
Muscle Function Restoration by Bioelectrical Stimulation
Xiao Li
Imperial College London, London, SW7 2AZ, U.K.
Keywords: Muscle Rehabilitation, Functional Electrical Stimulation, Implantable FES.
Abstract: There are millions of people who are suffered from neurological diseases such as stroke and spinal cord
injury, and their nervous systems are damaged from rapid muscle denervation, caused by neurological
diseases, which results paralysis or muscle weakness. In order to solve these problems and meditate
inconvenience of patients, muscle refunction and rehabilitation are necessary to be introduced. Besides, as
human stepping into electrical age, bioelectrical stimulation is developed. This paper covers the effect of
bioelectrical stimulation on muscle refunction and rehabilitation, as well as different applications for
bioelectrical stimulation. In addition, different topologies for bioelectrical stimulation are also included.
1 INTRODUCTION
Nerve cells and muscle cells are significant to human
body and these two types of cells are deeply
correlated with each other. Nervous system is
combined by Central Nervous System (CNS) and
Peripheral Nervous System (PNS). Central Nervous
system includes brain and spinal cord, and Peripheral
Nervous System includes autonomic nervous system,
which is involuntary, and somatic nervous system,
which is voluntary. CNS receives the sensory signals
from PNS, and also sends motor signals to PNS to
trigger movements. Neuron has three main parts.
Dendrites receive chemical input from other neurons;
soma, the main body of neuron, integrates the
information received from dendrites; and Axon
conveys electrical signals out of the cell. Electrical
signals include the signal which triggers muscle
contraction (Malmivuo et al. 1995).
From the perspective of biology, Nerves always
work along with action potentials, and damaged
nerves are not able to perform action potentials
regularly by themselves. Thus, electrical stimulation
is introduced to depolarizes neuron cell membrane,
by creating a localized electric field, to reach a
critical threshold and generate action potential which
propagates in both directions away from where it
stimulated. Stimulation to motor nerves triggers
muscle contraction which can be used to rehabilitate
muscle functions. Luigi Galvani applied electrical
wires to leg muscles which cleaved from the frog
body in 1790 and the motion was observed. Michael
Faraday demonstrated that movement can be created
by using electrical currents to stimulate nerves in
1831 (Cambridge NA, 1997). Moreover, Electrical
stimulation for peroneal nerve in hemiplegia patients
attempted to correct foot drop during ambulation in
early clinical experiments (Liberson et al. 1961).
Thus, electrical stimulation has the great potential in
the field of rehabilitation recovery, including muscle
strength improvement, range of motion increment,
edema reducing, tissue healing, pain relieving, etc.
(Doucet et al. 2012).
In this paper, the topic is focused on how to use
bioelectrical stimulation to help people with mobility
dysfunction, such as patients with spinal cord
injuries, to reactivate muscle contraction and
partially restore their mobility. This review firstly
aims to introduce backgrounds for bioelectrical
stimulation, mainly functional electrical stimulation,
which pairing a functional task with electrical
stimulation, as well as implantable FES. Secondly,
this review explains how to stimulate tissues by
electrical input with examples of different topologies.
Moreover, prospects for the future of this technology
are also included in the conclusion aiming to provide
any constructive suggestions for future studies.
2 FUNCTIONAL ELECTRICAL
STIMULATION
Functional electrical stimulation (FES), which is
similar to neuromuscular electrical stimulation but
Li, X.
Muscle Function Restoration by Bioelectrical Stimulation.
