
Genes Associated with Metabolism in Gestational Diabetes Mellitus:
A New Categorization According to Risk Factors
Qianyan Zheng
School of Public Health, University of Washington, Seattle, WA, 98105, U.S.A.
Keywords: Genes, Metabolism, GDM, Risk Factors.
Abstract:
Gestational Diabetes Mellitus (GDM) is a rising public health concern with a highly increased prevalence
over the last decade. Previous genetic studies on GDM mainly focused on identifying genes associated with
the shared genetic architecture between Type 2 Diabetes Mellitus (T2DM) and GDM. There is a relative lack
of research on the unique genetic architecture of GDM. Thus, to shed light on the traits of GDM, this review
provided a new categorization of genes with determined association with GDM based on their correspondence
to some important risk factors, through combining out the related references. It was concluded that most
genetic evidence concentrated in a history of GDM and a strong family history of diabetes. Evidence in
obesity, polycystic ovary syndrome (PCOS), and ethnicity gave insights on other underlying mechanisms of
GDM that are worth exploration.
1 INTRODUCTION
Diabetes Mellitus is one of most significant public
health concerns because of its wide age range and
diverse complications. As one of the three essential
classifications of Diabetes Mellitus, although GDM
experiences a similar increase in the prevalence as
T2DM, this medical complication did not receive as
much exclusive research.
To date, most genetic studies on GDM were
extended research based on genes previously
demonstrated to be associated with T2DM.
Therefore, it cannot be denied that there may be a
focus bias leading to the much higher number of
genes related to the shared metabolic mechanisms
with T2DM compared to genes uniquely related to
GDM. In short, research on genetics about exclusive
traits of GDM is insufficient. This review introduced
genes associated with GDM and categorized these
genes according to their corresponding risk factors—
history of GDM and a strong family history of
diabetes, obesity, PCOS, and ethnicity. New insights
on the underlying genetic mechanisms of GDM and
further identification of more genes unique for GDM
are in hope. Such identification could contribute to
the personalization of therapy and created new drugs
to target specific mechanisms. (Rosik 2020) What is
more, even though clinical genetic testing procedure
for GDM is not available yet, this review could offer
references for the range of genes considered in future
genetic testing of GDM.
2 THE CONCEPTION OF GDM
GDM is the type of diabetes developed or first
recognized during pregnancy, in which glucose
intolerance results in different severities of
hyperglycemia. The prevalence of GDM has a
noticeable rise during last decade. Because of its
obvious regional difference and inconsistency in the
diagnostic criteria, the prevalence of GDM is varied
all over the world, ranging from 1 to 20%. (Alfadhli
2015) There’s no sign that the trend will stop
worsening. Thus, inventing procedures for the
prevention, diagnosis, and treatment of GDM is of
urgency.
Typically, GDM doesn’t have overt symptoms.
Even at present, it’s difficult to distinguish them from
normal pregnancy symptoms, making a uniform and
sound screening for GDM crucial. However,
currently, a global standard strategy for screening and
diagnosing GDM is not available. The most adopted
diagnostic criterion is the one recommended by WHO
in 2013. An oral glucose tolerance test (OGTT) is
usually required for diagnosis, during which the
plasma glucose concentrations are measured
regularly after ingesting 75 grams of glucose.
Zheng, Q.
Genes Associated with Metabolism in Gestational Diabetes Mellitus: A New Categorization According to Risk Factors.
DOI: 10.5220/0011202300003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 295-302
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
295
(Alfadhli 2015) GDM is diagnosed when blood sugar
levels range from 92 mg/dl fasting, ≥180 mg/dl in the
60th minute of the OGTT, or ≥153 mg/dl in the 120th
minute of OGTT. (Rosik 2020) Once GDM is
diagnosed, lifestyle management like dietary changes
and physical exercise should be initiated. Long-term
self-monitoring of blood glucose levels is another
necessary part of GDM management. When these
interventions fail to control the patient’s blood
glucose level within an acceptable range, medical
therapies with insulin, glyburide, and metformin will
be introduced. (Alfadhli 2015) In general, GDM is a
disease requiring a lot of individual effort. This trait
leads to a relatively low-costing treatment for GDM
as well as some uncontrollability in its treatment
because of the huge variation in people’s self-
discipline.
Undiagnosed GDM increases the risk for
gestational hypertension, pre-eclampsia,
macrosomia, birth defects, and polyhydramnios.
