
Research on the Role of Dopamine and Noradrenaline in Alzheimer’s
Disease and Their Changes in the Aging Brain
Guangmiao Jin
Department of Life Sciences, Imperial College London, SW7 2AZ, U.K.
Keywords: Alzheimer Disease (AD), Aging, Noradrenaline (NA), Dopamine (DA), Neurodegernative Diseases.
Abstract: Alzheimer’s disease (AD) particularly affects the aged generations on a global scale. Dopamine (DA) and
noradrenaline (NA) are the two essential components in regulating human behaviours, cognition and memory
formation. The correlation between the two neuromodulators and AD was intensively studied in this paper. A
wide range of genetically modified animal models were adopted in combination with monitoring methods.
Because AD occurs more frequently in older age, it is suspected that the aging might be a potential factor of
AD. in this review, abundant literatures regarding AD, NA, DA and aging were summarized to generate an
insight. Although the relationship between AD and the NA system remains vague, AD is a long-term affecting
disease and may not be induced simply by aging.
1 INTRODUCTION
According to Global Health Estimates of World
Health Organization (WHO) in 2019, Alzheimer’s
disease (AD) and other dementias rated sixth place
among all the diseases with the highest mortality rate,
causing 814,000 deaths annually. For high-income
countries, neurodegenerative diseases overtook
stroke and became the second most lethal disease
(World Health Organization. (2020). Past research
has identified that beta-amyloid (Aβ) plaque has
strong association with AD and is often regarded as a
histopathological hallmark (Jack 2013). Genetic
studies revealed that the presence of Aβ is frequently
associated with synaptic dysfunction, interrupting
neuronal connectivity and neuronal death in a region-
specific manner (Murphy, & LeVine 2010). AD onset
is often diagnosed in the older age. Similar to AD,
aging is accompanied by the changes in neural
circuits. To reveal the mechanism of how AD is
gradually developed and if there is similarity between
the two factors, some studies related to dopamine
(DA) and noradrenaline (NA) are listed, analysed and
compared. The review aims to guide readers to a more
comprehensive view of NA and DA functioning and
their potential roles in regulating AD and aging. As a
consequence, the clinical trials based on the two
neuromodulator might be attempted, benefiting the
AD patients. In the following text, dopaminergic and
noradrenergic systems with linkage to aging will be
discussed in more detail.
2 DA PATHWAY
Tyrosine is the precursor amino acid in DA
biosynthesis. Tyrosine hydroxylase (TH) catalyzes
the conversion of L-tyrosine to L-
dihydroxyphenylalanine (L-DOPA). Consequently,
the decarboxylation reaction catalyzed by DOPA
decarboxylase (DDAP) removes one molecule of
carbon dioxide from the L-DOPA molecule and
converts L-DOPA to dopamine (Pan, Kaminga, Wen,
Wu, Acheampong, & Liu 2019). Synthesized DA is
immediately transported out from the cytosol of the
dopaminergic neurons into the monoaminergic
synaptic vesicles by vesicular monoamine
transporters (VMAT-2). VMAT-2 is located at the
membrane of the vesicle. DA uptake into
monoaminergic vesicles prevents the accumulation of
free DA and the oxidation of DA to o-quinone, as
VMAT-2-coupled ATPase actively pumps protons
into the vesicles to build up a high proton gradient.
DA stored in the dopaminergic vesicle is released into
the synaptic cleft and binds onto the DA receptor on
the postsynaptic neuron membrane. DA remained in
the cleft is cleaved by a DA transporter which
Jin, G.
Research on the Role of Dopamine and Noradrenaline in Alzheimer’s Disease and Their Changes in the Aging Brain.
DOI: 10.5220/0011248100003438
In Proceedings of the 1st International Conference on Health Big Data and Intelligent Healthcare (ICHIH 2022), pages 221-227
ISBN: 978-989-758-596-8
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
221
localizes on the dopaminergic membrane (Segura-
Aguilar, Paris, Muñoz, Ferrari, Zecca, & Zucca
2014).
