
Botulinum Toxin’s using in Treatment of Cerebral Palsy
Haitao Tian
1a
, Zehao Wu
2b
and Chen Yang
3c
1
Macleans college, Auckland, 2010, New Zealand
2
University of Washington, Seattle, WA, 98109, U.S.A.
3
Biological Science, University of California Davis, Davis, CA, 95618, U.S.A.
†
These authors contributed equally
Keywords: Botulinum Toxin, Cerebral Palsy.
Abstract: Using botulinum toxin (BTX) to treat CP patients has become more mature and widely used recently. In this
article, the authors first briefly introduce the CP and BTX, then summarize the mechanisms of BTX on the
treatment of CP through a flow chart, following by opinions driven from recent clinical treatment results.
Most articles mentioned by the authors conclude that BTX therapy is one of the most important treatments
for CP patients, and the opposed research shows that it is rebuttable. Through careful analysis, the data
confirms the effectiveness of BTX therapy in the treatment of CP patients and concludes that BTX
treatment should be treated as a supportive treatment monitoring with the involvement of other therapies.
Further studies are needed to discover which 1. Impact of cerebral palsy on infant therapy is the best for a
company with BTX therapy in different situations.
1 INTRODUCTION
1
Cerebral palsy (CP), which is a group of disorders
that could affect one’s ability to move and maintain
balance and posture, is the most common motor
disability in childhood, about 1 in 345 children has
been identified with CP according to CDC’s data.
Such disorders have symptoms of muscle spasms,
stiff feeling, poor muscle control, feeding difficulties
and so on, forcing patients to live with assistance
equipment like wheelchairs and walking sticks,
reducing life quality dramatically. Although CP
could not be cured, physical therapy (PT), as the
main part of rehabilitation treatment, could improve
the situation of patients dramatically. However, the
symptom of CP makes the patients under hypertonia
situation, making their unable to control the body,
including undergoing PT treatment.
Since the hypertonia situation prevent CP
patients from PT treatment, which is one of the most
direct treatment towards CP, the way of eliminating
such negative impact become the hot topic of
treating CP patients. Botulinum toxins (BTX), the
most poisonous neurotoxic protein know produced
a
https://orcid.org/0000-0003-1773-8047
b
https://orcid.org/0000-0002-5449-8516
c
https://orcid.org/0000-0001-8498-1456
by the bacteria “Clostridium botulinum”, are
discovered by the scientists and seems to become a
promising drug for such situation with its two
special characters. Firstly, BTX could block the
release of acetylcholine neurotransmitter, prevent
the transmission of action potential through the
neuron system, reduce the muscle stimulation. With
lower muscle stimulation, the symptom of
hypertonia reduced dramatically. Without
hypertonia, patients regain the ability to move
normally and undergo the physical therapy. More
importantly, scientists discovered that even though
BTX is the most poisonous neurotoxic protein, the
damage it brings to the neuron system is totally
reversible, granting the drug ability to undergo
clinical trials. In this review, we highlight the
mechanism of BTX under the treatment of CP,
examine the research have done so far on the
effectiveness of BTX for CP treatment, compare
pros and cons of such treatment, and provide our
own insight of future treatment directions.
2 IMPACT OF CEREBRAL PALSY
ON INFANT
As introduced before, Cerebral Palsy (CP) is defined
as motor impairment, including a broad range of
Tian, H., Wu, Z. and Yang, C.
Botulinum Toxin’s using in Treatment of Cerebral Palsy.
DOI: 10.5220/0011252400003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 661-665
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
661

muscle and movement disorders. The aetiology is
mainly attributed to non-progressive disturbances
during brain development in foetuses or infants, for
example, the lack of oxygen to the brain, gene
mutation or head trauma. These injuries can all lead
to abnormal development. The injuries can also
cause other illnesses. CP is frequently accompanied
by impaired cognition, communication and sensory
perception, behavioural abnormalities, seizure
disorders, or a combination of these features.
According to the main type of movement
disorder involved, CP is classified into four main
types, depending on which brain areas are affected:
spastic, dyskinetic, ataxic, and mixed. Spastic
cerebral palsy is the most common type of CP,
which affects about 80% of the patients. People with
this type of CP have increased muscle tone, which
means their muscles are stiff, and their movements
can be awkward as a result.
