
Effective Prediction of Neurofeedback based on Functional
Connection Characteristics of Brain Network in Insomnia
Kai Li
1
, Zhi Zou
2
, Huan Zhang
3
, Linyuan Wang
1
, Ying Zeng
1
, Fei Qi
2
and Chi Zhang
1,*
1
Henan Key Laboratory of Imaging and Intelligent Processing, PLA Strategic Support Force Information Engineering
University, Zhengzhou, Henan, China
2
Henan Provincial People’s Hospital, Zhengzhou, Henan, China
3
Center for Healthcare Data Science, Zhejiang Lab Zhongtai Street, Yuhang District, Hangzhou, 311100, Zhejiang, China
Keywords: Insomnia, Neurofeedback, Brain Network Connection.
Abstract: Real-time functional magnetic resonance imaging neurofeedback (rt-fMRI-nf) is a new means of emotion
regulation in insomnia, however, due to personal physiological and psychological differences, the effect of
neurofeedback training on different patients is significantly different. Using brain imaging data to predict the
curative effect is of great significance to improve the individual adaptability of clinical application of
neurofeedback training, reduce the treatment cost and reduce the burden of patients. In this article, we raise a
neurofeedback training effectiveness prediction method based on brain network functional connection. In this
method, network connection matrices of the default mode network (DMN), salience network (SAN),
executive control network (ECN), basal ganglia (BG), sensorimotor (SM) et.al. in insomnia are used as
features to construct the prediction model, so as to predict the training effect of patients' neurofeedback by
using machine learning method. The experimental results through the cross validation of CatBoost model
with leave one method show that, the prediction accuracy of whether an insomnia patient can benefit from
emotion regulation method produced by rt-fMRI-nf is 75%. This method can initially provide a reference
basis for insomnia patients to choose treatment methods.
1 INTRODUCTION
The drug treatment effect of patients with mental
diseases such as insomnia, depressive disorder and
anxiety shows that more than 50% of patients have
not been relieved after treatment (Li 2007). In recent
years, rt-fMRI-nf has become a conventional method
for the treatment of mental diseases. Due to the large
differences in individual treatment success rates,
some participants did not learn to control their brain
responses, resulting in "ineffective neurofeedback".
Ineffective regulation reduces the overall efficiency
of neurofeedback training and hinders its
transformation into clinical interventions (Haugg
2020). In neurofeedback studies, these participants
are often referred to as "non-responders", accounting
for 30% to 50% of the total population in study
(Alkoby 2017). For example, in 2021, Direito
(
Ramos 2019
) et al. explored whether a personalized
fMRI neurofeedback framework will have a positive
impact on the success of neurofeedback, the
adjustment threshold is defined for every subject
according to the maximum change of blood oxygen
level dependency (BOLD) area during positioning
operation in visual motion, it is found that 40% of the
subjects can successfully adjust the activation of
visual motor area, while 60% of the subjects do not.
Therefore, exploring the prediction model of the
effectiveness of neurofeedback therapy is of great
significance to guide individual clinical treatment
decisions.
In recent years, brain imaging indexes, combined
with traditional machine learning approach such as
support vector machine and random forest, have been
widely used to predict the prognosis of diseases. In
2017, Kesler et al. (Kesler 2017) used rt-fMRI data
and random forests to predict the long-term cognitive
ability of breast cancer patients after drug therapy. It
was found that the prediction accuracy of the training
model could reach 100% in the network and attention
network. In 2019, Zhutovsky et al. (Zhutovsky 2019)
used the functional connectivity of resting state brain
network components and Gaussian process classifier
to predict the treatment response of psychotherapy to
patients with post-traumatic stress disorder. It was
found that the network centered on auxiliary motor
Li, K., Zou, Z., Zhang, H., Wang, L., Zeng, Y., Qi, F. and Zhang, C.
Effective Prediction of Neurofeedback based on Functional Connection Characteristics of Brain Network in Insomnia.