DOI: 10.5220/0011207400003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 349-354
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
349
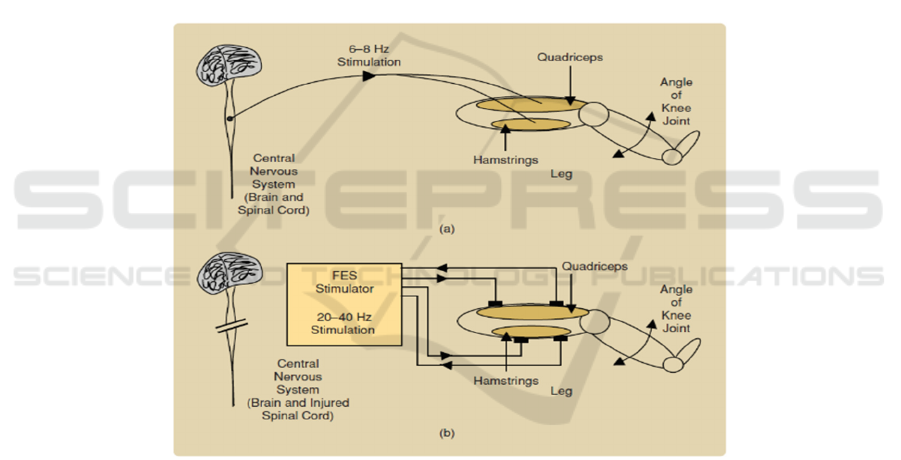
pairing the stimulation with a functional task.
Paralyzed muscle contractions, triggered by using
short electrical pulses, is programed to fit with
different tasks. For instance, multiple muscles,
flexor muscles and extensor muscles, connected
with the joint are stimulated with different
stimulation intensity delivered to achieve different
joint angles. Wrist extensors and finger flexors are
stimulated to contract the fingers around an object in
order to facilitate a grasping task. Flexion of the
shoulder and elbow extension is also triggered by
FES to produce a forward reaching motion. FES is
used most commonly for spinal cord injured (SCI)
individuals to improve their motor functions. For
example, FES can be used to reproduce the
activation pattern of lower extremity muscles to
produce human gait (Lynch et al. 2008).
Electrical pulses are delivered via electrodes.
Electrodes can be placed in four ways:
transcutaneous (on the skin surface), epimysial (on
the surface of the muscle), percutaneous (in the
muscle), or cuff (motor nerve surrounding) (Popovic
et al. 2000). Frequency and intensity, which is the
amount of charge input in muscle, of stimulation
decide the level of the contraction (Lynch et al.
2008).
The stimulus pulses for FES usually have both
cathodic phase and anodic phase. Cathodic phase
triggers the action potential along with harmful
electrochemical processes occurred at the interface
between electrode and tissue. Anodic phase
followed by cathodic phase reverses those damage
by neutralizing the charge accumulated during
cathodic phase, to avoid tissue damage, and the
charge of two phases are ideally equal. Figure 1
illustrated three types of stimulus waveforms.
Figure 1: Stimulus waveforms. (a) Monophasic. (b) Biphasic with active cathodic and active anodic phases. (c) Biphasic
with active cathodic phase and passive anodic phase (exponential decay) (Demosthenous, 2014).
People with spinal cord injury might not able to
complete fundamental behavior tasks such as
walking, standing, jumping, due to their insufficiency
of lower extremities strength. Thus, FES is
introduced to facilitated with finishing fundamental
tasks. Figure 2(b) shows how FES works to
compensate the nonfunction of muscle caused by
injury. For intact nervous system, each motor unit are
stimulated at a frequency of 6-8 Hz, and adjacent
units are sequentially stimulated, which overall
triggers muscles to produce tetanic contractions, and
finally triggers the change of knee angle. Previous
mechanism is called asynchronous recruitment. For
SCI individuals, motor units are stimulated by FES at
the same time, which what we called synchronous
recruitment, and different from the asynchronous
recruitment happens in complete nervous system.