(Metzger 2008) Also, evidence show that women
with GDM have six times higher possibility to
develop diabetes after pregnancy, which accordingly
makes GDM a significant predictor for T2DM
(Damm 2016). Furthermore, children of patients with
GDM are two to eight times more likely to develop
obesity, metabolic syndrome, and T2DM, compared
to children of mothers without GDM (Damm 2016).
If the worsening trend of GDM continues, a
widespread deterioration in blood glucose control is
in expectation due to the impact from generation to
generation.
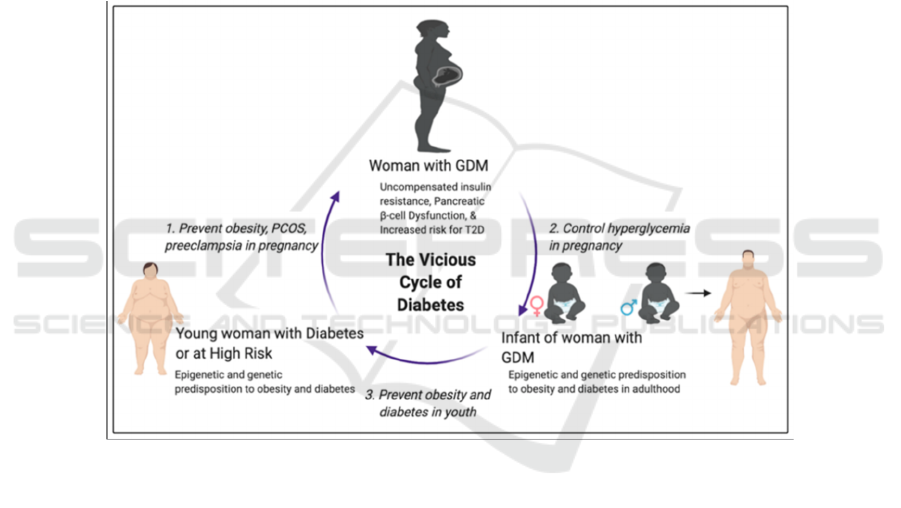
Figure 1: A vicious cycle of obesity and diabetes from generation to generation (Alejandro 2020).
A vicious cycle of obesity and diabetes from
generation to generation exists. As evidence show,
obesity, a history of GDM and a strong family history
of diabetes are all strong factors leading to the
occurrence of GDM. Then, children of patients with
GDM are two to eight times more likely to develop
obesity and T2DM. However, there are still three
major windows of opportunities to take interventions
to break the vicious cycle. First, prevent risk factors
for GDM, like obesity and PCOS. Second, manage
and control blood sugar levels during pregnancy.
Third, control weight gain and prevent obesity during
adolescence. A break of the vicious cycle is critical as
the prevalence of diabetes including GDM continues
to increase over years.
3 THE NORMAL PREGNANCY
METABOLISM AND
PATHOPHYSIOLOGY OF GDM
During pregnancy, maternal metabolism changes
greatly to provide and store enough nutrients for
different stages of pregnancy. For instance, an
increased lipid storage often occurs. The key
metabolic alteration is a substantial increase in insulin
resistance. Placenta and other hormones, including
human placental lactogen, placental growth hormone,
progesterone, leptin, cortisol, prolactin, human
chorionic gonadotropin, and estradiol, are possible
factors leading to increased insulin resistance. [6-9]
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
296
Other factors include tumor necrosis factor-alpha
(TNF-α) and other inflammatory mediators which are
produced by the placenta and other tissues. (Lowe
2014)
To compensate for the pregnancy-induced insulin
resistance, an enhancement in insulin secretion is
initiated. GDM develops when beta cells fail to
secrete as much insulin as needed in pregnancy.
