (OD600=1) PSBs and cultured at
28°C and 130 r/min for 20 h. Established a chart
between OD600 and the different salt
concentrations.
2.5 Pot Experiment for Evaluation of
PSBs Application on Micro-Tom
Growth
Collected seeds of Micro-Tom (Solanum
lycopersicum L. cv Micro-Tom) from School of life
science, Zhengzhou University, Henan, China.
Seeds were surface-sterilized by soaking in 5%
NaClO solution for 10 min and rinsing with sterile
distilled water. Then transferred them to sterile
dishes filled with double-layer wet filter paper and
incubated for 7 d at 26℃ after germination.
Seedlings of uniform size were transferred to pots
(diameter 15 cm, height 18 cm) filled with 1 kg of
soil (river sand: vermiculite=1:1).
The experiment was divided into 6 treatments:
CK, NK2, NK3, TCP, NK2 (TCP), NK3 (TCP), with
six replications each. CK was un-inoculated
controls. NK2 and NK3 were soil treated with 100ml
of NK2 and NK3 (OD
600
=1), respectively. TCP was
soil treated with 1% Ca
3
(PO4)
2
. NK2 (TCP) and
NK3 (TCP) were soil treated with TCP and 100 ml
of NK2, NK3 (OD
600
=1), respectively.
In pot experiments, the effects of PSBs on
Micro-Tom and soil of rhizosphere were studied.
Different growth parameters including plant height,
biomass in the plants, the total chlorophyll contents
(SPAD value), root indexes and soil available
phosphorus were examined at 15 d and 30 d after
inoculation. Replicates were not pooled. A 5 g (dry
weight) aliquot of the sampled soil was suspended in
50 ml of sodium bicarbonate solution (PH=8.5) by
Effects of Phosphate-solubilizing Bacteria on Micro-Tom and Soil of Micro-Tom Rhizosphere
255
shock (170 rpm) for 30 min at 25°C(Olsen, 1954).
The soluble phosphorus content in soil was
evaluated by Mo-Sb anti colorimetry.
3 RESULTS
3.1 Isolation of PSB and Phosphorus
Solubility of Two Selected Strains
Selected the PSBs showing greater solubilization
(both qualitatively and quantitatively) of insoluble P
under in vitro conditions. Each isolate was purified
in LB and working cultures were maintained at 4℃.
Isolates with a larger halo zone of solubilization in
NBRIP were selected for further studies. The
dissolved phosphorus ratio (D/d) of two strains
exceeded 2.0, and these were quantitatively assayed
for phosphate solubilization potential. A total of 6
different bacterial isolates were obtained from
different samples of corn soil (Table 1).
Table 1: Dissolved phosphorus ratio of 6 isolates isolated
from the near-root corn soil samples on NBRIP.
Strain D/mm d/mm D/d
NK2 2.45±0.07 1.15±0.07 2.13±0.19 b
NK3 2.73±0.15 0.67±0.20 4.35±1.26 a
NK22 7.60±0.42 5.40±0.28 1.41±0.15 c
N
2
K2 8.40±0.28 5.75±0.21 1.46±0.01 c
N
2
K3 5.45±0.21 4.35±0.35 1.26±0.15 d
N
2
K8 5.85±0.07 4.00±0.14 1.46±0.07 c
Note: D, dissolved phosphorus circle diameter; d, colony
diameter; D/d, dissolved phosphorus ratio equal to
diameter of hydrolysis circle divided by diameter of
colony.
