
Combination Therapies of Metastatic Melanoma and Melanoma
Brain Metastases
Jiarui Jiang
a
Department of Biology, University of Washington, Seattle, Washington, U.S.A.
Keywords: Immune Checkpoint Inhibitors, Combination Therapies, Metastatic Melanoma, Melanoma Brain
Metastases.
Abstract: Melanoma is a fatal cancer that develops in melanocytes and has the highest mortality rates in all types of
skin cancers. It generally starts as a primary tumor, spreads to adjacent lymph nodes, migrates to distant
sites of body through bloodstream, and becomes metastatic in this case. Melanoma brain metastases occur
when melanoma cancer cells disperse to the brain. However, compared to the survival rate of melanoma that
is localized (stage I, II) or regional (stage III), the survival rate in metastatic melanoma (stage IV) decreases
a lot, and it is imperative to find an effective treatment to prolong the survival. As immunotherapy has been
developed, one of the methods, checkpoint inhibitors provide higher overall survival and more enduring
objective response, which can be both used as monotherapies and combination therapies. In this review, we
will focus on the combination of checkpoint inhibitors as an impressive therapy for metastatic melanoma
and melanoma brain metastases and summarize its effect on the overall survival and response rate in
different clinical trials. The combination of radiotherapy and checkpoint inhibitors for treating melanoma
brain metastases is also explored. These combination therapies serve as potent treatments of metastatic
melanoma and its brain metastases.
1 INTRODUCTION
1
Ultraviolet rays (UV) from the sunlight are the
major cause of melanoma. Excessive sunlight
exposure leads to mutations in DNA in the skin
cells, and the mutation in the BRAF oncogene is the
most common one that found in almost half of the
melanomas. NRAS, CDKN2A, and NF1 are the
other genes affected by UVs. When mutations occur,
they keep turning on the oncogenes and make
melanocytes grow and divide abnormally, which
stimulates them to become cancerous (What Causes
Melanoma?, n.d.).
Melanoma develops from a primary tumor and
then propagate to distant sites of the body, such as
lymph nodes, the skin, brain, lungs, bones, and liver,
and such condition is called metastatic melanoma.
Symptoms are shown when there are metastases in
distant sites of the body. Melanoma is lethal, and
metastatic melanoma is the one that has extremely
poor survival rate in contrast to the melanoma that is
localized. Conventionally, surgery was utilized when
a
https://orcid.org/0000-0002-9651-0843
the tumor is dispersed to only one or several sites of
the body, and they were resectable. However, for
metastatic melanoma, surgery is impotent, and the
treatments of chemotherapy, radiotherapy, and
immunotherapy have become more appropriate.
Chemo drugs are applied in chemotherapy for
slowing the growth of cancer cells, such as
dacarbazine (DTIC) and temozolomide (Temodar).
Radiotherapy is given when the tumors are in the
location that can’t be moved easily, such as brain, or
in many sites of the body, and it is able to shrink
tumors. As the field of immunotherapy advances, it
has become the preferred treatment for metastatic
melanoma. Immune checkpoint inhibitors (ICI),
ipilimumab and nivolumab, are one of them that are
helpful and provide improved survival and
prolonged responses. Nivolumab and
pembrolizumab are anti-PD-1 checkpoint inhibitors,
which both can be employed as monotherapies or in
combination with ipilimumab. Ipilimumab is an anti-
CTLA-4 immune checkpoint inhibitor that triggers
the immune system to attack melanoma cells, but it
is less efficient than nivolumab and pembrolizumab
when it is using alone. Many studies have stated the
686
Jiang, J.
Combination Therapies of Metastatic Melanoma and Melanoma Brain Metastases.
DOI: 10.5220/0011255600003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 686-693
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

beneficial effects of using ipilimumab or nivolumab
monotherapy as treatments for metastatic cancers.
However, these monotherapies are not effective for
all patients with metastatic melanoma and melanoma
brain metastases. Therefore, in this review, we will
not only discuss the combination of nivolumab and
ipilimumab for treating metastatic uveal melanoma
and mucosal melanoma and its effect on patients in
clinical trials, but also explore the effect of
radiotherapy plus anti-PD-1 inhibitors or anti-
CTLA-4 inhibitors for treating melanoma brain
metastases.
2 MELANOMA
Melanoma is the deadliest form of skin cancer, and
it is brought about by the malignant tumor derive
from melanocytes which is a type of cell derived
from neural crest stem cells (NCSCs) that produces
and contains a UV absorbing pigment called
melanin. There are two forms of melanin generated
by melanocytes. One is, a black or brown pigment,
eumelanin. Since this pigment is dark, it serves as a
great shield for UV radiation, and that is why people
with darker skin have lower risk of skin cancer.