Another possible mechanism leading to GDM is that
pregnancy-induced insulin resistance triggers a
genetic predisposition of impairment of beta cell
function. (Lambrinoudaki 2010)
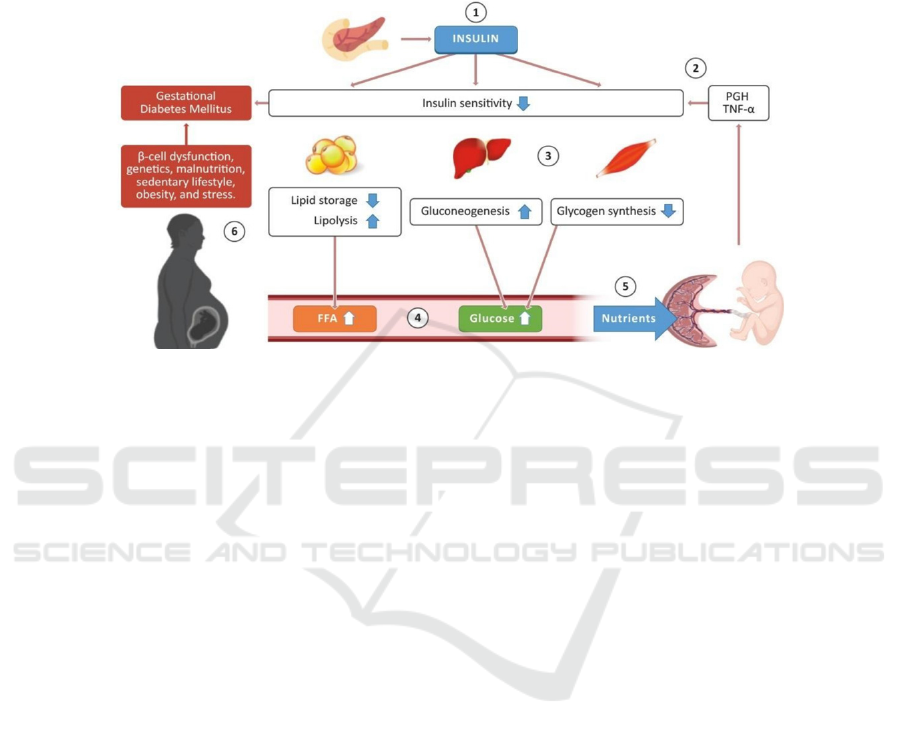
Figure 2: Normal glucose-insulin metabolism during pregnancy. (Lizárraga 2021)
Part 1. The pancreas produces insulin which can
act on multiple organs, adipose tissue, and muscle to
control blood glucose levels. Part 2. Placenta and
other hormones, proinflammatory cytokines like
TNF-α cause increased insulin resistance/decreased
insulin sensitivity. Part 3&4. Then, there’s an
accordingly decrease in lipid storage and an increase
in lipolysis activity, resulting in higher amounts of
free fatty acids (FFA) in the bloodstream. Meanwhile,
promoted gluconeogenesis occurs in the liver and
depressed glycogen synthesis occurs in skeletal
muscles, leading to higher amounts of glucose in the
bloodstream. Part 5. FFA and glucose are transported
into the placenta to provide enough nutrients to
support fetal growth. Part 6. GDM develops when
factors like beta-cell dysfunction and genetics lead to
insufficient insulin secretion that fails to compensate
for the increased insulin resistance.
4 GENETICS IN VARIOUS RISK
FACTORS
Common GDM risk factors include a history of
GDM, a strong family history of diabetes, obesity,
PCOS, ethnicity, and advanced maternal age.
(Alfadhli 2015) Except for advanced maternal age,
other risk factors are all supported with genetic
evidence. This study aims to achieve a new
categorization of genes that have been identified to be
related to GDM according to their correspondence to
different risk factors. Because of an uneven
distribution of efforts in studies on these risk factors,
a ranking of the importance of the risk factors cannot
be safely concluded. Based on current evidence, a
history of GDM and a strong family history of
diabetes are the biggest risk factors. The following
ordering of risk factors is primarily according to the
amount of available evidence.
4.1 A History of GDM & a Strong
Family History of Diabetes
A history of GDM and a strong family history of
diabetes are two risk factors that share many
physiological traits. Previous studies achieved most
findings of genes responsible for glucose metabolism
and mechanisms regarding insulin like beta cell
function. It provides a sound explanation for why a
history of GDM and a strong family history of
diabetes, which contribute to progressive
dysfunctions of these mechanisms, are significant
risk factors of GDM. Interestingly, GDM has a
similar impact on the risk of T2DM.
4.1.1 Transcription Factor 7–like 2
(TCF7L2)
Many TCF7L2 polymorphisms have threatening
Genes Associated with Metabolism in Gestational Diabetes Mellitus: A New Categorization According to Risk Factors
297
effects on the risk of GDM. In their study, Zhang et
al. showed that rs7903146 (OR=1.44, p < 0.001) and
rs12255372 (OR=1.46, P = 0.002) are strongly
correlated with increased GDM risk. (Zhang 2021)
Reducing insulin secretion is the potential pathway.