DA neuron loss has been a widely accepted
concept in explaining AD progression. Hippocampus
which controls the memory formation and voluntary
movement receives input from both cortical and
subcortical regions. Consistent with the observation,
hippocampal DA is released from the ventral
tegmental area (VTA) which contributes to the
subcortical input. Decreased levels of DA neurons
and DA receptors are often detected in AD patients’
brains, in agreement with the changes in the
midbrain-located DA system of Tg2576 mouse
model. Tg2576 mice were genetically modified to
overexpress mutated amyloid precursor proteins
(APPs). Also, DA is a well-recognized modulator for
hippocampal plasticity. The memory formation is
encoded by the binding of DA at the hippocampal DA
receptors (Nobili 2017). In addition, previous studies
applying the 18F - fluorodeoxyglucose Positron
Emission Tomography indicated that human neuronal
function loss occurs prior to the onset of AD.
Synaptic dysfunction is one of the early indicators,
marking the initiation of pathology. Spine loss in
mice harboring the human familial gene mutation is
positively correlated with the appearance of cognitive
impairment. Synaptic connectivity determines the
signal transmission efficiency, further impacting on
the learning and memory (Kashyap, Bapat, Das,
Gowaikar, Amritkar, Rangarajan, Ravindranath, &
Ambika 2019). In line with these finding, aging is
positively correlated with neuronal degeneration.
Noda et al. reported the age-dependent DA neuronal
loss and mitochondrial dysfunction in DA neurons of
C57BL/6 mice (Noda, Sato, Fukuda, Tada, & Hattori
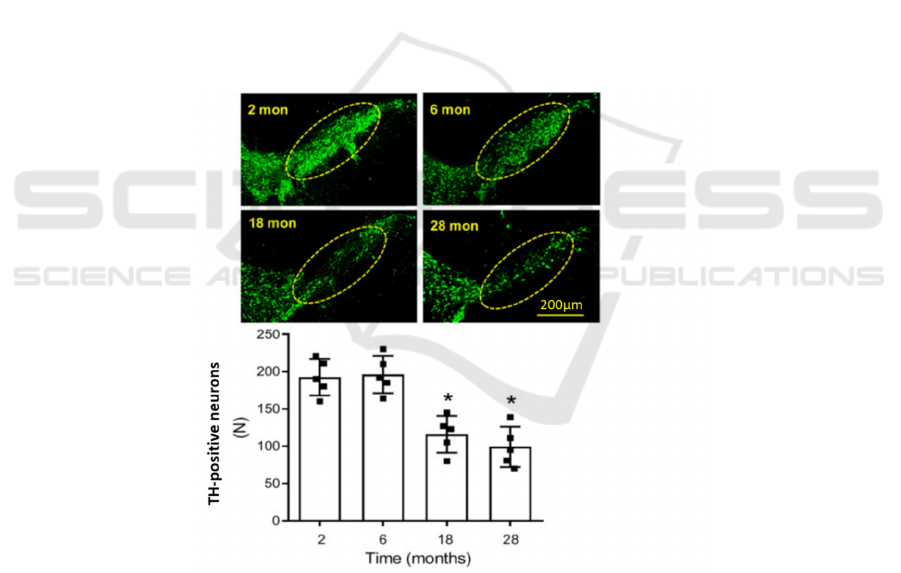
2020). Wang et al. in 2019 also demonstrated that
normally aged rat brains contained fewer DA
neurons, as the number of TH positive neurons
declined significantly in 18-month and 28-month than
their younger counterparts which were 2- and 6-
month-old (Figure 1) (Wang, Zhou, Wang, Li, Liu, &
Zhang 2019).
Figure 1. The image shows the stained neurons using anti-TH in four age groups: 2-month, 6-month, 18-month and 28-month-
old. Quantitative counting is summarized as a bar chart in which *P < .05 compared with 2-month-old rats (Wang, Zhou,
Wang, Li, Liu, & Zhang 2019).
