
Evaluation of 20 Citrus Varieties Resistance to Pseudofabraea
citricarpa
Quan Chen
1,2
, Wenjing Zhang
1,2
, Jiequn Ren
1,2
, Pingwei Xiang
1,2
and Jinhui He
3
1
Chongqing Three Gorges Academy of Agricultural Sciences, Wanzhou, 400401, Chongqing, China
2
College of Biology and Food Engineering, Chongqing Three Gorges University, Wanzhou, 404020, Chongqing, China
3
Plant Protection and Fruit Tree Technology Popularization Station in Wanzhou District of Chongqing, Wanzhou, 404155,
Chongqing, China
Keywords: Citrus Target Spot, Pseudofabraea citricarpa, Resistance Evaluation.
Abstract: Citrus target spot is one of the most destructive diseases on leaves, shoots and fruits of some citrus in China.
In this study, to evaluate the resistance against the disease, the separated leaf inoculation method was used
to inoculate Pseudofabraea citricarpa pathogen on abatial of citrus leaves under 10℃. Resistance of 20
citrus varieties were evaluated. The results indicated that among the 20 citrus varieties, 18 varieties were
classified into highly resistant varieties according to the diameter spots. Orah 091 was moderate resistant
and CRIC32-01 was resistant to Ps. citricarpa. The study could be used for the further study of citrus
resistance genes to Ps. Citricarpa and has given suggestions for structure in citrus production areas.
1 INTRODUCTION
Citrus target spot is a newly emerging citrus disease
caused by Pseudofabraea citricarpa (Yang, Fang,
Yu, Bi, Zhou, 2019); (Chen, 2016). And a newly
emerging leaf-spotting disease of citrus reported in
the year of 2004 which was occurred in late winter
and early spring in Chenggu County, Shanxi
Province of China (Zhu, Wang, Huang, Zhang, Li,
2012). This fungal pathogen could infect both
Satsuma mandarin (Citrus unshiu) and kumquat
(Fortunella margarita) in orchards (Zhu, Wang,
Huang, Zhang, Li, 2012). This disease caused
considerable economic losses in Wanzhou,
Chongqing City, Yichang City, Hubei Province and
Jishou City, Hunan Province, China in 2021 (Xiao,
Zeng, Wang, Cheng, Li, 2020). These morbidity
tendency demonstrated a trend of accelerating
propagation from north to south of China (Zhan,
2021). These incidence trend was the same as our
previous prediction of suitable area and risk analysis
for citrus target spot (Xu, Chen, 2020). Citrus target
spot occurs during late winter and early spring and
causes severe leaf spotting, defoliation or even
fruit-dropping and tree- dead. Once the disease
becomes epidemic, existing technology including
chemical agents, some agricultural control and
physical control are difficult to control effectively
(Xiao, Zeng, Wang, Cheng, Li, 2020); (Zhu, 2012).
Breeding resistant varieties is the most fundamental
means of this disease control.
At present, the research on citrus target spot is
still in the initial stage, mainly focusing on the
identification of pathogen, identification of
pathogenic factors and prediction of suitable areas
(Yang, Fang, Yu, Bi, Zhou, 2019). The SCAR
molecular detection provides a fast and easy method
for early identification of this disease (Yang, Hu,
2018). And the research integrated transcriptomic
and secretomic approaches revealed critical
pathogenicity factors in Ps. citricarpa inciting citrus
target spot (Yang, Fang, Yu, Bi, Zhou, 2019).
According to the latest research, citrus target spot is
at a high risk level in China, and the high and
middle suitable areas are mainly concentrated in the
citrus dominant area in the upper and middle reaches
of Yangtze River (Xu, Chen, 2020). However, there
is not a perfect and viable standardized system for
the identification of resistance to citrus target spot,
which leads to the slow process of screening
resistant germplasm and breeding resistant varieties.
Therefore, we took the average diameter of the
speckle and the incidence as the evaluation standard
for resistance (Chen, Liu, 2009); (Hu, Liu, 2015);
(Liu, Hu, 2013); (Zhang, Ding, 2013); (Ling, Huang,
2011); (Li, Zheng, 2009) and inoculated different
Chen, Q., Zhang, W., Ren, J., Xiang, P. and He, J.
Evaluation of 20 Citrus Varieties Resistance to Pseudofabraea Citricarpa.
DOI: 10.5220/0011212900003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 397-401
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
397

citrus varieties for identifying resistance to this
disease.