DOI: 10.5220/0011235400003438
In Proceedings of the 1st International Conference on Health Big Data and Intelligent Healthcare (ICHIH 2022), pages 151-156
ISBN: 978-989-758-596-8
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
151

area before treatment contributed the most to the
classification of non-responders and responders.
Using the characteristics of resting state brain activity
to establish machine learning prediction model plays
an important role in improving the efficiency of
treatment and reducing the cost of treatment. It can be
seen that the resting state brain activity characteristics
of functional magnetic resonance imaging can
effectively predict disease treatment.
Recent studies have shown that the DMN, ECN,
SAN and other connection modes of resting state are
abnormal in insomnia, depressive disorder and other
diseases (Ma 2018, Albert 2018). These networks are
closely related to emotion processing, executive
function and attention, and the regulation between
brain networks affects the behavior of patients. For
insomnia patients, not only the weak connection
between the SAN responsible for discovering the
surrounding sensory and emotional stimuli and the
DMN related to self-reference and meditation may
lead to the over processing of negative information,
but also the enhanced connection between the ECN
responsible for attention control and advanced
cognitive control tasks and the DMN may be related
to the large cognitive load (Zhang 2021). Research
has pointed previously that the internal brain
functional network connection based on fMRI can be
used to predict the performance of remission of major
depressive disorder after drug treatment (Korgaonkar
2020). Researchers used a fully connected group
method to explore the internal brain function network
that can predict the treatment outcomes of
antidepressants in patients with depression before the
treatment, which found that patients with high
connectivity among DMN, frontal parietal and motor
network were most likely to benefit from
antidepressant treatment. Chin et al. (Fatt 2019) found
that depressive disorder patients with high
connectivity between DMN and ECN support the
treatment of antidepressants. Therefore, the brain
network connection model before neurofeedback
training is expected to become a potential feature to
predict the training effect, and plays an important role
in predicting which patients may be effective for
neurofeedback training in insomnia.
Aiming at the rt-fMRI neurofeedback emotion
regulation training in insomnia, this paper constructs
a resting state brain network functional connectivity
feature set based on reflecting emotion correlation,
and proposes a neurofeedback training effectiveness
prediction method based on brain network
connection. The results show that this method can
accurately predict the training effect of insomnia
patients, and provide an important basis for patients'
treatment decision-making.
2 MATERIALS AND METHODS
2.1 Participants
The subjects of this experiment were recruited by
Henan Provincial People's hospital through outpatient
and advertising. A total of 24 patients with right-
handed insomnia (average age 47.13 ± 12.76 years, 5
males and 19 females) were enrolled, which met the
criteria of DSM (American 1994). The degree of
insomnia of all patients was consistent with the
Pittsburgh sleep quality index (PSQI) total score of
more than 10 or the insomnia severity index (ISI)
score of more than 8 (PSQI > 10 and ISI > 8 showed
insomnia disorder. The higher the score, the more
serious insomnia). They subjectively showed
symptoms such as difficulty in getting in and out of
sleep, dreaminess and easy to wake up, depression
and emotional abnormalities. The exclusion criteria
included serious suicidal ideation, history of mental
or cardiovascular diseases, drinking or taking drugs
affecting brain function during the experiment, and
MRI contraindications.
2.2 Experimental Paradigm
The neurofeedback training of insomnia disorder
requires subjects to complete six stages of
experiments, once a week, and fixed on weekends. At
visit1, insomnia patients asked to fill in a
demographic scale and took an overnight
Polysomnography test (PSG) measurement. At visit2,
subjects underwent baseline scans, including T1
structural images and a resting state scan of 6min20s.
During vivst2 to vivst6, subjects were asked to fill in
six scales to evaluate sleep, depression and emotional
status before and after the experiment, including
Pittsburgh sleep quality index (PSQI), insomnia
severity index (ISI), Hamilton Depression Scale
(HAMD), Hamilton Anxiety Scale (HAMA), positive
and Negative Emotion Scale (PANAS) and Baker
Depression Scale (BDI). From vivst3 to vivst5, each
subject underwent three neurofeedback training runs,
each session lasted about 50 minutes, including the
formal experimental run and the rest time between
each run.