Because FES has to stimulate the motor units
synchronously, a higher frequency is required which
ranges from 20 to 40 Hz to trigger tetanic
contractions.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
350
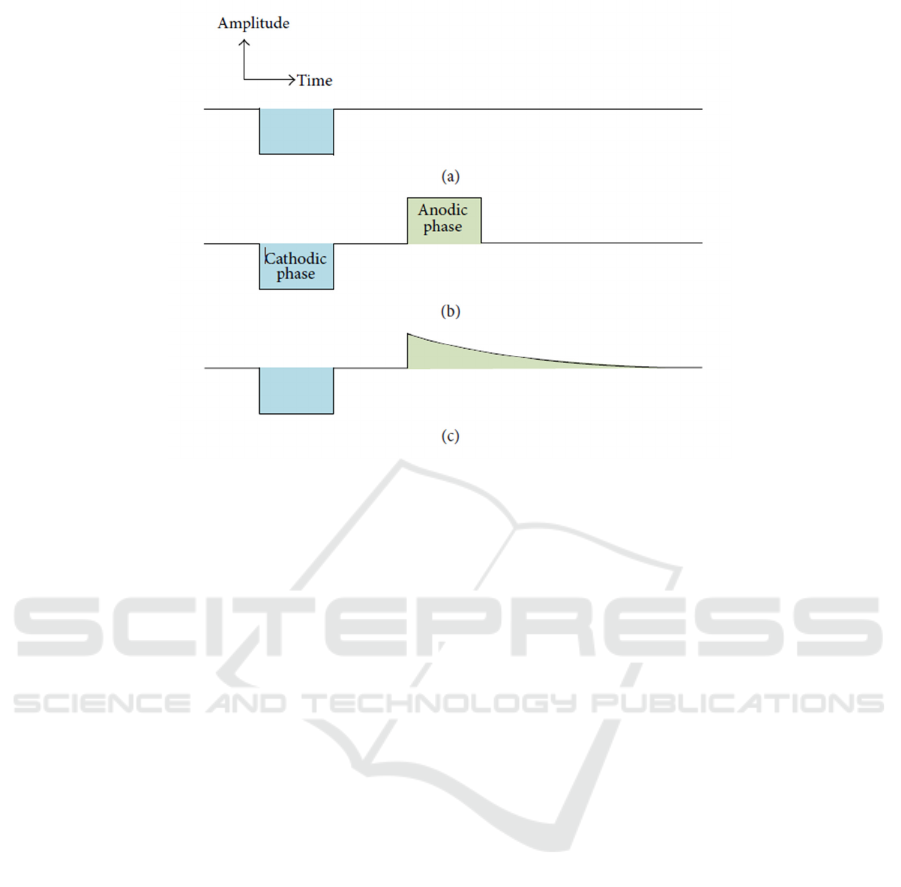
Figure 2: Different mechanisms for the production of tension in intact nerve system individuals and spinal cord injury
individuals.
A further application for FES is FES facilitated
muscle rehabilitation. As mentioned previously, SCI
individuals might not able to complete fundamental
behavior tasks. Thus, immobility will directly cause
insufficiency of physical activity which is always a
problem along with multiple syndromes such as
muscular atrophy, cardiovascular disease, type 2
diabetes, etc. To increase the level of physical
activities of SCI individual, FES training machine,
which is the combination of typical training machine
and FES system, is introduced. FES triggers
paralyzed muscles to produce tetanic contractions,
which is crucial for SCI individuals to complete the
training tasks. One representative product for FES
training machine is motor driven FES rowing
machine. SCI individuals are not able to complete
normal rowing machine training due to their
insufficiency of low extremities strength. Thus,
motor-driven FES machine is able to support them
to complete the rowing. This machine is constructed
by a chair with inclination control, control program,
leg supporter, motor system, and the most
importantly, the four-channel FES system (MEGA
XP, Cybermedic Corp.) which is surrounding the
legs to stimulate hamstring and quadriceps muscles.
As mentioned previously, FES synchronously
stimulates the motor units which leads the frequency
ranging from 20 to 40 Hz. The FES rowing machine
stimulates through surface electrodes to the
hamstring and quadriceps muscles with a frequency
of 30 Hz. An optical encoder which senses the seat
position and controls the input of stimulation is used
to construct a closed-loop and feedback control FES
system (Kim et al. 2014).
3 STIMULATION CIRCUIT
To achieve stimulation, at least two electrodes are
needed in the circuit to produce current flow.