The spastic CP is also described with the body
parts that are affected. For example, Spastic diplegia
is when the stiffness is mainly in the legs, the arms
are less affected or not affected at all. Dyskinetic
cerebral palsy means problems are controlling the
movements of hands, arms, feet, and legs. Patients
would be more difficult to sit and walk due to the
involuntary movements, which can either be slow
and writhing or rapid and jerky. The ataxic CP
stands for problems with balance and coordination.
Patients would have a hard time with quick
movements. They would be unsteady when walking,
actions which require lots of control, such as
writing, can also be difficult for them.
Some patients would get mixed CP, which means
they would have symptoms of more than one type of
CP, a very common type of mixed CP is spastic-
dyskinetic CP. The method we introduce in the later
text of injecting botulinum toxin (BTX) would only
work for the spastic, dyskinetic, and a few mixed
CP, as it works by blocking the patient’s muscle
stimulation.
Once the types of CP have been diagnosed, it is
then very important to evaluate the severity of the
disease. The Gross Motor Function Classification
System (GMFCS) is the most widely used clinical
foundational classification of CP. It is an ordinal
scale that categorizes a child’s mobility or lower
limb function in five levels, ranging from walking
without restrictions (level I) to inability to maintain
antigravity head and trunk postures (level V), below
is a summary of the criteria of GMFCS: (Table 1).
Table 1: Level of GMFCS’ Criteria.
Grade CLINICAL MANIFESTATIONS
Level 1 Walks without limitations
Level 2 Walks with limitations
Level 3 Walks using hand-held mobility device
Level 4 Self-mobility with limitations; may use
p
owered mobilit
y
Level 5 Transported in a wheelchair.
3 ILLUSTRATIONS OF BTX AS
AN EFFECTIVE TREATMENT
FOR CP
3.1 Basic Information about BTX
BTX is produced by Clostridium botulinum under
anaerobic conditions and consists of a complex
mixture of proteins containing botulinum neurotoxin
and various non-toxic proteins. As a neurotoxin,
BTX-Type-A(BTX-A) can target and control
unpredictable body movement. There are seven
different serotypes of BTX, and they could be
distinguished by the letters A to G. Different types
have a high degree of sequence homology, but their
toxicity and molecular action sites are different.
Different serotypes bind to different protein
receptors. These serotypes inhibit the release of
acetylcholine from nerve endings, intracellular
targets, action characteristics, and potency. The
indirect effects of BTX on the central nervous
system are reflex inhibition, reversal of reciprocal
inhibition changes, cortical inhibition and
somatosensory evoked potential, and formalin-
induced pain reduction, suggesting that TB has a
direct analgesic effect. In BTX-A, SV2 (isoforms A–
C) is the receptor for BTX-A. BTX -A has a
complex 3D structure. It is folded into three
domains: the heavy chain receptor-binding domain,
the heavy chain translocation, and the light chain
catalytic domain (Figure 1). It is the most widely
studied serotype for therapeutic purposes. BTX
interferes with the spinal stretch reflex by blocking
the fibres of the fusiform muscle, resulting in
reduced afferent signals carried by fibres IA and II
and decreased muscle tone. Treating CP with BTX-
A can influence the signals to transmit.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
662
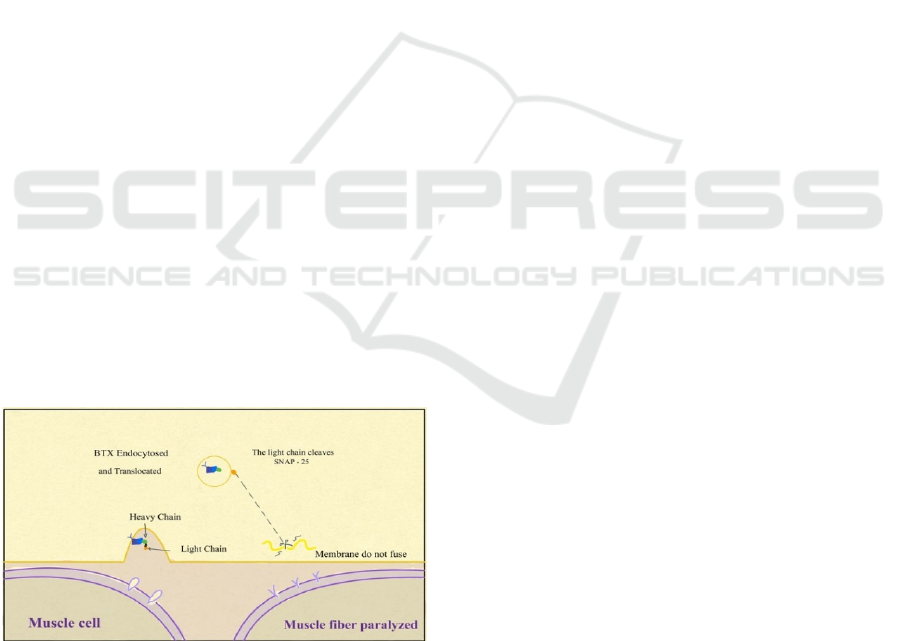
3.2 Mechanisms of BTX on the
Treatment of CP
BTX injection can reduce muscle spasms. It works