Before neurofeedback training, the subjects were
asked to write down three or more specific positive
autobiographical memories, and explain the specific
tasks of the experiment to the subjects. Each rt-fMRI
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
152

neurofeedback experiment session included
functional localization before training and 7 min
resting state scanning. Before the formal
neurofeedback training, there will be a pre-training
lasting for 6 min and 30s and subjects will train
without feedback signal. Then, three formal
neurofeedback trainings composed of seven 30s
"rest" blocks and six 30s "emotion regulation" blocks
guided by the prompt "happy" were conducted, each
lasting for 6 min and 30s. In the "rest" block, the
patient is required to look at the cross on the screen to
calm their emotions. In the "emotion regulation"
block, the activity signal of the left amygdala of the
insomnia patient is fed back to the subjects in the
shape of a bar column, and the patient is instructed to
adjust the height of the bar column on the screen by
specifically recalling positive autobiographical
memory. Each repetition time of the feedback signal
(TR = 2S) update once. Then a transfer training run
without feedback signal but the same as the formal
feedback run experimental paradigm was carried out,
which also lasted for 6 min and 30s. Finally, a 7 min
resting state scan was performed. Visit 6 is the
follow-up period, during which the T1 structure
image and resting state of the subjects were scanned
again. The neurofeedback process is shown in Figure
1:
Figure1 : The process of neurofeedback training in
insomnia
2.3 Experimental Acquisition
All fMRI data in this experiment are from the medical
imaging center of Henan people's hospital. The data
collection is completed through their magnetic
machine (Siemens prism 3T). The head coil is 64
channels. Before the experiment, we first fixed the
subject's head with a sponge pad, and then pasted
medical tape laterally on the subject's forehead to
both sides of the coil for fixation to prevent excessive
head movement from affecting the experimental
results. This paper uses the resting state data of
insomnia patients before and after neurofeedback.
2.4 Data Processing
The DPABI is used to process resting state fMRI data,
including converting DICOM raw data to NII data
(deleting the first 10 time points), slice timing,
realignment, reorientation, coregister, brain
component segment, smooth, detrending, filter, etc.
This paper performed group ICA analysis by gift
v3.0b toolbox (Calhoun2010) on the preprocessed
data to gain the resting state brain network
components of the whole brain. The minimum
description length criterion (MDL) (Li 2007) was
used to calculate the optimal number of resting state
components in the whole brain, and the INFOMAX
algorithm was used to decompose the fMRI data. The
reliability and robustness of component analysis are
improved through repeated calculation for 20 times
by ICASSO (Himberg 2003). Then, the simplified
data of principal component analysis (PCA) is
inversely reconstructed and decomposed into a series
of spatially independent components and their
corresponding time processes. Normalization
converts each independent component to a z-value.
The brain network of interest is selected by the
method of maximum spatial correlation, and the brain
network template is used as the spatial template of the
component. We single out 10 brain networks
involved in emotional cognitive processing and
sensory motor in the resting brain network template
proposed by Stanford cognitive and System
Neuroscience Laboratory in 2012 (Shirer 2012),
anterior salience network (ASN), posterior salience
network (PSN), dorsal default mode network
(DDMN), ventral default mode network (VDMN),
left executive control network (LECN) and right
executive control network (RECN), basal
ganglia(BG), sensorimotor(SM), precuneus and
auditory. The Pearson correlation between each
network time series is calculated, and the correlation
coefficient is transformed by fisher-z transform.
Finally, the functional connection matrix of the brain
network before neurofeedback is used as the
characteristic input of the prediction model.