In neurons with high DA concentration, reactive
oxygen species (ROS) is often generated by DA
autoxidation, including superoxide anion radical
(O2•–) and hydrogen peroxide (H2O2) which
severely damages cellular activities (Linert,
Herlinger, Jameson, Kienzl, Jellinger, & Youdim
1996). ROS can bring a series of toxic consequences:
such as mitochondrial dysfunction, oxidative stress
and protein denaturation. Not only Aβ, ROS is also a
potential pathological factor that intertwines with Aβ
through numerous routes. Aβ complex binding to
metal ions such as Cu (I/II) and Zn (II) facilitates Aβ
aggregation and toxic oligomer formation. In
addition, the redox-active metal bound Aβ complex
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
222

promotes the overproduction of ROS. Consequently,
ROS overproduction would impose detrimental
effects on nucleic acids, lipids and cellular organelles
(Han, Lee, Kim, Lee, Suh, Cho, Chae, & Lim 2018).
These factors can individually or mutually contribute
to oxidative transformation of DA, bridging the gap
between DA and AD pathology. DA is responsible for
long-term memory and motor activities, therefore,
Aβ-included DA system dysfunction, as well as
degeneration of AD-releasing neurons in VTA region
were commonly reported in AD-affected brains. In
conclusion, DA, along with its oxidative derivatives,
would have a potential role in oxidizing metal-bound
or metal-free Aβ oligomers and regulating Aβ
aggregation pathways (Nam, Derrick, Lee, Kang,
Han, Lee, Chung, & Lim 2018).
Despite that aging is reported to be a major risk
factor of AD, normal aging still overlaps with AD in
terms of pathology and postulated mechanisms. For
instance, neurofibrillary tangles (NFTs) and plaques
are frequently found in brains of neurologically
normal individuals in postmortem studies (Figure 2)
(Davis, Schmitt, Wekstein, & Markesbery 1999).
Besides, tau pathology which has been confined in
LC and entorhinal cortex (EC) is also detected in
many aged brains. The deposit of phosphorylated tau
was visualized by staining and identified via the
immunocytochemistry techniques. The severity of
AD develops with aging until the symptoms are
diagnosable (Braak, Thal, Ghebremedhin, & Del
Tredici 2011). In addition, oxidative balance in brains
is disrupted with aging, as the capacity of
synthesizing anti-oxidants decreases significantly.
Thus, ROS accumulates and inhibits the
metabolically important pathways, such as the
synthesis of DNA, lipids and proteins. Moreover,
inefficient oxidative phosphorylation in mitochondria
of older individuals might aggravate the oxidative
stress (Sutherland, Chami, Youssef, & Witting 2013).
The close association between aging, mitochondrial
dysfunction and oxidative burden in AD formation
would implicate that antioxidants would be a
potential medical target.
3 NA PATHWAY
Noradrenergic pathway initiates from the cell bodies
in LC and propagates towards different cerebral
regions, spinal cord, and other areas, such as the
amygdala, hippocampus, and hypothalamus (Moret,
& Briley 2011). Unlike GABA or glutamate which
binds to ionotropic receptors in fast action, NA is a
neuromodulator and mainly activates metabotropic
receptors (Ranjbar-Slamloo, & Fazlali 2020). NA is
synthesized from precursor amino acid tyrosine by a
series of steps. Dopamine-β-hydroxylase is one of the
critical enzymes which catalyze the conversion of
dopamine to NA. Once the step is completed, the
vesicle packs NA and transports it across the
membrane by VMAT2 into the synaptic cleft via
exocytosis. Otherwise, catechol-O-
methyltransferases (COMT) or monoamine oxidases
(MAO) can enzymatically digest the extracellular NA
molecules (Figure 3) (Gannon, & Wang 2019). The
release of noradrenaline is mediated by adrenoceptors
which could generate different effects. For instance,
activation of presynaptic α2-adrenoceptors (α2-ARs)
and β2-adrenoceptors (β2-ARs) respectively inhibits
and promotes NA release. Transporter facilitates the
recycling of NA into the presynaptic neuron. The
complexity of NA is regulated by the density or
distribution of different adrenoceptor subtypes
(Gareri, De Fazio, & De Sarro 2002).
Figure 2. The process of NA synthesized in the presynaptic neuron and different fates of NA (Reprinted from “NA synthesis
and export”, by BioRender.com (2021).