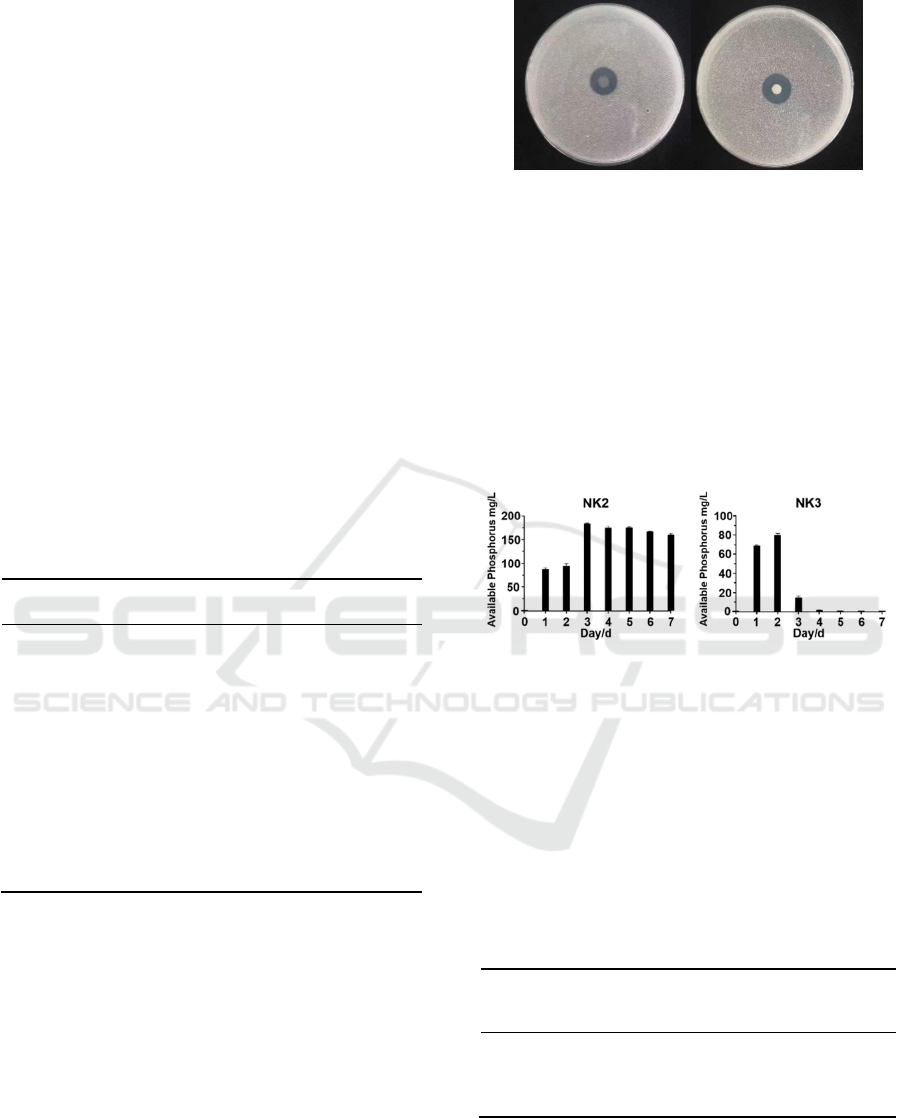
Bacterial strains exhibiting
phosphate-solubilizing activity are detected by the
formation of clear halo around their colonies. We
selected two PSBs and showed these morphological
characteristics in Fig.1. The colonies were circular.
Figure 1: Phosphate-solubilizing activity on NBRIP by
species of NK2 isolates (Plate left) and NK3 isolates
(Plate right).
Two of the bacterial strains exhibited higher
phosphate-solubilizing activity, we measured the
daily phosphate-solubilizing activity of the two
strains within 7 days (Fig.2). The NBRIP liquid
cultures of NK2 isolates contained 183.10 mg/L (the
highest concentration) P solubilized from insoluble
Ca
3
(PO4)
2
as the sole source of P in the medium,
followed by NK3 isolates containing 79.87 mg/L
(the highest concentration).
Figure 2: Phosphate-solubilizing activity of two PSBs.
3.2 Identification of Bacterial Isolates
Nucleotide sequencing of the 16S rDNA gene of two
selected isolates proved 98%-99% similarity with
species present in the GenBank database (Table
2)(G, 1991). According to phylogenetic analysis, the
similarity between NK2 isolates and Pseudomonas
sp. was 98.33%, and NK3 isolates was identified as
Acinetobacte sp.