2.5 Machine Learning Model for
Treatment Effect Prediction
In recent years, people are increasingly interested in
the application of machine learning technology in the
diagnosis, classification and effect prediction of
clinical diseases such as depression, schizophrenia,
bipolar disorder and autism disorder. We use machine
learning method combined with brain image
characteristics to predict the effect of neurofeedback
Effective Prediction of Neurofeedback based on Functional Connection Characteristics of Brain Network in Insomnia
153
training for insomnia patients, further promote the
decision-making of individual treatment plan for
insomnia, and accelerate the clinical transformation
and application of rt-fMRI neurofeedback
technology.
In this paper, 10 functional brain networks and 68
network sub-components of 24 insomnia patients
were analyzed in time, and twenty-four 78× 78
functional connection matrix were generated within
and between brain networks. We select 3003 non-
repeated function connection values in the generated
function connection matrix as features. The machine
learning method is adopted, and CatBoost,
RandomForest, LightGBM, XGboost, ExtraTrees,
KNeighbors and other models are used for secondary
classification. Label 0 represents the ineffective
neurofeedback treatment, and label 1 represents the
effective neurofeedback treatment.
3 RESULTS
Referring to the common segmentation proportion of
machine learning, 24 patients are divided into 14
patients for training and 10 patients for testing. Using
automatic machine learning technology, the
recognition accuracy of 11 machine learning models
under this data set is investigated.
For the generated 10 test sets, the prediction of
each model is shown in Table 1.
Table 1: Prediction of 10 subjects under each model.
model Light-GBMLarge
Light-
GBMXT
XG-
Boost
Light-
GBM
Cat-
Boost
accurac
y
90% 70% 50% 70% 60%
Random-
ForestEnt
r
KNeigh-
b
orsUnif
KNeigh-
b
orsDist
Random-
ForestGini
Extra-
TreesEnt
r
Extra-
TreesGini
40% 30% 30% 30% 20% 20%
Considering that the sample size of this
experiment is relatively small, the left one method
(LOOCV) is used for cross validation, that is, the
functional connection matrix of each subject is
recycled as the test set, and the brain network
characteristics of the other 23 subjects are input into
the automatic machine learning model as the training
set for training, with a total of 24 cyclic evaluations.
The prediction results of each model are shown in
Table 2.
Table 2: The prediction of each model of 24 subjects under LOOCV.
model Cat-Boost
Random-
ForestEnt
r
Random-
ForestGini
Light-
GBMXT
Light-
GBM
accuracy 75% 71% 71% 63% 63%
Extra-
TreesEnt
r
Light-
GBMLarge
XG-Boost
KNeigh-
b
orsDist
KNeigh-
b
orsUnif
Extra-
TreesGini
63% 58% 54% 54% 54% 54%
From table2, CatBoost, RandomForestEntr and
RandomForestGini models have higher prediction
accuracy among all models.
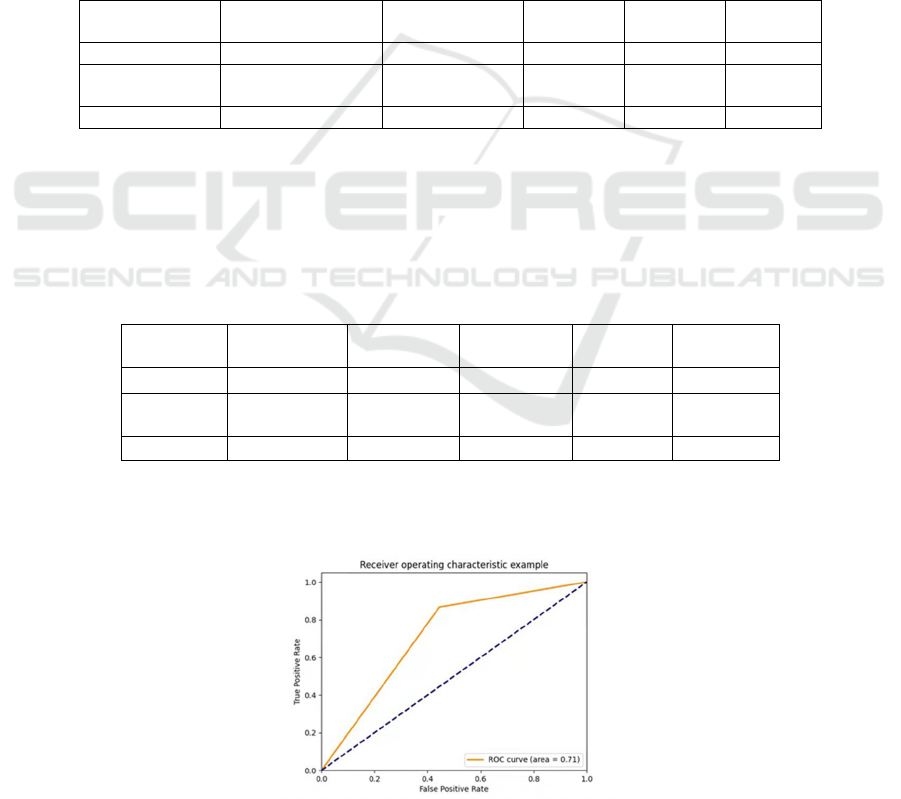
Figure 2: ROC curve of CatBoost under LOOCV.
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
154

As shown in Figure 1, the area is 0.84 under the
ROC curve of CatBoost, the accuracy of this model is
high. Compared with other models, CatBoost model