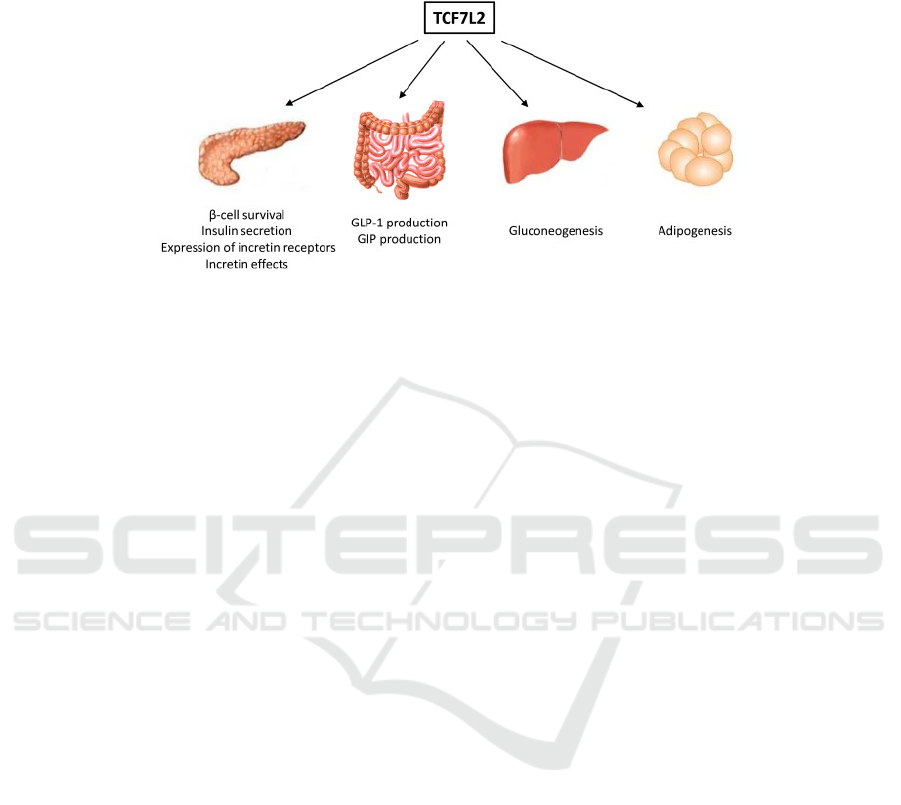
Figure 3. Potential metabolic pathways that TCF7L2 may contribute to. (Chiang 2012).
TCF7L2 is an important protein involved in Wnt
signaling pathway by contributing to the formation of
a key effector in this pathway. Wnt pathway impacts
glucose homeostasis by controlling the gene
expression and functioning of some hormones, like
GLP-1, GIP, and insulin. This pathway also affects
adipogenesis negatively. Even though the mechanism
of how TCF7L2 polymorphisms leads to increased
GDM risk is unclear, this finding has been
successfully replicated several times. Insulin down-
regulates the gene expression of TCF7L2 in
adipocytes. People with insulin resistance are
observed to have higher levels of TCF7L2 in adipose
tissue.
4.1.2 Melatonin Receptor 1B (MTNR1B)
MTNR1B has the potential effect of antagonizing
insulin release. (Dalfrà 2020) Its polymorphism
rs10830963 was more frequently found in GDM
patients compared to the controls (48.4% vs. 42.3%).
(Zhang 2014) The G allele carriers were observed to
increase the risk of GDM risk (OR=1.24, p <
0.00001). (Zhang 2014) A similar association was
found between rs1387153 single-nucleotide
polymorphism (SNP) and GDM risk. (Zhang 2014)
4.1.3 Glucokinase (GCK)
GCK amplifies the secretion when a rise in blood
glucose is detected to manage insulin secretion.
(Dalfrà 2020) A meta-analysis of recent studies found
a significant association between rs1799884 and
enhanced GDM risk with an OR of 1.29 (p < 0.001).