Research on the Role of Dopamine and Noradrenaline in Alzheimer’s Disease and Their Changes in the Aging Brain
223
As NA is mainly supplied from LC, the
degeneration of NA is marked as an early sign of
neurodegeneration. One of the most obvious changes
in the LC region is the decline in the number of NA
neurons. A significant NA neuron loss can lead to AD
progression (Holland, Robbins, & Rowe 2021). The
remaining NA neurons would initiate changes in
activity as a compensation. For instance, the mRNA
level of TH would increase in the remaining NA
neurons, since TH is involved in the rate-limiting
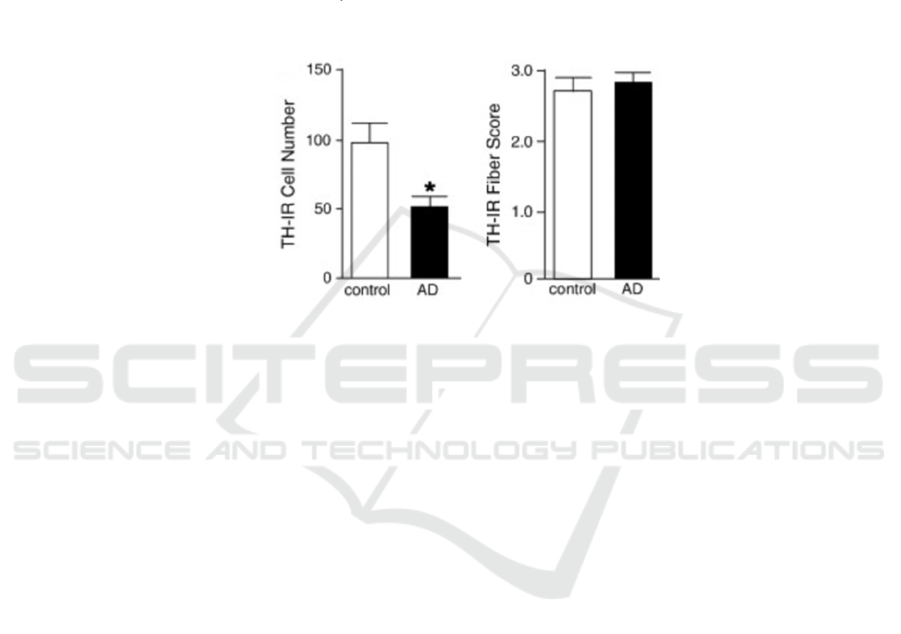
step. According to TH-immunoreactivity (TH-IR)
quantification, the number of TH-IR-positive neurons
reduced significantly in AD subjects (Figure 3). The
evidence further emphasizes the importance of NA in
maintaining the normal functioning of human brains.
Besides, NA negatively regulates the transcription of
pro-inflammatory genes in astrocytes and microglia
and the production of the cytokine and chemokine, as
well as controlling the microglial and phagocytosis
migration (McMillan, White, Franklin, Greenup,
Leverenz, Raskind, & Szot 2011).
Figure 3. Cell bodies are independently assessed as individual neurons. The number of TH-IR positive cells in AD is
significantly fewer than the control (Left histogram). TH-IR positive fiber score is an indicator of TH level. No clear difference
is observed between the two groups (Right histogram). The two results, in combination, would implicate a compensation
behaviour in AD-loss scenario (McMillan, White, Franklin, Greenup, Leverenz, Raskind, & Szot 2011).
In post-mortem studies of AD patients’ brains,
tissue separation and oligonucleotide probe which
targets at the AR of interest were performed
individually. It was found that various AR subtypes
undergo different changes. The expression level
ofα1A- and α2A-AR mRNA in the hippocampus
remains constant, whereas the expression level
ofα1D- and α2C-AR mRNA reduces profoundly
(Szot, White, Greenup, Leverenz, Peskind, &
Raskind 2006). Alterations in the AR are constantly
observed with the changes in receptor expression and
density, affecting sensitivity and amplitude of the NA
modulating abilities (Gannon, & Wang 2019).
Furthermore, AR subtypes were proved to be related
to the formation of Aβ. For instance, α2A-AR
activation interprets the interaction of APP with a
Vps10 family receptor which mediates the APP
sorting. Therefore, activation of inα2A-AR promotes
the amyloidogenic process (Chen, Peng, Che,
Gannon, Liu, Li, Bu, van Groen, Jiao, & Wang 2014).