Current stimulation mode and voltage stimulation
mode, which is shown in Figure 3, are two main
stimulation modes. voltage stimulation use voltage
as its output and current stimulation use current. The
output of voltage is constant, thus the generated
current delivered to the tissue, depends on the
inter-electrode impedance. A sequence of voltage
steps has to be introduced to control the charge
supply, which requires large number of capacitors
and not able to be on-chip. Thus, voltage stimulators
usually use with surface electrodes. The magnitude
of the current delivered to the tissue is controlled by
current stimulation and not dependents with
inter-electrode impedance, and current stimulation is
suitable with implanted electrodes.
Muscle Function Restoration by Bioelectrical Stimulation
351
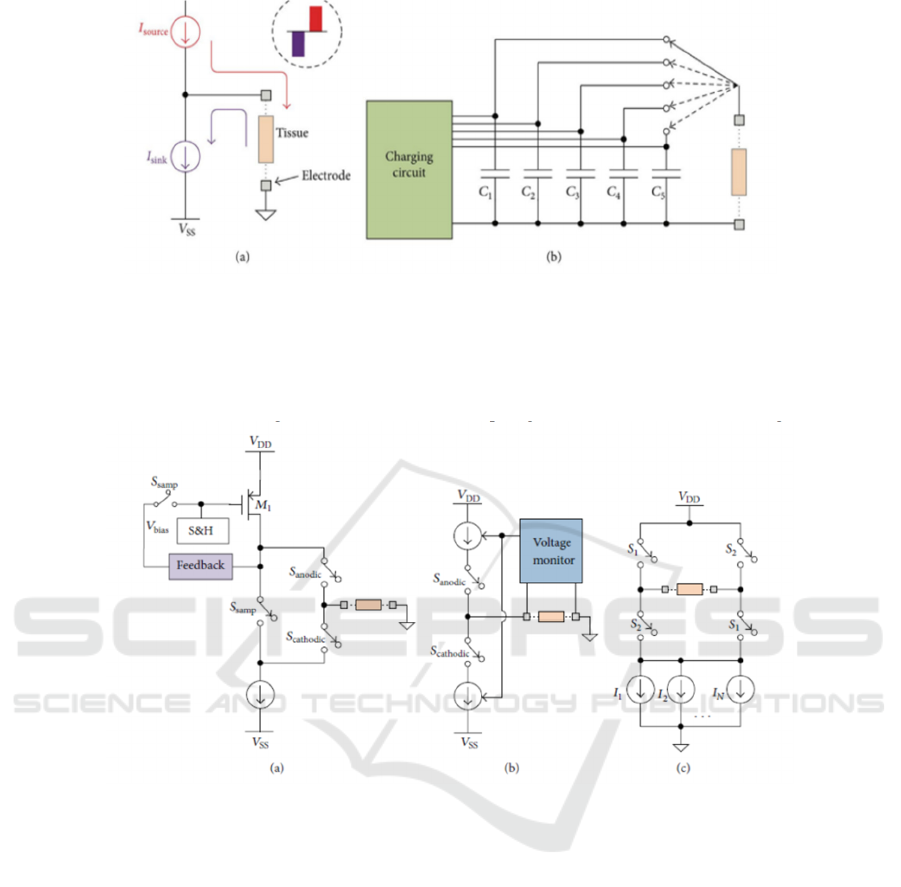
Figure 3: (a) current stimulation circuit. (b) voltage stimulation circuit (Suo et al. 2013).
As mentioned previously, biphasic stimulus
pulses are usually used in bioelectrical stimulation
because anodic phase followed by cathodic phase
neutralizing the accumulated charge and avoid tissue
damage. Thus, the total charge in cathodic phase and
anodic phase has to be balanced. To achieve charge
balancing, three classic biphasic current stimulators
topologies are introduced below in Figure 4.