(Zhang 2021)
4.1.4 Glucokinase Regulatory Protein
(GCKR)
GCKR encodes regulatory proteins that exert an
inhibiting effect on GCK in the liver and pancreatic
islet cells. (Dalfrà 2020) The polymorphism rs780094
C/T SNP was found to be associated with a decrease
in GDM risk in all populations in its dominance,
recessive, and allelic models. (Lin 2018)
4.1.5 Potassium Channel Inwardly
Rectifying Subfamily J member 11
(KCNJ11)
KCNJ11 contributes to the regulation of insulin
secretion. (Dalfrà 2020) A meta-analysis conducted
by Zhang et al. demonstrated a modest correlation
between KCNJ11 rs5219 (E23K) and increased GDM
risk (OR=1.15, P=0.002). (Zhang 2021)
4.1.6 CDK5 Regulatory Subunit
Associated Protein 1 Like 1
(CDKAL1)
CDKAL1 is involved in beta cell function and insulin
release. (Rosik 2020) Guo et al. demonstrated that
rs7754840 and rs7756992 were all significantly
correlated with GDM risk. (Guo 2018)
4.1.7 Solute Carrier Family 30, Member
8 (SLC30A8)
SLC30A8 is only expressed in the pancreas and is
responsible for insulin secretion. (Dalfrà 2020) In
their study, Lin et al. demonstrated a protective effect
of rs13266634 C/T SNP on GDM development. (Lin
2018)
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
298

4.1.8 Insulin Receptor Substrate 1
(IRS1)
IRS1 encodes insulin receptor substrate 1 which
impacts insulin signaling. (Dalfrà 2020) A meta-
analysis determined that the T allele of IRS1
rs1801278 was discovered more frequently in GDM
patients than in the control group (8.7% vs. 5.1%). It
leads to the conclusion that rs1801278 is strongly
correlated with GDM risk. (Zhang 2014)
4.1.9 Insulin Like Growth Factor 2
MRNA Binding Protein 2
(IGF2BP2)
IGF2BP2 encodes proteins which play a role in
insulin secretion. Zhang et al. identified the effect of
rs4402960 on increasing GDM risk (OR=1.21,
P<0.001). (Zhang 2021)
Many of the recent studies were testing the
association between T2DM susceptibility genes with
GDM, based on the assumption that there is a shared
genetic architecture between GDM and T2DM.
(Lowe 2014) Also, there is a potential bias because
the references this review relies on only included a
relatively limited number of women with GDM,
compared to a large number of patients with T2DM
involved in these references. Among tested genes, the
nine genetic loci listed are demonstrated to be also
associated with GDM and are supported with the
most evidence. However, many T2DM susceptibility
genes failed to show any evidence for their
correlation with GDM. (Lowe 2014) Therefore,
although further examination of the association
between other T2DM susceptibility genes and GDM
is of great significance to understand GDM,
exploration of other underlying genetic mechanisms
is still necessary.
4.2 Obesity
As a rising public health crisis aggravated by the
widespread sedentary lifestyles, obesity leads to
many life-threatening diseases, including GDM. It is
shown that the prevalence of GDM in normal-weight
women, defined as women whose pre-pregnancy
BMI is 18.5-24.9, is 3.6%, whereas that in women
with BMI over 40.0 is 13.9%. (Deputy 2018) Some
genes related to lipid metabolism showed evidence
for association with increased GDM risk.
FTO contributes to regulating fat mass,
adipogenesis and body weight. FTO rs9939609 T/A
SNP had an identified association with increased
GDM risk. (Dalfrà 2020) Moreover, according to
Yang et al.’s study, there were associations between
FTO gene rs11075995, rs3826169, rs74245270,
rs74018601, rs7205009 and rs9888758 and the
enhancement of the risk of GDM. (Yang 2020)
Furthermore, an enhancement in the gene
expression of the adipokines TNFα, IL-1β and or
leptin was investigated to increase in adipose tissue
from obese and GDM women. On the other hand, the
gene expression of LPL, FATP2, FATP6, ASCL1,
PNPLA2, PPARδ, PPARγ and RXRα was observed
to decrease in GDM patients. (Lappas 2014)
As findings of the association between FTO and
GDM risk are inconsistent, further studies are in need
to determine the association. Still, the association
between other genes involved in lipid metabolism and
GDM risk is worth examination.
4.3 PCOS
PCOS is a health condition characterized by
hyperandrogenism, anovulation, and insulin
resistance. Similar to the relationship between T2DM
and GDM, PCOS may contribute to an increase of the
risk of GDM.
Fibroblast growth factor (FGF) 19 & 21. FGF 19
and FGF 21, encoding adipokines which are involved
in insulin resistance and serum levels of adiponectin,
were identified to be correlated with GDM risk.
(Wang 2013) Moreover, GDM patients with PCOS
history were observed to have much lower levels of
FGF 19 than GDM patients without PCOS history
and controls without PCOS history. (Wang 2013)
Wang et al. indicated that a decrease in serum FGF19
level was a possible part of the pathophysiology of
GDM. (Wang 2013) On the other hand, increased
serum FGF 21 was potentially involved in a
compensatory response to GDM. (Wang 2013)
Even though only limited genes are found to
prove the association between PCOS and GDM
genetically, evidence for such genes’ involvement in
the pathophysiology of GDM is encouraging,
providing potential research directions of the
pathophysiology of GDM.