Indeed, the risk of getting skin cancer is related to
the color of skin, hair, and eyes. People who have
light skin, blond or red hair, and light eyes has
higher risk of gaining skin cancer than those with
darker ones. The other pigment is called
pheomelanin, which is red or yellow. Compared to
eumelanin, it offers less protection against UV
radiation, and the yielding of pheomelanin
stimulates the advent of cancer. This is due to
pheomelanin give more ultraviolet-A-induced
reactive oxidative species (ROS) which results in
more DNA damage reacting to UV radiation (Davis
et al., 2019).
2.1 Mortality Rates, Pathology, and
Clinical Manifestations of
Melanoma
Skin cancer is one of the most common cancers.
Although melanoma only occupies 1% of the skin
cancer, more than 75% mortalities was induced by it
among all types of skin cancers. According to
National Cancer Institute and SEER Database, there
are approximately 106,110 new cases of melanoma
in 2021, and 7,180 people will die due to this disease
(Melanoma of the Skin - Cancer Stat Facts, n.d.).
Melanoma is caused by the mutations in DNA which
are induced by excessive sun exposure. It can be
metastatic when melanocytes become malignant and
start to disperse to distant body parts, including soft
tissues (skin, muscle, and lymph nodes) and major
organs (liver, lung, and brain) (DiCaprio et al., 2020;
Rebecca et al., 2020). The first step of metastases is
to disseminate from the primary tumor to nearby
lymph nodes, and then the cancer cells enter the
bloodstream and travel to other body parts to form
new tumors (Melanoma Cells That Pass through
Lymph More Likely to Spread - National Cancer
Institute, 2020). Symptoms of melanoma that has
spread to other sites of body, for example, are
hardened lumps under skin, swollen or painful
lymph nodes, trouble breathing, bone pain, and
swelling of liver (Peri, n.d.). The survival rate of
metastatic melanoma is much lower than that of
melanoma that doesn’t spread based on statistics
from Cancer.Net, which drops from 80% to 27% in
metastatic melanoma (Melanoma - Statistics, 2012).
2.2 Conventional Treatments of
Melanoma
Conventional melanoma treatments include surgery,
chemotherapy, and radiation therapy. However,
surgery is not robust enough to treat metastatic
melanoma due to too many sites of tumors
throughout the body. Chemotherapy and
radiotherapy are more useful as the treatments for
metastatic melanoma. As Table 1 showed, there are
six chemotherapeutic drugs that are primarily used.
Dacarbazine (DTIC) was granted by Food and Drug
Administration (FDA) in 1975. However, when it
works as a single agent, it has poor efficacy on
treating metastatic melanoma, thus it doesn’t have
much effect on increasing the OS of patients. The
other chemotherapeutic drug temozolomide is a
substitute of DTIC and can be taken orally. There is
no significant difference on the effect of utilizing
DTIC and temozolomide. The major problem that
induces such low effectiveness is that melanoma
cells are intrinsically resistant to chemotherapy
(Mishra et al., 2018). Radiotherapy is mainly
utilized when melanoma spreads to the brain, which
is effective in shrinking the tumors. For melanoma
brain metastases, one of the radiotherapies called
stereotactic radiation therapy applies to only parts of
the brain that have tumor while preventing it from
damaging the surrounding normal brain cells
(Patient Education: Melanoma Treatment;
Advanced or Metastatic Melanoma (Beyond the
Basics) - UpToDate, n.d.). As the field of
immunology gets developed, the better
understanding of immune systems leads to a novel
Combination Therapies of Metastatic Melanoma and Melanoma Brain Metastases
687
method of treating metastatic cancers, which is
employing immune cells against cancers.
Table 1: The Chemo Drugs that Used to Treat Metastatic
Melanoma. (Commissioner, 2021).
Drug Name (Brand
Name
)
Years Approved by FDA
Dacarbazine (DTIC) May 1975 and January
1998
Temozolomide
(Temodar)
August 11th, 1999
Nab-paclitaxel
(
Abraxane
)
January 2005
Paclitaxel (Abraxane) January 25th, 2002
Cisplatin (Platinol) December 19th, 1978
Carbo
p
latin
(
Para
p
latin
)
Jul
y
14th, 2003
3 MONOTHERAPIES OF
MELANOMA
3.1 Nivolumab
Nivolumab is a fully human immunoglobulin (Ig)
G4 monoclonal antibody that binds to programmed
cell death protein 1(PD-1), a T-cell surface receptor,
and prevents it from being activated by the
programmed cell death ligand 1 (PD-L1) and
programmed cell death ligand 2 (PD-L2). Activated
PD-1 down regulates T-cell activation, helps tumors
evade from the immune attack, and promotes its
growth. By exploiting nivolumab, PD-1 remains
inactivated which enables T cell activation against
the tumors. Nivolumab is commonly used as a
remedy for melanoma, especially for the situation
when the tumor is unresectable or has metastasized.