Figure 4: Three main topologies for charge balancing. (a) Dynamic current balancer (Sit et al. 2007); (b) Active charge
balancer (Ortmanns et al. 2007); (c) H-bridge with multiple current sinks (Williams et al. 2013)
Dynamic current balancer includes two sampling
switches, and two more switches for cathodic phase
and anodic phase respectively. Two Ssamp close to
form a close circuit. Because of feedback, the
amplitude of M1 drain current is equals to the
current sink, and the bias voltage on M1 is sampled
and held. Then, two Ssamp open and only Scathodic
closes to form the current which stimulates the
tissue. Then Sanodic closes and Scathodic opens,
and the held bias voltage results the anodic current
has the same amplitude with cathodic current to
reach charge balancing. Active charge balancer
combines voltage monitor on electrodes and uses
two switches, Sanodic and Scathodic, to manipulate
the circuit to achieve charge balancing. only
Scathodic closes firstly and the current, get
measured by Voltage Monitor, reaches to the tissue
and go to VSS; then during anodic phase, Sanodic
closes and Scathodic opens, the monitored amount
of charges are coming out from VDD to the tissue
for the neutralization. “H-bridge” configuration uses
four switches in two groups to form cathodic current
and anodic current. S1 and S4 firstly close to form
cathodic current. Then, S1, S4 open and S2, S3 close
to form anodic current to neutralize the accumulated
charge.
Blocking capacitors are usually used in
stimulator circuits. When the circuit failed, blocking
capacitors avoid DC connection to supplies to
ensure safety. Secondly, they also ensure the
electrodes have no net charge. In addition, adding
blocking capacitors into stimulation circuit realize
active discharge function.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
352

4 IMPLANTABLE FUNCTIONAL
ELECTRICAL STIMULATION
Implantable functional electrical stimulation is
becoming a more popular direction of research due
to its high mobility. Moreover, because the FES
devices are implanted into human body, not
percutaneous, the impedance is lower which leads to
a lower power consumption directly. A typical
example for implantable FES is implantable device
for hand grasping which is shown in Figure 5. The
hand grasping device uses an external control unit,
which provides energy source and particular
program, that connects to an implantable stimulator.
The implantable stimulator connects with group of
electrodes used for neuromuscular stimulation to
trigger muscle contraction and finish tasks. The
stimulator also connects with electromyography
(EMG) recording electrodes to measure the
electrical activities of muscle in responding to nerve
stimulation. The connection between external
control unit and implant stimulator can be wireless.
The data and power transfer are via inductively
coupled power transfer. The efficiency of
Inductively coupled power transfer in its working
range is really high, especially for high power
delivery, which has a total efficiency to be greater
than 95%. Moreover, the range of its output power
levels is from milliwatts to tens of watts which
indicates that it can be used in a huge variety of
implantable devices (Schormans et al. 2018).
Figure 5: Diagram of implantable hand grasping FES
device (Hart et al. 1998).
5 CONCLUSIONS
In this paper, the fundamental principles and
applications about bioelectrical stimulation is
introduced. The development of bioelectrical
stimulation is boosted in these two decades.
However, the space of bioelectrical stimulation
development and application is big. For instance,
one problem for stimulator is power consumption.
Bioelectrical stimulation can be divided into
implanted and not implanted. The power
consumption for implanted stimulator is always a
breakthrough point because no direct power
transmission cable is connected. Thus, to achieve
wireless power transfer. Inductively coupled power
transfer (ICPT) is introduced as the most capable
way of power transfer for implanted stimulator
(Schormans et al. 2018). With the correct way of
power transfer, the efficiency of ICPT is the biggest
research field currently. The realization of wireless
fast charging safely is a meaningful goal. The
combination of bioelectrical stimulation with
close-loop control has brought bioelectrical
stimulation into the cross field of biotechnology,
electronic engineering, and programing. The
monitor can track different behaviors and muscle
movements and upload the feedback into computer,
and software engineers can analysis the big data and
development different programs which is able to
simulate the muscle movement. The simulation
program can be updated and transmitted back into
the stimulator to realize iteration.
In the future, big data analysis is able to
prognosis the next muscle movement and stimulate
with less lag to reduce delay of actions. Moreover, a
new innovation for bioelectrical stimulation is the
application of brain-computer interface (BCI) and
nanorobots. Trillions of nanorobots which controlled
and monitored by brain-computer interface is
launched into different part of human body. For
instance, when a SCI individual is willing to move
legs, electrical signal from brain is recorded and
decoded into binary language which controls
nanorobots, located on paralyzed leg muscle, to
stimulate the neuron and trigger muscle contraction,
and overall realize the leg movement. The
innovation sounds surrealistic, but with the rapid
development of scientific and technological level,
BCI and nanorobotic induced bioelectrical
stimulation is feasible and is able to be popularized
in several decades.