4.4 Ethnicity
Many statistics show that the prevalence of GDM
varies among different ethnic groups, even though the
genetic evidence is not obvious yet. Non-Hispanic
Asian women had the highest prevalence (11.1%).
(Deputy 2018) In general, GDM has higher frequency
among African, Hispanic, Indian, and Asian women
than among Caucasian women. (Alfadhli 2015)
PPARG is a gene that demonstrated some association
Genes Associated with Metabolism in Gestational Diabetes Mellitus: A New Categorization According to Risk Factors
299
with ethnicity.
Peroxisome proliferator-activated receptor γ
(PPARG). PPARG plays a role in the regulation of
adipocyte differentiation and glucose homeostasis. Its
polymorphism rs1801282 was demonstrated to be
associated with GDM risk only in the Asian
population. However, the association was not
identified in the Caucasian population. (Metzger
2008)
The lack of genetic evidence for this association
could be because many previous studies contained
groups of mixed ancestry or participants from a single
ethnic group. Further findings of such genes require
research incorporating and comparing various ethnic
groups.
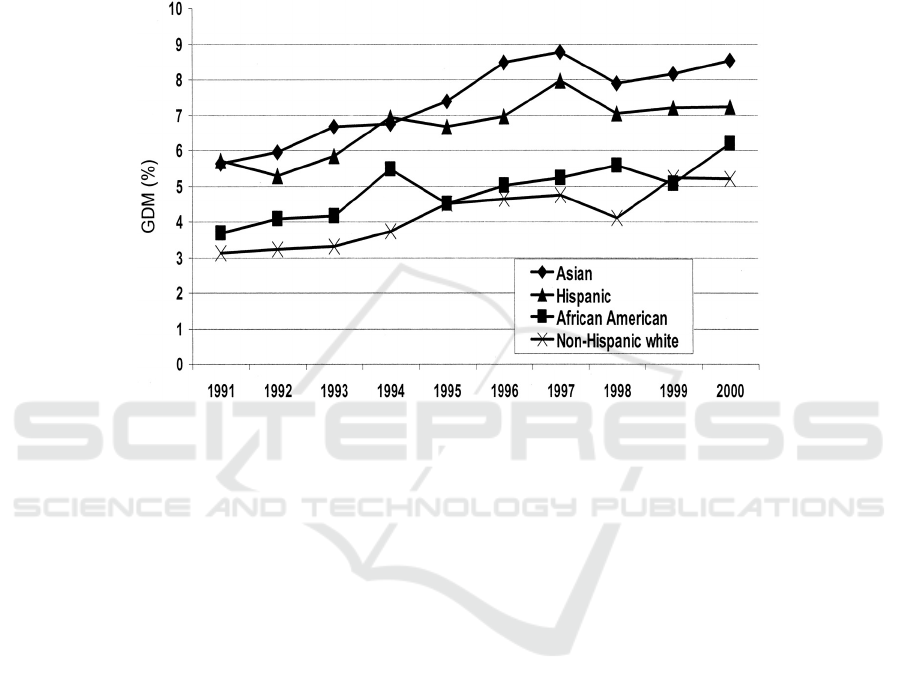
Figure 4: Age-adjusted prevalence of GDM by ethnicity and years: Northern California Kaiser Permanente, 1991-2000
(Ferrara 2007).
All ethnic groups were investigated to have a
similar increasing trend in their prevalence of GDM
in the Northern California Kaiser Permanente study
from 1991-2000. Asian and Hispanic women had
higher prevalence of GDM than other ethnic groups.
5 DISCUSSIONS
Clinical genetic testing is already available for
monogenic forms of diabetes, especially Maturity
Onset Diabetes of the Young (MODY). The
mutations of 14 genes are identified to be individually
associated with the occurrence of MODY. (Firdous
2018) Currently, the genetic diagnosis of MODY is
usually done through next-generation sequencing
(NGS), which analyzes the mutations in DNA
isolated from blood sample. This method usually
diagnoses MODY with almost 100% sensitivity.
(Firdous 2018)
However, in terms of polygenic forms of diabetes
like T2DM and GDM, the genetic etiology is much
more complicated. What further extends the difficulty
of clinical genetic testing for polygenic forms of
diabetes is the interactive impacts of environmental
and lifestyle factors on such diabetes.
Nonetheless, the application of genetic diagnosis of
MODY is still inspiring for the development of a
genetic diagnosis procedure of GDM in the future.