2 METHODS AND MATERIALS
Fungal Pathogens. Ps. citricarpa strains
Pc-WZBY1was isolated from Eureka lemon leaves
in Wanzhou District, Chongqing City. Direct tissue
isolation of causal agents was performed reported by
Zhu et al. (Zhu, Wang, Huang, Zhang, Li, 2012) The
materials were surface-sterilized with 75% ethanol
for 30 s, 1% NaOCl for 1 min, and then rinsed with
sterile distilled water for 5 times. Small sections (3 ×
5 mm) from the margins of diseased and healthy
tissues were placed onto potato dextrose agar (PDA)
in petri dishes. Small drops of suspension were
placed on a glass slide and examined under a
dissecting microscope at ×20 magnification. Drops
that contained only a single spore were transferred to
fresh PDA plates. The plates were incubated at 20°C
until the mycelium covered approximately
three-quarters of the plates (about 15 to 20 days).
Pure single-spore cultures grew on PDA were then
transferred onto fresh PDA and stored at 4°C for
further study.
Citrus Varieties. C.grandis (Shatianyou,
Hongbaoshiyou, Taiguoqingyou, Dianjiangwanyou,
DianjiangBaiyou, Liangpingyou, Sanhongyou),
C.sinensis (Tarocco Blood Orange NO.8, Tarocco
Blood Orange NO.9, Newhall Navel Orange),
C.reticulata (Shiranui, Aiyuan NO.38, Daya
mandarin, Tango, Orah 091, Gold Nugget) and
C.tangerine (Hongjv, CRJC32-01), C.junos(Tanaka),
C.aurantium(Poncirus trifoliata) were collected from
citrus germplasm resource nursery of Chongqing
Three Gorges Academy of Agricultural Sciences.
Resistance Identification Experiment. The
inoculation experiment was conducted in laboratory
of Chongqing Three Gorges Academy of Agricultural
Sciences in 2020-2021, following a modified method
of placing the mycelial plugs on the excised leaves
reported by Lin et al. (Lin, Huang, 2011).
15 separated leaves inoculated two sits on each
leaf, for 30 spots in total, were inoculated with a
typical strain WZBY1 in every variety. Leaves were
washed, air-dried, and surface-disinfested with 75%
ethanol using cotton swabs, and then put it into a
sterile tray covered with sterile gauze soaked with
distilled water. Subsequently, pricking ten times (but
not pierced) on the lower surface with an insect
needle. Two sclertiums (5 mm diameter) were placed
on the abaxial of each wounded leaf. For the
non-inoculated controls, leaves were put with the
same size of PDA only. All treatments were cultured
separately under 10 °C in a moisture box and
investigating the average diameter of the speckle and
incidence rate in in 28 days after inoculation. The
resistance was evaluated with the method of average
diameter of the speckle (Chen, Liu, 2009); (Hu, Liu,
2015); (Liu, Hu, 2013). The leaves were recorded as
infected if inoculated sits displaying disease
symptoms (Zhang, Ding, 2013); (Lin, Huang, 2011).
Assessment of the resistance grades of citrus
varieties to Ps. Citricarpa was according to Li et al.
(Li, Zheng, 2009). Incidence was obtained using the
formula: incidence (%) = (infected leaves/inoculated
leaves) × 100%.
All experiments were performed in triplicate. The
entire experiment was conducted triplicate.
Statistical Analysis. The database was analyzed by
IBM SPSS 16.0 (New York) and and Microsoft
Excel. The data are means of 30 replicates in
triplicate.