ACKNOWLEDGEMENTS
Grateful acknowledgement is made to professor
Andreas Demosthenous who gave me two weeks of
guidance and inspiration during the summer project:
Muscle Function Restoration by Bioelectrical Stimulation
353

Bioelectronics for Applications in Implantable and
Wearable Medical Devices.
REFERENCES
Cambridge NA, Electrical apparatus used in medicine
before 1900. Proc R Soc Med. 1997;70(9):635-41.
Demosthenous, A, Advances in Microelectronics for
Implantable Medical Devices. Advances in
Electronics, 2014, 1–21.
https://doi.org/10.1155/2014/981295.
Doucet, B. M., Lam, A., & Griffin, L., Neuromuscular
electrical stimulation for skeletal muscle function. The
Yale journal of biology and medicine, 2012: 85(2),
201–215.
Hart R. L., Kilgore K. L. and Peckham P. H., A
comparison between control methods for implanted
FES hand-grasp systems, in IEEE Transactions on
Rehabilitation Engineering, vol. 6, no. 2, pp. 208-218,
June 1998, doi: 10.1109/86.681187.
Kim, D. I., Park, D. S., Lee, B. S., & Jeon, J. Y., A
six-week motor-driven functional electronic
stimulation rowing program improves muscle strength
and body composition in people with spinal cord
injury: a pilot study. Spinal Cord, 52(8), 2014, 621–
624. https://doi.org/10.1038/sc.2014.76.
Liberson W, Holmquest H, Scot D, Dow M, Functional
electrotherapy: stimulation of the peroneal nerve
synchronized with the swing phase of the gait of
hemiplegic patients. Arch Phys Med Rehabil. 1961;
42:101.
Lynch C. L. and Popovic M. R., Functional Electrical
Stimulation. IEEE Control Systems Magazine, vol. 28,
no. 2, 2008, pp. 40-50. doi:
10.1109/MCS.2007.914689.
Malmivuo, J., & Plonsey, R., Bioelectromagnetism:
Principles and Applications of Bioelectric and
Biomagnetic Fields (1st ed.). Oxford University Press,
1995.
Ortmanns M., Rocke A., Gehrke M., and Tiedtke H. J., A
232- channel epiretinal stimulator ASIC, IEEE Journal
of Solid-State Circuits, vol. 42, no. 12, pp. 2946–2959,
2007.
Popovic D. B. and Sinkjaer T., Control of Movement for
the Physically Disabled. London, U.K.: Springer
Company, Inc., 2000.
Schormans, Matthew, et al. Practical Inductive Link
Design for Biomedical Wireless Power Transfer: A
Tutorial. IEEE Transactions on Biomedical Circuits
and Systems, vol. 12, no. 5, 2018, pp. 1112–30.
Crossref, doi:10.1109/tbcas.2018.2846020.
Sit J. J. and Sarpeshkar R., A low-power
blocking-capacitorfree charge-balanced
electrode-stimulator chip with lesst than 6 nA DC
error for 1-mA: full-scale stimulation, IEEE
Transactions on Biomedical Circuits and Systems, vol.
1, no. 3, pp. 172–183, 2007.
Suo Y., Zhang J., R. Etienne-Cummings, T. D. Tran, and
S. Chin, Energy-efficient two-stage compressed
sensing method for implantable neural recordings, in
Proceedings of the IEEE Biomedical Circuits and
Systems Conference (BiOCAS ’13), pp. 150–153,
Rotterdam, TheNetherlands, October-November 2013.
Williams I. and Constandinou T. G., An energy-efficient,
dynamic voltage scaling neural stimulator for a
proprioceptive prosthesis, IEEE Transactions on
Biomedical Circuits and Systems, vol. 7, no. 2, pp.
129–139, 2013.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
354