Table 2: Identification results for 16S rDNA sequencing.
Strain
Gram
+
or
Gram
−
Specific name
16SrDNA
homology/%
NK2 Gram
−
Pseudomonas
sp
.
98
NK3 Gram
−
Acinetobacter
sp
.
99
3.3 Growth Curve of Strains
The growth curve of NK2 isolates and NK3 isolates
was shown in Figure 3 (left). The bacteria grow
slowly within 0~2h and are in the growth delay
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
256
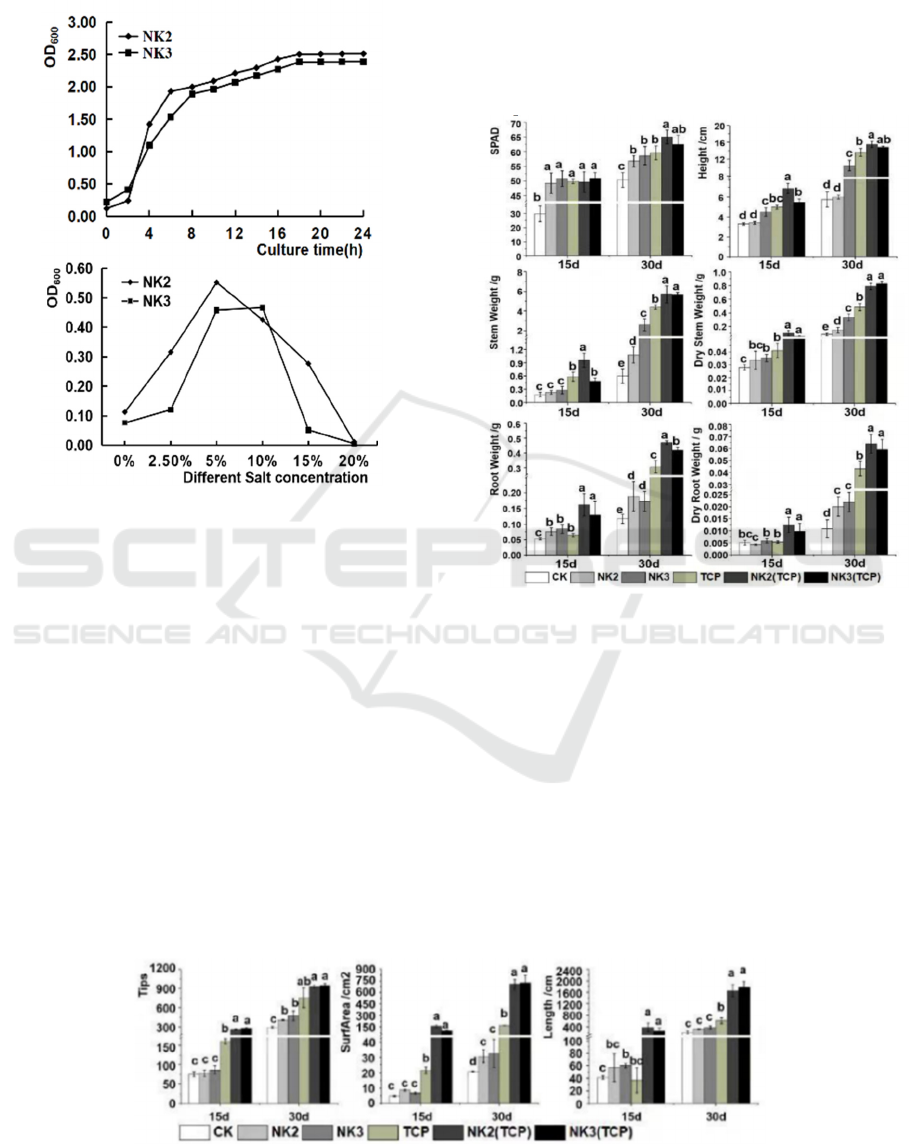
period; after 3h, the strain proliferates rapidly and
enters the logarithmic growth phase. The curve tends
to be flat after 18h.