as an inhibitor. BTX-A can bind to the presynaptic
membrane through gangliosides and protein
receptors and then is internalized into the endosome
through endocytosis. After this, the light chain is
transferred across the membrane to the cytosol,
where it acts as a specific endopeptidase against any
SNARE (Soluble N-ethylmaleimide-sensitive factor
attachment protein receptor) protein. BTX cleaves
its substrate before forming the SNARE complex.
After BTX-A is incorporated within the early
endosomes, the acidic environment of the
endocytosis vesicles is believed to induce a
conformational change in the neurotoxin structure.
The heavy chain is inserted into the synaptic vesicle
membrane to form a transmembrane protein-
conducting channel that translocates the light chain
into the cytosol. After BTX-A is internalized into the
cytosol of neurons, BTX-A exerts its toxic effect by
virtue of the metalloprotease activity of the light
chain. Light chains can specifically cleave three
kinds of soluble N-ethylmaleimide. One of the
imine-sensitive factor attachment protein receptor
SNARE proteins is an indispensable part of vesicle
transport and neurotransmitters. BTX-A specifically
cleaves SNAP-25 (Synaptosome-associated protein
of 25 kDa) at the unique peptide bond. By cutting
SNAP-25, BTX-A can block the transmission of
some nerve signals in the neurotransmitter and thus
act as an inhibitor. Using this specialty, BTX-A is
effective in the targeted treatment of CP's
uncontrollable muscle tremor, as shown in figure 1.
Figure 1: Mechanism of BTX. Mechanism of BTX light
chain cleave SNAP-25 then cause muscle membrane do
not fuse and the muscle will not be going to be fiber
paralyzed.
BTX type A has two different types under the
serotype classification, and they could be named by
A1LL and A2NTX. According to the article by
Norio Akaike et al., when the newly developed
BTX-A2 (A2NTX) was injected into the foreleg
muscle of a rat, it was transported to the
contralateral muscle. This finding is consistent with
the retrograde propagation of neurotoxins through
spinal cord neurons and then through motor neurons
across synapses to reach the contralateral motor
neurons in the spinal cord and reach the soleus
muscle. The muscle relaxation on the ipsilateral side
of the injected toxin was faster and stronger in
A2NTX-treated rats than A1LL. This is because
A1LL is transported to the contralateral muscle
almost equally through nerve pathways and blood
flow. A2NTX is mainly delivered to the
contralateral muscle through blood. A1LL is more
successfully transported to the contralateral spinal
neuron than A2NTX. From this, by comparing that
A1LL can be transported faster compared to
A2NTX. However, the actual use effect of A2NTX
may be better than that of A1LL. In an article,
people experimented with A1LL could cause a
decrease in the grip strength of rats’ foreleg, but the
A2NTX could not make that. Therefore, the actual
use effect of BTX-A1 will be better than BTX-A2.
3.3 Clinical Treatment Result
Pl After it was found that BTX could be a promising
medicine for CP patients, researchers almost head to
clinical trial directly. That is because BTX has been
used to treat different diseases regarding to
hypertonia for decades, beginning with the treatment
of strabismus in the 1970s. The long history of
utilizing BTX to treat hypertonia situations gives
physicians rich experiences to handle similar
situations. Also, the paralysis caused by BTX could
be long-lasting but reversible via the administration
of small amounts locally, making such therapy
relatively safe to be conducted.