(National Cancer Institute).
As a monotherapy, nivolumab improves the
objective response rate (ORR), progression free
survival (PFS), and overall survival (OS) compared
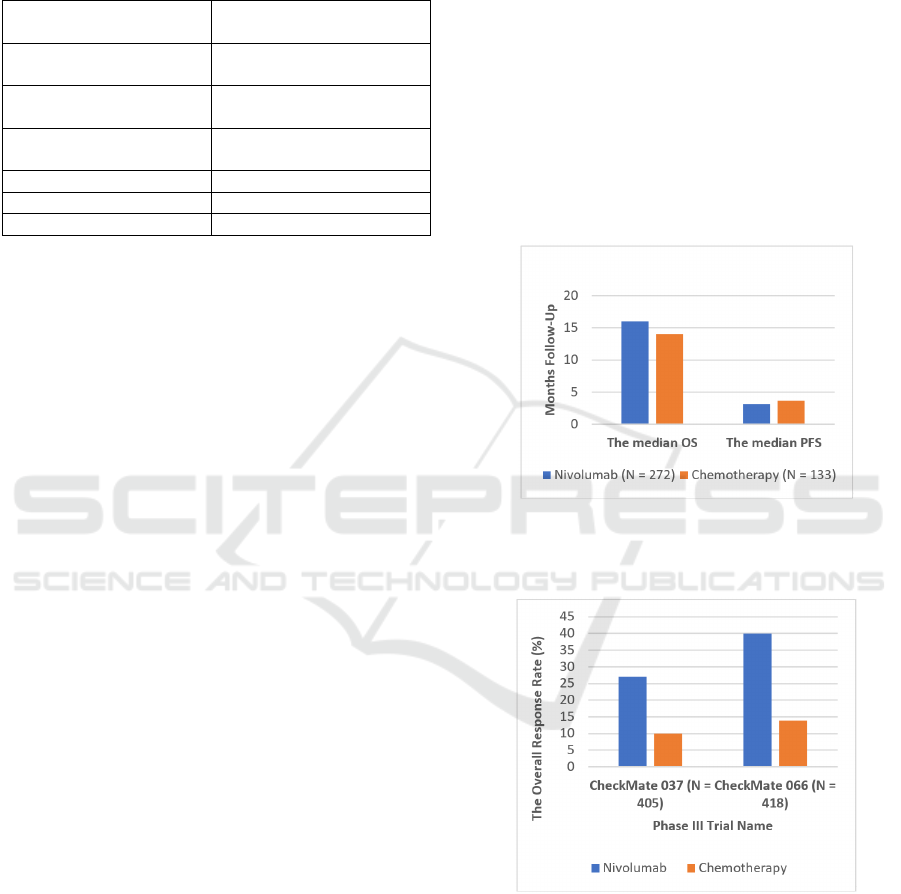
to chemotherapy. As Figure 1 showed, Larkin et al.
compared the overall survival in patients with
advanced melanoma using nivolumab versus
chemotherapy as a treatment in a phase III trial
(CheckMate 037). There were 272 patients treated
with nivolumab, and 133 patients were involved in
chemotherapy. The median OS was 16 months for
nivolumab and 14 months for chemotherapy. The
median PFS was 3.1 months in patients with
nivolumab and 3.7 months with chemotherapy.
Besides, the ORR was higher in nivolumab than in
chemotherapy (27% vs. 10%) (Larkin et al., 2018).
Another phase III trial, CheckMate 066, also
justified the increment in survival. There were 418
patients with metastatic melanoma, the ORR was
40% in those who had nivolumab and 13.9% for
patients with dacarbazine, a chemotherapy drug. The
median PFS was 5.1 months in nivolumab and 2.2
months in people with chemotherapy (O’Reilly &
Larkin, 2017). Figure 2 demonstrated that the overall
response rate was higher in melanoma patients
treated with nivolumab than those of treated with
chemotherapy in both phase III trials. Due to the
nivolumab’s durable response, this indication has
been approved by authorities, such as Food and
Drug Administration (FDA) and European Union
(EU). On December 20, 2017, nivolumab was
approved by FDA as a treatment for melanoma.
(Food and Drug Administration). Nivolumab was
also granted for treating advanced melanoma in
adults in EU. (European Medicines Agency).
Figure 1: The Overall Survival of Advanced Melanoma
Patients Using Nivolumab vs. Chemotherapy in
CheckMate 037.
Figure 2: The Overall Response Rate (ORR) of Melanoma
Patients Treating with Nivolumab or Chemotherapy in
CheckMate 037 vs. CheckMate 066.