3 TEST RESULTS
3.1 Incidence Rate of Different Citrus
Cultivars
There were various degrees of differences on the
pathogenicity to test-cultivars, and even got
significant difference(P<0.05) among some
varieties. There was 8 varieties of incidence rate
were under 20%, 7 varieties among 21-50%, 2
varieties among 51-80%, 3 varieties displayed 100%
incidence. Newhall Navel Orange (C.sinensis), Orah
091 (C.reticulata) and CRIC32-01 (C.tangerine)
were got 100% incidence. The disease didn’t infect
Shatianyou, Hongbaoshiyou, Tarocco Blood Orange
No.8, Tanaka, Poncirus trifoliata. Incidence rate of
this 5 citrus cultivars were all 0%. Sanhongyou,
Daya mandarin and Taiguoqingyou displayed
3.33%, 6.67% and 20% for incidence. Incidence rate
of Newhall Navel Orange, CRIC32-01 and Orah 091
reached 100%, and Dianjiangwanyou,
DianjiangBaiyou and Liangpingyou were showed
40%-50%. The other varieties of C. grandis were
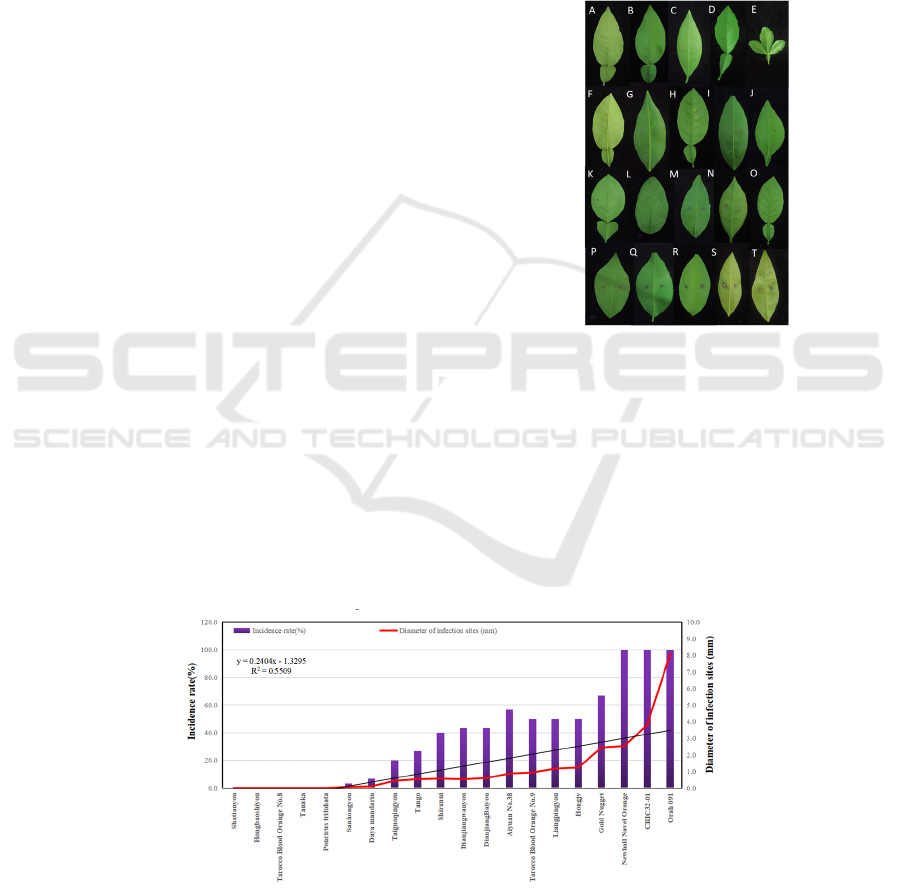
showed the incidence under 20% (Fig. 1, Fig. 2).
3.2 Diameter of Infection Sits of
Different Citrus Cultivars
Shatianyou, Hongbaoshiyou, Sanhongyou, Tarocco
Blood Orange No.8, Tanaka and Poncirus trifoliata
were 0mm in diameter of infection sits, and
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
398
displayed immune phenotype. Taiguoqingyou, and
Daya mandarin displayed 0.1m for average lesion
diameter, respectively. 6 varieties ranged from
0.2mm to 1.0mm for average diameters of lesions,
and 5 varieties were 1.1mm-5.0mm (Fig. 3).
In the 20 varieties, 18 (90 percent of all test
varieties) ranged from 0 to 3 mm in spots diameter
which can be classified into highly resistant varieties.
Orah 091 got 8.1mm for average lesion diameter of
which the maximal diameter could reach 14mm
which was defined as moderate resistant. CRIC32-01
gotting 3.8mm for average lesion diameter and 100%
for incidence which showed resistant to Ps.
Citricarpa. There was a particular example Newhall
Navel Orange, of which the average lesion diameter
got 1.8 mm but incidence was 100%, which was
identified with highly resistant variety according to
the diameter spots.