Figure 3: The growth curve of two PSBs.
It can be seen from Figure 3 (right) that with the
strengthening of salt stress conditions, the growth of
NK2 and NK3 strains increased firstly and then
decreased. NK2 and NK3 can tolerate a wide range
of salt concentration.
3.4 Effects of PSB on Micro-Tom
Growth
3.4.1 Growth Attributes of Micro-Tom
Two PSBs with sufficient P production were chosen
to determine their beneficial effects on Micro-Tom
growth under greenhouse conditions. The
Micro-Tom grew better than CK when NK2 isolates
and NK3 isolates were colonized in the rhizosphere
of Micro-Tom seedlings (Fig.4). The NK2 (TCP)
and NK3 (TCP) significantly promoted plant height
by 170% and 160% at 30 d. Plant height was
measured from stem base to top. Leaves at a certain
fixed node height were marked and evaluated. The
total chlorophyll contents (SPAD value) increased
by 35% and 24%, the stem biomass by 850% and
800%, the root biomass by 480% and 440%,
compared with CK.
Figure 4: Growth Attributes of Micro-Tom.
3.4.2 Root Indexes
The effects of the two PSBs in the rhizosphere of
plant, measured as root length, root surface area, and
the number of root tips in the root of plant, are
presented in Fig.5. Application of NK2 (TCP) had
the strongest effect on root length of Micro-Tom,
with an increase by 39.73% at 30 d (P ≤ 0.05)
compared with CK. NK3(TCP) resulted in an
increase of 20.10 times at 30d for root surface area.
NK3 (TCP) resulted in an increase of 1.39 times at
30d for root tips (Fig. 6). Therefore, PSBs effectively
improved root development in Micro-Tom.
Figure 5: Root indexes of Micro-Tom.
Effects of Phosphate-solubilizing Bacteria on Micro-Tom and Soil of Micro-Tom Rhizosphere
257
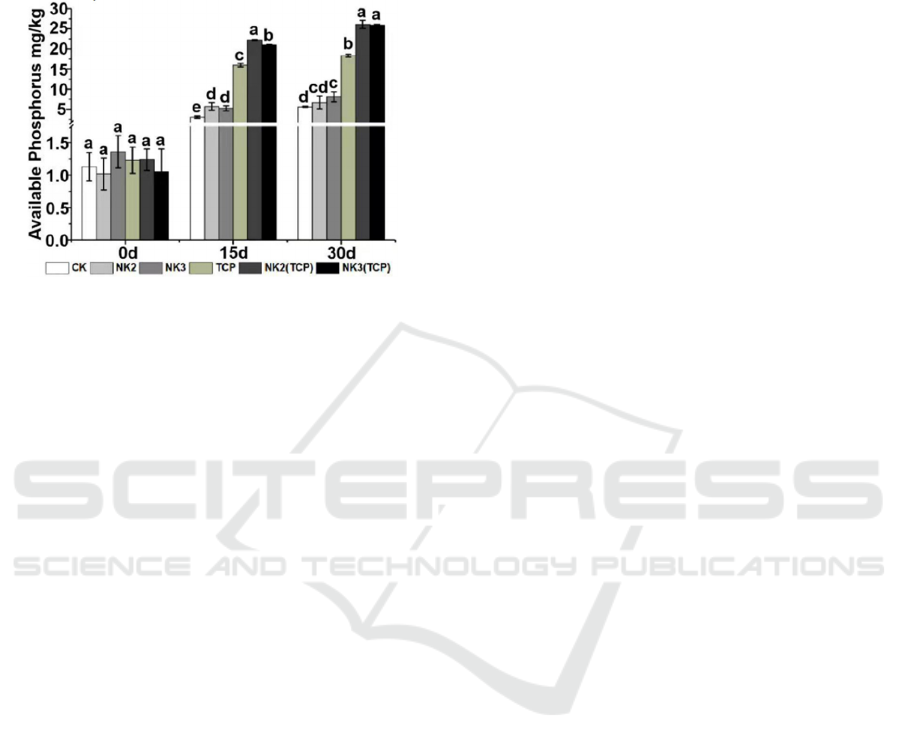
3.4.3 Postharvest Soil Available Phosphorus
The available phosphorus content in soil treated with
NK2 (TCP) and NK3 (TCP) was at a high level at 30
d. NK2 (TCP) and NK3 (TCP) increased
significantly relative to CK at 15 d and 30 d.