Through years of application in the treatment of
CP patients, many clinical data have proven the
effectiveness of BTX injection therapy. In 2009, an
article conducted by Lukban and colleagues revied
cases of class I and class II CP patients, involving
115 children with spasticity of upper limb and 360
children with spasticity of lower limbs. After
reviewing the database of such, it concludes that
these data provide growing evidence for the
effectiveness of BTX treatment in reducing
spasticity degrees and could provide a time-limited
improvement of function in the upper and lower
limbs for children with CP. Another study conducted
in 2020 also concludes that BTX therapy has
significant improvement in both clinical and
Botulinum Toxin’s using in Treatment of Cerebral Palsy
663

functional outcomes by utilizing different systems to
measure functional gains. Through the utilizing of
manual ability classification system (MACS) and
Canadian occupational performance measure
(COPM), such study provides clear and convincing
evidence that BTX therapy is one of the most
important treatments for CP patients.
Even though some research concluded that BTX
treatment is not effective, this research may
overlook certain key points. In 2020, an article
published by Farag and colleagues denied the
effectiveness of BTX in the field of CP; it reports
that although there is a positive effect for spasticity
degrees after BTX injection treatment for upper
limbs spasticity in children, the effect with respect to
the function gains or improvement of quality of life
remained insignificant or conflicting. Such
conclusion seems to be convincing, but researchers
actually overvalued what BTX really supposed to
do. What BTX treatment could do is to reduce the
spasticity degrees for the patients, which does not
necessarily mean that could improve the life quality
or function situations of patients. Rehabilitation
training like walking and running should really take
charge of improving patients’ quality of life. For CP,
it is not that a single therapy or medicine could cure
the diseases on its own, it has to be the way that
many therapies are used together alone the way. For
these patients who could not walk or run because of
hypertonia, BTX therapy could eliminate the
hypertonia situation as much as possible; however,
for these who have never walk or run for their entire
life, it is fair to assume that they could not gain the
function even without hypertonia situation, which
means that there should be someone here teaching
them how to do that, and rehabilitation treatment
would be “the person” to do that. In conclusion,
BTX therapy grants patients the possibility to walk
or run, but it does not teach them how to walk or
run. In this article, the result has already proved the
effectiveness of BTX therapy, and the analysis on
functional gains and quality of life seems to be too
far ahead.
As mentioned previously, for treatment of CP, it
is not that a single therapy or medicine could cure on
its own; most of the time, a combination of therapies
is so important that it could directly affect the result
from experiments. In 2019, a trail performed by
Cahlin and colleagues led to the conclusion that the
effect of BTX-A compared with placebo on outcome
variables was unsignificant at the group level and
the evidence could not prove the used of BTX-A as
a therapy of affected masticatory muscle in CP.
While in 2017, a study conducted by Dursun and
colleagues regarding to the treatment of spastic
equinus foot due to CP leads to the conclusion that
BTX-A injection treatment with physical therapy
provided additional benefit for the patients. One
significant difference between these two
experiments is that the latter experiment conducts
more than BTX treatments but also physical therapy
along the way. More importantly, even though the
former experiment leads to the conclusion that there
is no objective improvement, researchers reported
that most patients request for continuing BTX
injection treatment, implying that there is a subject
effect on patients. One plausible explanation would
be that patients feel better while undergoing other
treatments after BTX eliminates the hypertonia
situation. Through the comparison, the importance
of the combination of different therapies has been
revealed.
Above all, many clinical research have proven
the effectiveness of BTX therapy as a treatment for
CP patients. Although some research may provide
contradictory evidence, there are certain points that
we should be aware of, and further research are
needed. One example here would be the
combination of different treatments with BTX
therapy, and such a topic should be something
researchers want to focus on in future studies.
4 CONCLUSIONS
In conclusion, here provides information for how CP
affects infant and a flowchart for how BTX therapy
could be a treatment to eliminate such effect. There
are convincing evidences used from other articles
that BTX therapy could be one of the most important
treatments for CP patients. Evidence shows that
there are some opposed conclusions could be driven
by overlooking certain key points and combining
BTX therapy with other therapy like physical
therapy or hydrotherapy is the best way to monitor.