Similarly, β2-AR up-regulates the γ-secretase
activity. Activated γ-secretase, along with β-
secretase, cleaves APP to produce Aβ. Thereby β2-
AR accelerates the pathology of AD by stimulating
more Aβ plaques formed in the brains (Ni, Zhao, Bao,
Zou, Teng, Wang, Song, Xiong, Bai, & Pei 2006).
The β2-AR also functions to influence the microglial
dynamics. Strikingly, the effect of β2-AR imposed on
microglia depends on the stress level. By comparing
mice during wakefulness and sleeping, β2-AR was
found to diminish the activity and clearance ability of
microglia for awake mice. In contrast, β2-AR
inhibitors down-regulate the stress-induced activities
of microglia (Mather 2021).
Even though a clear relationship between the
postmortem LC neuron count and aging has not been
established, several other indicators have implied the
decline in the LC-NA system. One of the indicators is
the decreased NA level with aging in some brain
regions, such as the cingulate gyrus, hippocampus,
hippocampus and hindbrain (Mather, Gutchess, &
Thomas 2019). Complementarily, NA level in
cerebrospinal fluid and blood grows with age
progression. Seals & Esler estimated that a 15-20%
increase per decade in plasma NA would be observed
over the adult range. More NA spillover into plasma
was reported in accord with aging (Seals, & Esler
2000). The degree of increase is even more obvious
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
224

in AD patients than in healthily aged adults (Elrod,
Peskind, DiGiacomo, Brodkin, Veith, & Raskind
(1997). Strikingly, hyperactivation of LC-NA
systems would also occur in the early AD stage. LC
hyperactivation promotes the Ca2+ influx and
mitochondrial toxicity. Furthermore, the pacemaker
activity is interpreted, associated with an elevation in
bioenergetic demand which would potentially induce
a significant level of oxidative stress (Weinshenker
2018). Moreover, tau pathology continues to progress
in LC with aging. Increasing tau proteins are
hyperphosphorylated and integrate as oligomers. The
tubulin-binding affinity of hyperphosphorylated tau
decreases. Instead, tau proteins tend to aggregate.
Consistently, aggregated tau is often involved in the
initial phase of AD and other neurodegenerative
diseases (Iqbal, Liu, & Gong 2016). At least, by
current studies, clinical signs of AD are detectable in
an early age but can also be symptomless, since pre-
tangles and NFTs in nerves can exist for decades.
Although AD is not likely to be age-dependent, it
develops in a long-term mode and extends in old ages
(Braak, & Del Tredici 2011).
4 CONCLUSIONS
The article reviews the past investigations on the DA
and NA. In conclusion, DA and NA, being provided
from VTA and LC, are both crucial neuromodulators
in terms of modulating brain states and memory
formation. They influence the brain functioning from
several mechanisms: (1) reduction or lack of neurons
synthesizing DA or NA; (2) adding burden to
oxidative stress in the neurons; (3) variation in the
density and expression levels of different AR
subtypes. These findings provide new strategies for
designing drugs which target specifically at AD
patients in the hope that their brains’ normal functions
could be recovered. Although brain changes during
normal aging overlap partially with changes in AD
patients’ brain, there is still no clear evidence to prove
that aging is directly correlated to AD progress. But
instead, AD is more likely to affect an individual from
an early age until the symptoms reveal in a relatively
older age. Thus, it would be worth exploring if there
is any other factors correlated to aging.
REFERENCES
Braak, H. & Del Tredici, K. (2011) The pathological
process underlying Alzheimer's disease in individuals
under thirty. Acta Neuropathologica. 121 (2), 171-181.
Available from: doi: 10.1007/s00401-010-0789-4 [doi].
Braak, H., Thal, D. R., Ghebremedhin, E. & Del Tredici, K.
(2011) Stages of the Pathologic Process in Alzheimer
Disease: Age Categories From 1 to 100 Years. Journal
of Neuropathology & Experimental Neurology. 70
(11), 960-969. Available
from: https://doi.org/10.1097/NEN.0b013e318232a37
9. Available from: doi:
10.1097/NEN.0b013e318232a379. [Accessed
9/28/2021].