Figure 6: Soil available phosphorus.
4 DISCUSSIONS
It is an urgent thing to establish a sustainable
agriculture industry that maintains the ecological
balance of soil systems for a long time. As the basic
element of fertilizer, soil available phosphorus plays
an important role in the ecosystem. In this study,
rhizosphere soil samples from corn plants were
screened by PSB isolation. Among the
phosphate-solubilizing isolates, two efficient PSB
strains were selected for the further studies. The
strains were identified as Pseudomonas (NK2) and
Acinetobacter (NK3) by 16S rDNA sequencing
technologies.
The data presented in this paper showed that two
isolates significantly promoted the growth of the
plant seedlings and root of Micro-Tom under the
greenhouse conditions. It may be due to the greater
absorption of nutrients, especially P element. The
results in this study were similar to those reported
studies (Datta, 1982, Asea, 1988). However, some
researchers obtained contrary conclusions by
inoculating PSBs to plants. Inoculating PSBs to the
seed of Chinese cabbage will promote its growth, but
there had no effect on the absorption of P element in
plants. In fact, the beneficial effects of PSBs in plant
growth largely depend on the environmental
conditions, type of strain, host plant and condition of
soil (Khan, 2009), which cannot be tested and
evaluated separately (Liu, 2014). In this work, it has
remained a significant challenge to obtain a
complete root from the soil, which caused to too
obvious differences in the measured values of root
surface area between NK3 (TCP) and CK. There is
an urgent need to use an effective method to analyze
and survey the rhizosphere. In any case, it is
necessary to explore the mechanism of NK2 and
NK3 attained a regulating balance, promoting the
growth of plants and the development of
rhizosphere. The application of PSBs with TCP
showed an obvious effect compared to control. It is
possible that the soil environment had changed and
caused a steady increase in nutrients. It is required to
explore the effect of these PSBs either alone or in
combination with other bio-fertilizers on growth of
Micro-Tom under field conditions. Further studies
under field conditions are needed to confirm the
present findings and recommend strains for
commercial applications.
5 CONCLUSIONS
NK2 and NK3 were screened in this experiment,
which have relatively strong Phosphate-solubilizing
activity and high salt tolerance. A pot experiment of
NK2 and NK3 strains were carried out. Some
indexes of the Micro-Tom in the growth period (15
d) and flowering period (30 d) were studied. The
result of pot experiment indicated that the growth
attributes and the root indexes of the Micro-Tom, as
well as the content of soil available phosphorus
treated by NK2 (TCP) and NK3 (TCP) were
significantly higher than CK (P<0.05). These imply
that both strains could promote plant growth.
REFERENCES
ASEA, P., KUCEY, R. & STEWART, J. 1988. Inorganic
phosphate solubilization by two Penicillium species
in solution culture and soil. Soil Biology &
Biochemistry, 20, 459-464.
BOJINOVA, D., VELKOVA, R. & IVANOVA, R. 2008.
Solubilization of Morocco phosphorite by Aspergillus
niger. Bioresource Technology, 99, 7348-7353.
DATTA, M., BANIK, S. & GUPTA, R. K. 1982. Studies
on the efficacy of a phytohormone producing
phosphate solubilizing Bacillus firmus in augmenting
paddy yield in acid soils of Nagaland. Plant and Soil,
69, 365-373.