solves the problems of gradient deviation and
prediction offset, so the occurrence of over fitting is
reduced and the accuracy and generalization ability of
the algorithm are improved. Randomforestentr and
RandomForestGini belong to random forest models,
which can be over fitted by reducing randomness, so
their prediction accuracy is high. However, due to the
high randomness of their parameters, their accuracy
is lower than CatBoost. Xgboost model uses
approximate algorithm to improve operation speed.
Kneigborsdist and Kneigborsunif are too simple in
structure, resulting in insufficient fitting of data.
Extratreesgini model adopts randomization for model
structure and model parameters, resulting in too
strong randomization of model. Therefore, the
prediction accuracy of Xgboost, Kneigborsdist,
Kneigborsunif and Extratreesgini are lower.
4 DISCUSSION
The results of machine learning prediction model
based on the functional connection characteristics of
brain network show that the effectiveness of
neurofeedback in insomnia patients is significantly
higher than the random probability. The clinical
practical significance of the model can be explained
as whether an individual with insomnia can be
predicted to be suitable for neural feedback training
after resting state MRI scanning and brain network
data analysis, which provided a reliable basis for the
selection of treatment schemes for insomnia patients.
However, our study has some limitations. On the
one hand, the degree of each subject is different in
insomnia, and there are individual differences in the
treatment effect after neurofeedback training. For the
ineffective treatment of patients after neurofeedback,
whether it is caused by internal factors or external
factors such as errors in the experimental process,
these problems need to be further discussed. For
example, we can divide the subjects' disease degree
more carefully, explore the impact of different
severity of insomnia on the effect of neurofeedback
training, or adjust the neurofeedback experimental
design by changing the neurofeedback target area. On
the other hand, due to the limited experimental
conditions and the limited number of samples
included in the experiment, 24 subjects participated
in neurofeedback training. With the development of
more clinical experiments, this method is expected to
achieve more accurate prediction.
5 CONCLUSIONS
In the paper, we propose a neurofeedback
effectiveness prediction method based on the
functional connectivity of resting state brain
networks. The functional connections of brain
networks such as DMN, ECN, SAN and SM et.al.
before neural feedback in insomnia are extracted as
features, and a prediction model based on automatic
machine learning is constructed to predict the neural
feedback training effect of insomnia patients, so as to
realize the prediction of the effectiveness of neural
feedback training of insomnia patients based on
machine learning. The experimental results show that
the highest prediction accuracy of all machine
learning models reaches 75%, which provides an
important support for insomnia patients to make
decisions in the treatment plan.