Chen, Y., Peng, Y., Che, P., Gannon, M., Liu, Y., Li, L.,
Bu, G., van Groen, T., Jiao, K. & Wang, Q. (2014)
α2A adrenergic receptor promotes amyloidogenesis
through disrupting APP-SorLA
interaction. Proceedings of the National Academy of
Sciences. 111 (48), 17296-17301. Available
from: http://www.pnas.org/content/111/48/17296.abstr
act. Available from: doi: 10.1073/pnas.1409513111.
Davis, D. G., Schmitt, F. A., Wekstein, D. R. &
Markesbery, W. R. (1999) Alzheimer Neuropathologic
Alterations in Aged Cognitively Normal
Subjects. Journal of Neuropathology & Experimental
Neurology. 58 (4), 376-388. Available
from: https://doi.org/10.1097/00005072-199904000-
00008. Available from: doi: 10.1097/00005072-
199904000-00008. [Accessed 9/30/2021].
Elrod, R., Peskind, E. R., DiGiacomo, L., Brodkin, K. I.,
Veith, R. C. & Raskind, M. A. (1997) Effects of
Alzheimer's disease severity on cerebrospinal fluid
norepinephrine concentration. The American Journal of
Psychiatry. 154 (1), 25-30. Available from: doi:
10.1176/ajp.154.1.25 [doi].
Gannon, M. & Wang, Q. (2019) Complex noradrenergic
dysfunction in Alzheimer's disease: Low
norepinephrine input is not always to blame. Brain
Research. 1702 12-16. Available
from: https://pubmed.ncbi.nlm.nih.gov/29307592 https
://www.ncbi.nlm.nih.gov/pmc/articles/PMC6855395/.
Available from: doi: 10.1016/j.brainres.2018.01.001.
Gareri, P., De Fazio, P. & De Sarro, G. (2002)
Neuropharmacology of depression in aging and age-
related diseases. Ageing Research Reviews. 1 (1), 113-
134. Available from: doi: S0047637401003700 [pii].
Han, J., Lee, H. J., Kim, K. Y., Lee, S. J. C., Suh, J., Cho,
J., Chae, J. & Lim, M. H. (2018) Tuning Structures and
Properties for Developing Novel Chemical Tools
toward Distinct Pathogenic Elements in Alzheimer’s
Disease. ACS Chemical Neuroscience. 9 (4), 800-808.
Available
from: https://doi.org/10.1021/acschemneuro.7b00454.
Available from: doi: 10.1021/acschemneuro.7b00454.
Holland, N., Robbins, T. W. & Rowe, J. B. (2021) The role
of noradrenaline in cognition and cognitive
disorders. Brain. 144 (8), 2243-2256. Available
from: https://doi.org/10.1093/brain/awab111.
Available from: doi: 10.1093/brain/awab111.
[Accessed 9/24/2021].
Iqbal, K., Liu, F. & Gong, C. (2016) Tau and
neurodegenerative disease: the story so far. Nature
Research on the Role of Dopamine and Noradrenaline in Alzheimer’s Disease and Their Changes in the Aging Brain
225

Reviews Neurology. 12 (1), 15-27. Available
from: https://doi.org/10.1038/nrneurol.2015.225.
Available from: doi: 10.1038/nrneurol.2015.225.
Jack, C. R., Knopman, D. S., Jagust, W. J., Petersen, R. C.,
Weiner, M. W., Aisen, P. S., Shaw, L. M., Vemuri, P.,
Wiste, H. J., Weigand, S. D., Lesnick, T. G., Pankratz,
V. S., Donohue, M. C. & Trojanowski, J. Q. (2013)
Tracking pathophysiological processes in Alzheimer's
disease: an updated hypothetical model of dynamic
biomarkers. The Lancet Neurology. 12 (2), 207-216.
Available
from: https://www.sciencedirect.com/science/article/pi
i/S1474442212702910. Available from:
doi: https://doi.org/10.1016/S1474-4422(12)70291-0.
Kashyap, G., Bapat, D., Das, D., Gowaikar, R., Amritkar,
R. E., Rangarajan, G., Ravindranath, V. & Ambika, G.
(2019) Synapse loss and progress of Alzheimer’s
disease -A network model. Scientific Reports. 9 (1),
6555.10.1038/s41598-019-43076-y.