FITRIATIN, B. N., YUNIARTI, A., TURMUKTINI, T.
& RUSWANDI, F. K. 2014. The effect of phosphate
solubilizing microbe producing growth regulators on
soil phosphate, growth and yield of maize and
fertilizer efficiency on Ultisol. Eurasian Journal of
Soilence, 3, 101-107.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
258

G, W. W., M, B. S., A, P. D. & J, L. D. 1991. 16S
ribosomal DNA amplification for phylogenetic study.
Journal of bacteriology, 173.
HAMEEDA, B., HARINI, G., RUPELA, O. P., WANI,
S. P. & REDDY, G. 2008. Growth promotion of
maize by phosphate-solubilizing bacteria isolated
from composts and macrofauna. Microbiological
Research, 163, 234-242.
HENRI, F., ANNETTE, D., JOHN, Q., WOLFGANG,
M. & FRANOIS-XAVIER, E. 2008. Solubilization of
inorganic phosphates and plant growth promotion by
strains of Pseudomonas fluorescens isolated from
acidic soils of Cameroon. African journal of
microbiology research, 2, 171-178.
HUSSAIN, M. I., ASGHAR, H. N., AKHTAR, M. J. &
ARSHAD, M. 2013. Impact of phosphate solubilizing
bacteria on growth and yield of maize. Soil &
Environment, 32, 71-78.
KHAN, M. S., RIZVI, A., SAIF, S. & ZAIDI, A. 2009.
Role of Phosphate Solubilizing Microorganisms in
Sustainable Agriculture - A Review. Springer
Netherlands.
KHAN, M. S., WANI, P. A. & ZAIDI, A. 2007. Role of
phosphate-solubilizing microorganisms in sustainable
agriculture - A review. Agronomy for Sustainable
Development, 27, 29-43.
LI, X. & SHENG, Y. 2003. OD Value Assay was Used to
Determine Bacterial Biomass in the Real-Time
Detection During Microbial Transformation. Journal
of Nanjing Normal University(Natural Science
Edition).
LIU, F. P., LIU, H. Q., ZHOU, H. L., DONG, Z. G., BAI,
X. H., BAI, P. & QIAO, J. J. 2014. Isolation and
characterization of phosphate-solubilizing bacteria
from betel nut (Areca catechu) and their effects on
plant growth and phosphorus mobilization in tropical
soils. Biology & Fertility of Soils, 50, 927-937.
OLIVEIRA, C. A., ALVES, V., MARRIEL, I. E.,
GOMES, E. A., SCOTTI, M. R., CARNEIRO, N. P.,
GUIMAR?ES, C. T., SCHAFFERT, R. E. & Sá, N.
2009. Phosphate solubilizing microorganisms isolated
from rhizosphere of maize cultivated in an oxisol of
the Brazilian Cerrado Biome. Soil Biology &
Biochemistry, 41, 1782-1787.
OLSEN, S. R. 1954. Estimation of available phosphorus
in soils by extraction with sodium bicarbonate.
Miscellaneous Paper Institute for Agricultural
Research Samaru Pp.
SHEKHAR, N. C., SANGEETA, M. & PALPU, P. 2003.
Composition for qualitative screening of phosphate
solubilizing microorganisms and a qualitative method
for screening microorganisms. Official Gazette of the
United States Patent & Trademark Office Patents.
SUNDARARAO, W. 1963. Phosphate dissolving
organisms in the soil and rhizosphere. Indian.jour.of
Agr, 33.
WANG, X., XIE, H., KU, Y., YANG, X. & CAO, C.
2020. Chemotaxis of Bacillus cereus YL6 and its
colonization of Chinese cabbage seedlings. Plant and
Soil, 447, 413–430.
Effects of Phosphate-solubilizing Bacteria on Micro-Tom and Soil of Micro-Tom Rhizosphere
259