ACKNOWLEDGEMENTS
This work was supported by the National Natural
Science Foundation of China [grant number
82071884]; and the Key Project of Medical Science
and Technology of Henan Province [grant number
LHGJ20200060].
REFERENCES
Alkoby, O., Abu-Rmileh, A., Shriki, O., & Todder, D..
(2017). Can we predict who will respond to
neurofeedback? a review of the inefficacy problem and
existing predictors for successful eeg neurofeedback
learning. Neuroscience, S0306452216307576.
Albert, K. M. , GG Potter, Boyd, B. D. , Kang, H. , &
Taylor, W. D. . (2018). Brain network functional
connectivity and cognitive performance in major
depressive disorder. Journal of Psychiatric Research.
American, P. . (1994). Diagnostic and statistical manual of
mental disorders. Encyclopedia of the Neurological
Sciences, 25(2), 4-8.
Calhoun, V. D. , Adali, T. , Pearlson, G. D. , & Pekar, J. J.
. (2010). A method for making group inferences from
functional mri data using independent component
analysis. Human Brain Mapping, 14(2), 140.
Fatt, C. R. C. , Jha, M. K. , Cooper, C. M. , Fonzo, G. , &
Trivedi, M. H. . (2019). Effect of intrinsic patterns of
functional brain connectivity in moderating
antidepressant treatment response in major depression.
American Journal of Psychiatry, 177(2),
appi.ajp.2019.1.
Haugg, A., Sladky, R., Skouras, S., Mcdonald, A., &
Scharnowski, F.. (2020). Can we predict real‐time fmri
Effective Prediction of Neurofeedback based on Functional Connection Characteristics of Brain Network in Insomnia
155

neurofeedback learning success from pretraining brain
activity?. Human Brain Mapping(28).
Himberg, J. , & Hyvarinen, A. . (2003). Icasso: software for
investigating the reliability of ICA estimates by
clustering and visualization. Neural Networks for
Signal Processing, 2003. NNSP'03. 2003 IEEE 13th
Workshop on. IEEE.
Kesler, S. R. , Rao, A. , Blayney, D. W. , Oakley-Girvan, I.
A. , Meghan, K. , & Oxana, P. . (2017). Predicting long-
term cognitive outcome following breast cancer with
pre-treatment resting state fmri and random forest
machine learning. Frontiers in Human Neuroscience,
11, 555.
Korgaonkar, M. S. , Goldstein-Piekarski, A. N. , Fornito, A.
, & Williams, L. M. . (2020). Intrinsic connectomes are
a predictive biomarker of remission in major depressive
disorder. NPG Open Access , 25(7).
Li, Y. O., Tülay Adal, & Calhoun, V. D. (2007). Estimating
the number of independent components for functional
magnetic resonance imaging data. Human Brain
Mapping, 28(11), 1251-1266.
Ma, X. , Jiang, G. , Fu, S. , Jin, F. , Wu, Y. , & Liu, M. , et
al. (2018). Enhanced network efficiency of functional
brain networks in primary insomnia patients. Frontiers
in Psychiatry, 9, 46.
Ramos, M. , Pereira, J. , Direito, B. , Sousa, T. , & Sayal,
A. . (2019). Directly exploring the neural correlates of
feedback-related reward saliency during fMRI-based
Neurofeedback.
W., R., Shirer, S., Ryali, & E., et al. (2012). Decoding
subject-driven cognitive states with whole-brain
connectivity patterns. Cerebral Cortex.
Zhutovsky, P. , Thomas, R. M. , Olff, M. , Rooij, S. ,
Kennis, M. , & Wingen, G. , et al. (2019). Individual
prediction of psychotherapy outcome in posttraumatic
stress disorder using neuroimaging data. Translational
Psychiatry, 9.
Zhang, Gao, et al. (2021). A research on resting-state
functional network connectivity after rt-fmri
neurofeedback in insomnia. Journal of Physics:
Conference Series, 1907(1), 012013 (7pp).
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
156