Linert, W., Herlinger, E., Jameson, R. F., Kienzl, E.,
Jellinger, K. & Youdim, M. B. H. (1996) Dopamine, 6-
hydroxydopamine, iron, and dioxygen — their mutual
interactions and possible implication in the
development of Parkinson's disease. Biochimica Et
Biophysica Acta (BBA) - Molecular Basis of
Disease. 1316 (3), 160-168. Available
from: https://www.sciencedirect.com/science/article/pi
i/0925443996000208. Available from:
doi: https://doi.org/10.1016/0925-4439(96)00020-8.
Mather, M. (2021) Noradrenaline in the aging brain:
Promoting cognitive reserve or accelerating
Alzheimer's disease? Seminars in Cell &
Developmental Biology. 116 108-124. Available
from: https://www.sciencedirect.com/science/article/pi
i/S1084952121001221. Available from:
doi: https://doi.org/10.1016/j.semcdb.2021.05.013.
Mather, M., Gutchess, A. & Thomas, A. E. (2019) How
arousal-related neurotransmitter systems compensate
for age-related decline. The Cambridge Handbook of
Cognitive Aging: A Life Course Perspective.
McMillan, P. J., White, S. S., Franklin, A., Greenup, J. L.,
Leverenz, J. B., Raskind, M. A. & Szot, P. (2011)
Differential response of the central noradrenergic
nervous system to the loss of locus coeruleus neurons
in Parkinson's disease and Alzheimer's disease. Brain
Research. 1373 240-252. Available
from: https://www.sciencedirect.com/science/article/pi
i/S0006899310026545. Available from:
doi: https://doi.org/10.1016/j.brainres.2010.12.015.
Moret, C. & Briley, M. (2011) The importance of
norepinephrine in depression. Neuropsychiatric
Disease and Treatment. 7 9-13. Available
from: https://pubmed.ncbi.nlm.nih.gov/21750623 https
://www.ncbi.nlm.nih.gov/pmc/articles/PMC3131098/.
Available from: doi: 10.2147/NDT.S19619.
Murphy, M. P. & LeVine, H., 3rd. (2010) Alzheimer's
disease and the amyloid-beta peptide. Journal of
Alzheimer's Disease: JAD. 19 (1), 311-323. Available
from: https://pubmed.ncbi.nlm.nih.gov/20061647 https
://www.ncbi.nlm.nih.gov/pmc/articles/PMC2813509/.
Available from: doi: 10.3233/JAD-2010-1221.
Nam, E., Derrick, J. S., Lee, S., Kang, J., Han, J., Lee, S. J.
C., Chung, S. W. & Lim, M. H. (2018) Regulatory
Activities of Dopamine and Its Derivatives toward
Metal-Free and Metal-Induced Amyloid-β
Aggregation, Oxidative Stress, and Inflammation in
Alzheimer’s Disease. ACS Chemical Neuroscience. 9
(11), 2655-2666. Available
from: https://doi.org/10.1021/acschemneuro.8b00122.
Available from: doi: 10.1021/acschemneuro.8b00122.
Ni, Y., Zhao, X., Bao, G., Zou, L., Teng, L., Wang, Z.,
Song, M., Xiong, J., Bai, Y. & Pei, G. (2006) Activation
of β2-adrenergic receptor stimulates γ-secretase activity
and accelerates amyloid plaque formation. Nature
Medicine. 12 (12), 1390-1396. Available
from: https://doi.org/10.1038/nm1485. Available from:
doi: 10.1038/nm1485.
Nobili, A., Latagliata, E. C., Viscomi, M. T., Cavallucci,
V., Cutuli, D., Giacovazzo, G., Krashia, P., Rizzo, F.
R., Marino, R., Federici, M., De Bartolo, P., Aversa, D.,
Dell’Acqua, M. C., Cordella, A., Sancandi, M., Keller,
F., Petrosini, L., Puglisi-Allegra, S., Mercuri, N. B.,
Coccurello, R., Berretta, N. & D’Amelio, M. (2017)
Dopamine neuronal loss contributes to memory and
reward dysfunction in a model of Alzheimer’s
disease. Nature Communications. 8 (1), 14727.