For future research, the study design for BTX
therapy should treat it as supportive therapy and
involve it with other therapy in order to produce the
maximum positive effect for the patients because it
is too demanding to test whether a supportive
therapy could play an important role alone. Also,
more research should be done to discover which
therapy is the best for the BTX therapy company.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
664

REFERENCES
Aisen, Mindy Lipson et al., (2011). “Cerebral palsy:
clinical care and neurological rehabilitation.” The
Lancet. Neurology vol. 10,9
Akaike N, Shin MC, Wakita M, Torii Y, Harakawa T,
Ginnaga A, Kato K, Kaji R, Kozaki S., (2013).
Transsynaptic inhibition of spinal transmission by A2
botulinum toxin. J Physiol.;591(4):1031-43.
CDC, “What is Cerebral Palsy?”
Singh BR., (2006). Botulinum neurotoxin structure,
engineering, and novel cellular trafficking and
targeting. Neurotox Res.; 209:73–92.
Cahlin BJ, Lindberg C, Dahlström L., (2019). Cerebral
palsy and bruxism: Effects of botulinum toxin
injections-A randomized controlled trial. Clin Exp
Dent Res.;5(5):460-468.
Dursun N, Gokbel T, Akarsu M, Dursun E., (2017).
Randomized Controlled Trial on Effectiveness of
Intermittent Serial Casting on Spastic Equinus Foot in
Children with Cerebral Palsy After Botulinum Toxin-
A Treatment. Am J Phys Med Rehabil.;96(4):221-225.
Dong M, Yeh F, Tepp WH, et al., (2006). SV2 is the
protein receptor for botulinum neurotoxin A. Science.;
312(5773):595–596.
Dressler D, Saberi FA, Barbosa ER., (2005). Botulinum
toxin: mechanisms of action. Arq Neuropsiquiatr.;
63(1):180-5.
Farag SM, Mohammed MO, El-Sobky TA, ElKadery NA,
ElZohiery AK., (2020). Botulinum Toxin A Injection
in Treatment of Upper Limb Spasticity in Children
with Cerebral Palsy: A Systematic Review of
Randomized Controlled Trials. JBJS Rev. 8(3): e0119.
Lukban MB, Rosales RL, Dressler D., (2009).
Effectiveness of botulinum toxin A for upper and
lower limb spasticity in children with cerebral palsy:
a summary of evidence. J Neural Transm (Vienna).;
116(3):319-31.
Montal M., (2010). Botulinum neurotoxin: a marvel of
protein design. Annu Rev Biochem.; 79:591–617.
Moreau NG, Bodkin AW, Bjornson K, Hobbs A, Soileau
M, Lahasky K., (2016). Effectiveness of
Rehabilitation Interventions to Improve Gait Speed in
Children with Cerebral Palsy: Systematic Review and
Meta-analysis. Phys Ther.; 96(12): 1938-1954.
Palisano, R et al., (1997). “Development and reliability of
a system to classify gross motor function in children
with cerebral palsy.” Developmental medicine and
child neurology vol. 39,4
Palisano, R. J., et al., (2007). "GMFCS—Expanded &
Revised© 2007." Can Child Centre for Childhood
Disability Research, McMaster University
Park J, Park HJ., (2017). Botulinum Toxin for the
Treatment of Neuropathic Pain. Toxins (Basel).
2017;9(9):260.
Torii Y, Akaike N, Harakawa T, Kato K, Sugimoto N,
Goto Y, Nakahira S, Kohda T, Kozaki S, Kaji R,
Ginnaga A., (2011). Type A1 but not type A2
botulinum toxin decreases the grip strength of the
contralateral foreleg through axonal transport from the
toxin-treated foreleg of rats. J Pharmacol
Sci.;117(4):275-85.
Thenganatt, M.A., Fahn, S., (2012). Botulinum Toxin for
the Treatment of Movement Disorders. Curr Neurol
Neurosci Rep 12, 399–409
Yadav S, Chand S, Majumdar R, Sud A., (2020). Effect
of botulinum toxin type-A in spasticity and functional
outcome of upper limbs in cerebral palsy. J Clin
Orthop Trauma.; 11(2):208-212.
Botulinum Toxin’s using in Treatment of Cerebral Palsy
665