In C.grandis, except Liangpingyou, the other 6
test varieties were all classified to highly resistant. In
C.reticulata, Orha 091 was the most susceptible
variety(100% for the incidence, 8.1mm for the
diameter spots). Daya mandarin was classified to
highly resistant to Ps. Citricarpa according to the
diameter spots. In C.tangerine, incidence and
epidemic degree of CRIC32-01 was more severe,
gotting second diameter(3.8mm) in the test varieties
and reaching 100% for incidence. While the Hongjv
displayed significantly lower incidence (50%) of Ps.
Citricarpa (Fig. 2, Fig. 3), which was classified into
highly resistant group.
The results also suggested that the incidence rate
of varieties correlated linearly with lesion
diameter(R
2
=0.55) (Fig. 2). In all test objects, the
high incidence, such as CRIC32-01 and Orah 091,
existed a tendency to strong virulent and a high risk
to infected by Ps. Citricarpa in citrus production
areas in our country. In addition, for reducing the risk
of disease and economic losses, late-maturing,
suitable and strong resistant varieties should be
planted, including increasing Tarocco Blood Orange
No.8, Tango, Shiranui in upper and middle reaches of
Yangtze River. In western Hubei and western Hunan,
according to the current structure of citrus industry
and climatic conditions, the susceptible citrus, such
as Orah 091 should be appropriately reduced.
Depending on the natural advantages, Aiyuan No.38,
CRIC32-01 and Taiguoqingyou should be with due
consideration.
Figure 1: Symptoms of some citrus varieties after
inoculation.
A, Shatianyou; B, Hongbaoshiyou; C, Tarocco
Blood Orange No.8; D, Tanaka; E, Poncirus
trifoliata; F, Sanhongyou; G, Daya mandarin; H,
Taiguoqingyou; I, Tango; J,Shiranui;
K,Dianjiangwanyou; L, DianjiangBaiyou; M,
Aiyuan No.38; N, Tarocco Blood Orange No.9; O,
Liangpingyou; P, Hongjv; Q, Gold Nugget; R,
Newhall Navel Orange; S,CRIC32-01; T, Orah 091
Note: The data are means of 30 replicates. The experiment was conducted triplicate.
Figure 2: Correlational analyses of the incidence and diameter of infection sits different citrus varieties.
Evaluation of 20 Citrus Varieties Resistance to Pseudofabraea Citricarpa
399
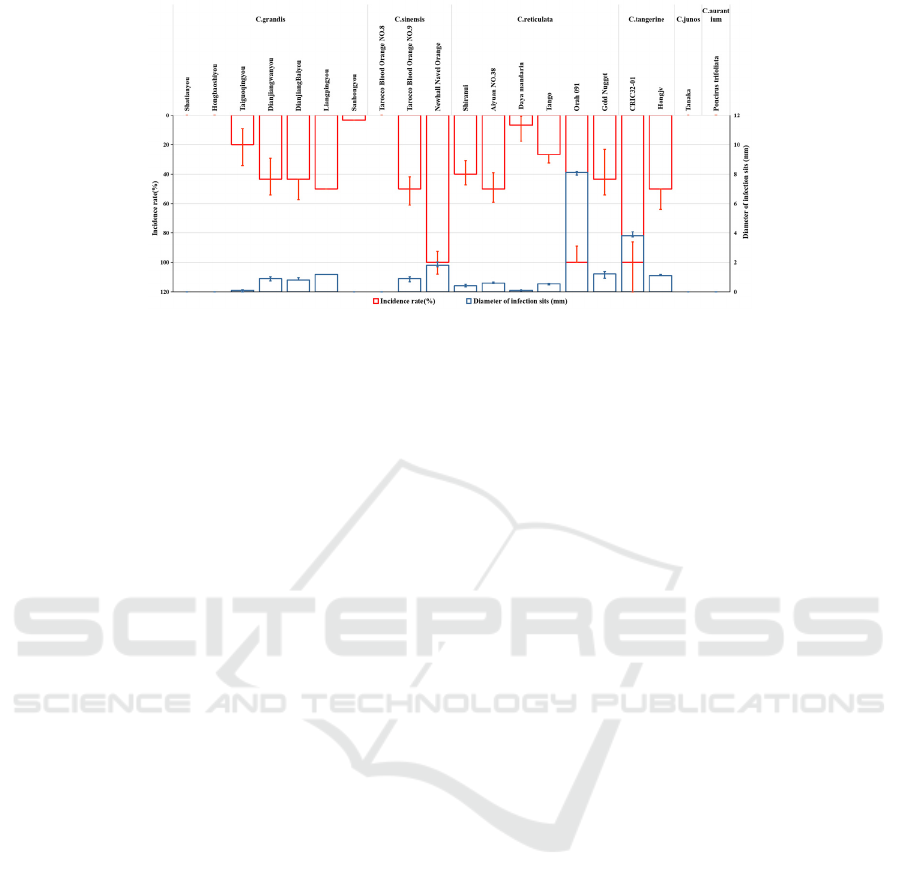
Note: Columns represented means of 30 replicates, and bars represent standard deviation. The experiment was conducted
triplicate.