Available
from: https://doi.org/10.1038/ncomms14727.
Available from: doi: 10.1038/ncomms14727.
Noda, S., Sato, S., Fukuda, T., Tada, N. & Hattori, N.
(2020) Aging-related motor function and dopaminergic
neuronal loss in C57BL/6 mice. Molecular Brain. 13
(1), 46. Available
from: https://doi.org/10.1186/s13041-020-00585-6.
Available from: doi: 10.1186/s13041-020-00585-6.
Pan, X., Kaminga, A. C., Wen, S. W., Wu, X.,
Acheampong, K. & Liu, A. (2019) Dopamine and
Dopamine Receptors in Alzheimer's Disease: A
Systematic Review and Network Meta-
Analysis. Frontiers in Aging Neuroscience. 11 175.
Available
from: https://pubmed.ncbi.nlm.nih.gov/31354471 https
://www.ncbi.nlm.nih.gov/pmc/articles/PMC6637734/.
Available from: doi: 10.3389/fnagi.2019.00175.
Ranjbar-Slamloo, Y. & Fazlali, Z. (2020) Dopamine and
Noradrenaline in the Brain; Overlapping or Dissociate
Functions? Frontiers in Molecular Neuroscience. 12
334. Available
from: https://www.frontiersin.org/article/10.3389/fnm
ol.2019.00334.
Reprinted from “NA synthesis and export”, by
BioRender.com (2021). Retrieved from:
https://app.biorender.com/illustrations/602cf1f6c8e56
800a3c62566
Seals, D. R. & Esler, M. D. (2000) Human ageing and the
sympathoadrenal system. The Journal of
Physiology. 528 407-417. Available
from: https://pubmed.ncbi.nlm.nih.gov/11060120 https
://www.ncbi.nlm.nih.gov/pmc/articles/PMC2270159/.
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
226

Available from: doi: 10.1111/j.1469-
7793.2000.00407.x.
Segura-Aguilar, J., Paris, I., Muñoz, P., Ferrari, E., Zecca,
L. & Zucca, F. A. (2014) Protective and toxic roles of
dopamine in Parkinson's disease. Journal of
Neurochemistry. 129 (6), 898-915. Available
from: https://doi.org/10.1111/jnc.12686. Available
from: doi: https://doi.org/10.1111/jnc.12686.
Sutherland, G. T., Chami, B., Youssef, P. & Witting, P. K.
(2013) Oxidative stress in Alzheimer's disease: Primary
villain or physiological by-product? Null. 18 (4), 134-
141. Available
from: https://doi.org/10.1179/1351000213Y.00000000
52. Available from: doi:
10.1179/1351000213Y.0000000052.
Szot, P., White, S. S., Greenup, J. L., Leverenz, J. B.,
Peskind, E. R. & Raskind, M. A. (2006) Compensatory
Changes in the Noradrenergic Nervous System in the
Locus Ceruleus and Hippocampus of Postmortem
Subjects with Alzheimer's Disease and Dementia with
Lewy Bodies. The Journal of Neuroscience. 26 (2),
467-478. Available
from: http://www.jneurosci.org/content/26/2/467.abstr
act. Available from: doi: 10.1523/JNEUROSCI.4265-
05.2006.
Wang, G., Zhou, Y., Wang, Y., Li, D., Liu, J. & Zhang, F.
(2019) Age-Associated Dopaminergic Neuron Loss and
Midbrain Glia Cell Phenotypic
Polarization. Neuroscience. 415 89-
96.https://doi.org/10.1016/j.neuroscience.2019.07.021.
Weinshenker, D. (2018) Long Road to Ruin: Noradrenergic
Dysfunction in Neurodegenerative Disease. Trends in
Neurosciences. 41 (4), 211-223. Available from: doi:
S0166-2236(18)30034-1 [pii].
World Health Organization. (2020) The top 10 causes of
death. https://www.who.int/news-room/fact-
sheets/detail/the-top-10-causes-of-
death#.YUio2eFUdhU.link [Accessed 20th September
2021]
Research on the Role of Dopamine and Noradrenaline in Alzheimer’s Disease and Their Changes in the Aging Brain
227