There could be a huge step forward if some genes
unique to the etiology of GDM are identified. This is
also in accordance with the aim of this review—to
provide insights on underlying mechanisms (other
than the shared genetic architecture with T2DM) that
have the potential to find genes associated with GDM
exclusively.
6 CONCLUSIONS
In this paper, through the combining of related
references, a new classification of genes that
influence GDM was made and based on their
correspondence to various risk factors. The
underlying mechanisms that are associated with
obesity (lipid metabolism), PCOS, and race were also
revealed in this paper. Finally, it was concluded that
except for advanced maternal age, other risk factors
such as history of GDM, a strong family history of
diabetes, obesity, PCOS, ethnicity were identified to
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
300

have genetic evidence associated with an increase in
GDM risk. Among them, history of GDM and a
strong family of history combined (due to their
similarity in physiological mechanism) had the
highest number of genes with a demonstrated
association with GDM. This trend could be attributed
to the concentrated research on the similarity between
the genetic architecture of T2DM and GDM.
However, this paper could be further improved with
more research on obesity, PCOS, race and other
related genes, as well as an in-depth explanation of
their metabolic mechanisms. In the future, with more
samplings and diverse ethnic groups, more associated
genes may be found by related researchers and may
contributing to the genetic testing for GDM.
REFERENCES
Alejandro, E.U., Mamerto, T.P., Chung, G., Villavieja, A.,
Gaus, N.L., Morgan, E., & Pineda-Cortel, M.R.B.
(2020) Gestational Diabetes Mellitus: A Harbinger of
the Vicious Cycle of Diabetes. International Journal of
Molecular Sciences. 2020; 21(14):5003.
https://doi.org/10.3390/ijms21145003
Alfadhli, E.M. (2015). Gestational diabetes mellitus. Saudi
Med J. 2015 Apr;36(4):399-406. doi:
10.15537/smj.2015.4.10307.
Butte, N.F. (2000). Carbohydrate and lipid metabolism in
pregnancy: normal compared with gestational diabetes
mellitus. Am J Clin Nutr. 2000; 71: 1256S–61. doi:
10.1093/ajcn/71.5.1256s.
Dalfrà, M.G., Burlina, S., Del Vescovo, G.G., & Lapolla,
A. (2020). Genetics and Epigenetics: New Insight on
Gestational Diabetes Mellitus. Front Endocrinol
(Lausanne). 2020 Dec 1; 11: 602477. doi:
10.3389/fendo.2020.602477.
Damm, P., Houshmand-Oeregaard, A., Kelstrup, L.,
Lauenborg, J., Mathiesen, E.R., & Clausen, T.D.
(2016). Gestational diabetes mellitus and long-term
consequences for mother and offspring: a view from
Denmark. Diabetologia. 2016 Jul;59(7):1396-1399.
doi: 10.1007/s00125-016-3985-5.
Deputy, N.P., Kim, S.Y., Conrey, E.J., & Bullard, K.M.
(2018). Prevalence and changes in preexisting diabetes
and gestational diabetes among women who had a live
birth — United States, 2012–2016. MMWR Morb
Mortal Wkly Rep 2018; 67:1201–7.
https://doi.org/10.15585/mmwr.mm6743a2.
Di Cianni, G., Miccoli, R., Volpe, L., Lencioni, C., & Del
Prato, S. (2003). Intermediate metabolism in normal
pregnancy and in gestational diabetes. Diabetes Metab
Res Rev. 2003; 19:259–70.
Ferrara, A. (2007) Increasing Prevalence of Gestational
Diabetes Mellitus. Diabetes Care 2007 Jul;
30(Supplement 2): S141-
S146.https://doi.org/10.2337/dc07-s206
Firdous, P., Nissar, K., Ali, S., Ganai, B. A., Shabir, U.,
Hassan, T., & Masoodi, S. R. (2018). Genetic Testing
of Maturity-Onset Diabetes of the Young Current Status
and Future Perspectives. Frontiers in endocrinology, 9,
253. https://doi.org/10.3389/fendo.2018.00253Rosik,
J., Szostak, B., Machaj, F., & Pawlik, A. (2020). The
role of genetics and epigenetics in the pathogenesis of
gestational diabetes mellitus. Ann Hum Genet. 2020
Mar;84(2):114-124. doi: 10.1111/ahg.12356.
Guo, F., Long, W., Zhou, W., Zhang, B., Liu, J., & Yu, B.