Figure 3: The incidence of different citrus varieties after inoculation.
4 DISCUSSIONS
Citrus target spot has become an important disease
infecting such as Satsuma mandarin, kumquat as
well as Eureka lemon et al. in some citrus-producing
regions reported in Shanxi Province (Zhu, Wang,
Huang, Zhang, Li, 2012) Chongqing City (Zhan,
2021), Hubei Province and Hunan Province (Xiao,
Zeng, Wang, Cheng, Li, 2020), China, which is
leading to substantial economic losses to citrus
production. Unlike the most diseases, the disease
infected by Ps. Citricarpa, prevails only in late
winter and early spring (Zhu, Wang, Huang, Zhang,
Li, 2012)
.
It causes defoliation, twig dieback,
fruit-dropping, and dramatically market value
reduction of infected fruits, especially on susceptible
citrus varieties. Furthermore, a high proportion of
trees become diseased or dead, and some orchards
have been destroyed.
Gene is the primary cause of resistance.
According to the characteristic of conserved domain
of resistance genes, sequence amplification of
conserved domain is a common method for
identification and discovery of resistance genes
(Fenillet, 1997), which has been applied to field
pepper (Zhang, Chen, 2008), soybean (Garzon,
2013), wheat (Xi, Wang, 2021) and other plants. The
key to study the resistance differences among citrus
germplasm, the resistance mechanism and even the
interaction between citrus and Ps. citricarpa is to
study the resistance genes of citrus. Study the
resistance genes was the basis of understanding the
resistance mechanism which needed to further
research.
5 CONCLUSIONS
Based on the results and discussions presented
above, the conclusions are obtained as below:
(1) Through the resistance evaluation of
dominant citrus varieties with the established
method, 18 varieties such as Shatianyou,
Hongbaoshiyou, Tarocco Blood Orange No.8,
Tanaka, Poncirus trifoliata, Sanhongyou, Daya
mandarin, Taiguoqingyou, Tango, Shiranui,
Dianjiangwanyou, DianjiangBaiyou, Aiyuan No.38,
Tarocco Blood Orange No.9, Liangpingyou, Hongjv,
Gold Nugget and Newhall Navel Orange were
classified into highly resistant varieties according to
the diameter spots. Only Orah 091 was defined as
moderate resistant. And CRIC32-01 was resistant
cultivars.
(2) According the results in this study, we
suggest increasing Tarocco Blood Orange No.8,
Tango in upper and middle reaches of Yangtze
River. In western Hubei and western Hunan, Orah
091 should be appropriately reduced. Aiyuan No.38,
CRIC32-01 and Taiguoqingyou should be with due
consideration.
ACKNOWLEDGMENTS
This research was funded by Science and
Technology Research Program of Chongqing
Municipal Education Commission (Grant
No.KJ202101254125241) and Chongqing Wanzhou
Science and Technology Program(wzstc-20210211).
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
400

REFERENCES
Chen Chen, Gerard J, M Verkley, Sun Guangyu, Johannes
Z. Groenewald, Pedro W. Crous. (2016) Redefining
common endophytes and plant pathogens in
Neofabraea, Pezicula, and related genera. Fungal
Biology, 120(11):1291-1322.
Chen Zhiyi, Liu Yongfeng, Liu Fengquan, Luo Chuping,
Nie Yafeng. (2009) Evaluation of rice varieties
resistant to bacterial leaf streak in Jiangsu.Journal of
Plant Protection, 36(04):315-318.
Fenillet C, Schachermayr G, Keller B. (1997) Molecular
cloning of a new receptor-like kinase gene encoded at
the Lr10 disease resistance locus of wheat. The Plant
Journal, 11(1):45-52.
Garzon L N. , Oliveros OA., Rosen B, Ligarreto G A.,
Cook D R., Blair MW. (2013) Isolation and
characterization of nucleotide-binding site resistance
gene homologues in common bean (Phaseolus
vulgaris). Phytopathology, 103(02):156- 168.
Hu Junhua, Liu Rongping, Wang Xuelian, Zhou Na, Hong
Qibin, Yao Tingshan, Li Taisheng, JIANG Dong, Cao
Li, Li Hongjun. (2015) Evaluation of Citrus
germplasm resistance to Alternaria alternata. Journal
of Fruit Science, 32(4): 672-680
Liu Rong-ping, Hu Jun-hua, Yao Tin-shan, Wang
Xue-lian, Zuo Pei-pei, Wang Yanjie, Li Hong-jun.
(2013) A rapid laboratory evaluation method of citrus
brown spot caused by Alternaria alternate. Journal of
Fruit Science, 30(5): 889-892
Lin Yueli, Huang Lili, Suolang Lamu, Gao Xiaoning,
Chen Yinchao, Kang Zhensheng. (2011) A rapid
laboratory evaluation system for apple ring rot.
Journal of Plant Protection, 38(01):37-41.
Li Wenyang, Zheng Chunyao, Li Chaoping, Cai Zhiying,
Lin Chunhua, Huang Guixiu. (2009) Resistance
identification of main rubber cultivars and some
rubber germplasm in china to colletotri⁃chum
acutatum in laboratory. Tropical Agricultural
Engineering, 33 (5): 31-36.
Xiao Xiaoe, Zeng Yating, Wang Wen, Cheng Lan, Li
Hongye. (2020) First Report and New Hosts of
Pseudofabraea citricarpa Causing Citrus Target Spot
in China, Plant Health Progress, doi. org/10.
1094/PHP-07-20-0056-RS.
Xu Yonghong, Chen Li, Tang Song, Ding Dekuan, Yang
Yuheng. (2020) Prediction of Suitable Area and Risk
Analysis for Citrus Target Spot, Scientia Agricultura
Sinica, 53(21):4430-4439.
Xi Ling, Wang Yuqi, Yang Xiu, Zhu Wei, Chen Guoyue,
Wang Yi, Qin Peng, Zhou Yonghong, Kang Houyang.
(2021) Evaluation of Resistance to Stripe Rust and
Molecular Detection of Resistance Gene(s) in 243
Common Wheat Landraces from the Yunnan
Province. Scientia Agricultura Sinica, 54(4): 684-695.
Yang Yuheng, Fang Anfei, Yu Yang, Bi Chaowei, Zhou
Changyong. (2019) Integrated transcriptomic and
secretomic approaches reveal critical pathogenicity
factors in Pseudofabraea citricarpa inciting citrus
target spot. Microbial Biotechnology, 12(6):1260–
1273.
Yang Yuheng, Hu Junhua, Chen Fajing, Ding Dekuan,
Zhou Changyong. (2018) Development of a SCAR
Marker-Based Diagnostic Method for the Detection of
the Citrus Target Spot Pathogen Pseudofabraea
citricarpa. BioMed Research International, 7128903.
Zhu L, Wang Xinghong, Huang Feng, Zhang Jinze, Li
Hongye. (2012) A Destructive New Disease of Citrus
in China Caused by Cryptosporiopsis citricarpa sp.
nov. Plant Disease. 96:804-812.
Zhan Shuang, Wu Wang, Hu Junhua, Wu Yuzhu ,Qiao
Xinhua, Chen Li, Cheng Lan, Zhou Yan. (2021)
Pathogen identification and screening of control agent
of suspected citrus target spot in Wanzhou,
Chongqing. Fruit trees in southern China, 50(1): 1-7.
Zhu Li. (2012) Identification of Five Pathogens Causing
Citrus Disease in china. Hangzhou: Zhejiang
University.
Zhang Changwei, Ding Guoxiang, Ni Xianlin, Liu
Tianpeng, Chen Guoming, Zhao Ganlin. (2013)
Resistance identification of the liquor-feedstock
sorghum varieties, hybrids and parents to sorghum
head smut. Journal of Plant Protection, 40(03):
219-224.
Zhang Liying, Chen Rugang, Zhang Junhong, Ouyang Bo,
Xiao Jinghua, Li Hanxia, Ye Zhibiao. (2008) Cloning
and Analysis of Resistance Gene Analogs from Pepper
(Capsicum annuum L.). Scientia Agricultura Sinica,
41(1):169-175.
Evaluation of 20 Citrus Varieties Resistance to Pseudofabraea Citricarpa
401