(2018). FTO, GCKR, CDKAL1 and CDKN2A/B gene
polymorphisms and the risk of gestational diabetes
mellitus: A meta-analysis. Archives of Gynecology and
Obstetrics, 298(4), 705–715. Retrieved from
https://doi.org/10.1007/s00404-018-4857-7
Ip, W., Chiang, Y. & Jin, T. (2012). The involvement of the
wnt signaling pathway and TCF7L2 in diabetes
mellitus: The current understanding, dispute, and
perspective. Cell & bioscience. 2. 28. 10.1186/2045-
3701-2-28.
Lain, K.Y., & Catalano, P.M. (2007). Metabolic changes in
pregnancy. Clin Obstet Gynecol. 2007; 50:938–48. doi:
10.1097/GRF.0b013e31815a5494
Lambrinoudaki, I., Vlachou, S. A., & Creatsas, G. (2010).
Genetics in Gestational Diabetes Mellitus: Association
with Incidence, Severity, Pregnancy Outcome and
Response to Treatment. Current Diabetes Reviews
2010; 6(6).
https://doi.org/10.2174/157339910793499155
Lappas, M. (2014) Effect of pre-existing maternal obesity,
gestational diabetes and adipokines on the expression
of genes involved in lipid metabolism in adipose tissue.
Metabolism (2014) 63:250–2. doi:
10.1016/j.metabol.2013.10.001
Lin, Z., Wang, Y., Zhang, B., & Jin, Z. (2018). Association
of type 2 diabetes susceptible genes GCKR, SLC30A8,
and FTO polymorphisms with gestational diabetes
mellitus risk: A meta-analysis. Endocrine, 62(1), 34–
35. Retrieved from https://doi.org/10.1007/s12020-
018-1651-z
Lizárraga, D., García-Gasca, A. (2021). The Placenta as a
Target of Epigenetic Alterations in Women with
Gestational Diabetes Mellitus and Potential
Implications for the Offspring. Epigenomes. 2021;
5(2):13. https://doi.org/10.3390/epigenomes5020013
Lowe, W.L. Jr., & Karban, J. (2014). Genetics, genomics
and metabolomics: new insights into maternal
metabolism during pregnancy. Diabet Med. 2014
Mar;31(3):254-62. doi: 10.1111/dme.12352.
Metzger, B.E., Lowe, L.P., Dyer, A.R., Trimble, E.R.,
Chaovarindr, U., et al. Hyperglycemia and adverse
pregnancy outcomes. N Engl J Med 2008; 358: 1991-
2002.
Newbern, D., & Freemark, M. (2011) Placental hormones
and the control of maternal metabolism and fetal
growth. Curr Opin Endocrinol Diabet Obes. 2011;
18:409–16.
Rosik, J., Szostak, B., Machaj, F., & Pawlik, A. (2020). The
role of genetics and epigenetics in the pathogenesis of
Genes Associated with Metabolism in Gestational Diabetes Mellitus: A New Categorization According to Risk Factors
301

gestational diabetes mellitus. Ann Hum Genet. 2020
Mar;84(2):114-124.
Wang, D., Zhu, W., Li, J., An, C., & Wang, Z. (2013).
Serum concentrations of fibroblast growth factors 19
and 21 in women with gestational diabetes mellitus:
association with insulin resistance, adiponectin, and
polycystic ovary syndrome history. PLoS One. 2013
Nov 19;8(11): e81190. doi:
10.1371/journal.pone.0081190.
Yang, F.F., Han, T.B., Du, W.Q., Zhao, F., Wang, Y., Feng,
Y.L., Yang, H.L., Wang, S.P., Wu, W.W., & Zhang, Y.W.
(2020). Association of fat mass and obesity associated
gene polymorphism with the risk of gestational
diabetes. Zhonghua Liu Xing Bing Xue Za Zhi. 2020
Jul 10;41(7):1097-1102. Chinese. doi:
10.3760/cma.j.cn112338-20200305-00263.
Zhang, C., Bao, W., Rong, Y., Yang, H., Bowers, K., Yeung,
E., & Kiely, M. (2013). Genetic variants and the risk of
gestational diabetes melli- tus: A systematic review.
Human Reproduction Update, 19(4), 376–390.
Retrieved from
https://doi.org/10.1093/humupd/dmt013
Zhang, Y., Sun, C.-M., Hu, X.-Q., & Zhao, Y. (2014).
Relationship between melatonin receptor 1B and
insulin receptor substrate 1 polymorphisms with
gestational diabetes mellitus: A systematic review and
meta-analysis. Scientific Reports, 4, 6113. Retrieved
from https://doi.org/10.1038/srep06113
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
302