3.2 Ipilimumab
Ipilimumab is a recombinant human
immunoglobulin (Ig) G1 monoclonal antibody that
targets cytotoxic T-lymphocyte-associated antigen 4
(CTLA-4), a surface receptor on T-cell. Since
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
688
CTLA-4 down regulates the immune system,
ipilimumab binds to and inhibits it in order to
activate the immune responses. Ipilimumab also
shuts down the inhibitory mechanisms on cytotoxic
T lymphocytes (CTLs), which kill cancer cells, thus
immune responses against cancer are boosted up.
Ipilimumab can be favorable for patients who have
had surgery for removing melanoma, in which it can
work as an adjuvant therapy. Similar to nivolumab,
it can also be effective for adults who have
metastatic melanoma. (National Cancer Institute).
In contrast to nivolumab monotherapy,
ipilimumab monotherapy has shorter PFS and
ORRs. In a phase III trial, CheckMate 238, there
were 906 patients who were undergone IV
melanoma. At 18 months follow-up, the 12 months
recurrence-free survival was 70.5% in patients with
nivolumab and 60.8% in ipilimumab (Weber et al.,
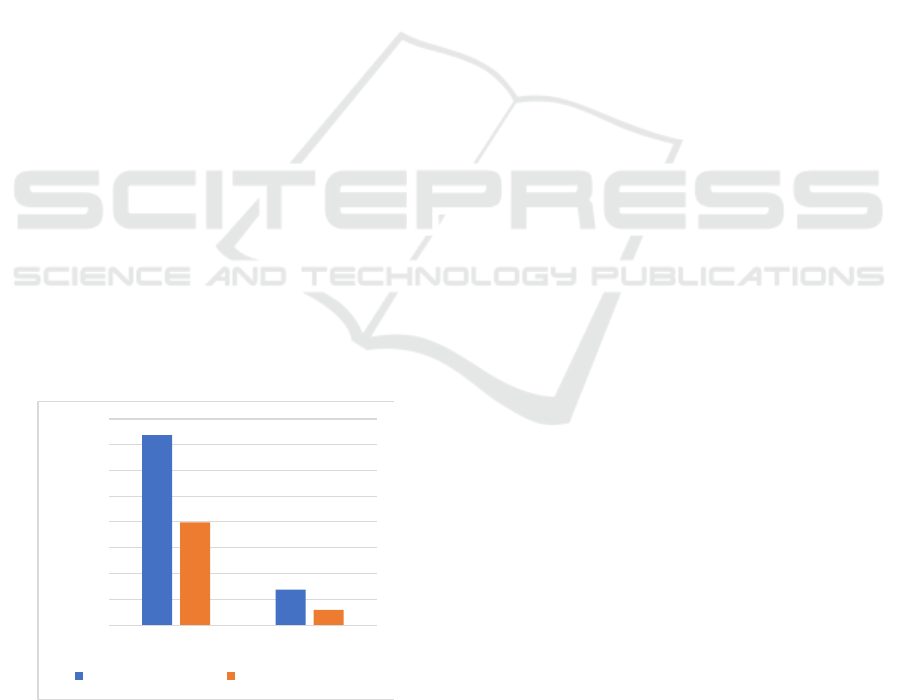
2017). As Figure 3 displayed, a phase III trial
(CheckMate 067) conducted by Hodi et al. also
proved that nivolumab had greater overall survival
than ipilimumab. There were 945 patients involved
in the study. Three hundred and sixteen patients
were assigned with nivolumab, 315 patients were
given ipilimumab, and other patients gained
nivolumab plus ipilimumab. The median OS was
36.9 months in the nivolumab group and 19.9
months in the ipilimumab group. The median PFS
was 6.9 months in nivolumab while the ipilimumab
group was much lower (2.9 months) (Hodi et al.,
2018). Ipilimumab was approved by FDA as a
therapy for melanoma prior to nivolumab. (Food and
Drug Administration). Many recent studies
illustrated that nivolumab plus ipilimumab as first-
line therapy can achieve a more durable and
continuous survival benefit in patients with
advanced melanoma.
Figure 3: The Overall Survival of Advanced Melanoma
Patients Treated with Nivolumab vs. Ipilimumab in
CheckMate 067.
4 COMBINATION OF
ANTI-CTLA-4 ANTIBODY AND
ANTI-PD-1 ANTIBODY
To minimize adverse events and increase the
response rate using immunotherapies, it is vital to
discover decent checkpoints to adjust the immune
response and monoclonal antibodies that target the
checkpoints. CTLA-4 and PD-1 are the two potent
checkpoints, and there are drugs that are developed
modulate the effect of CTLA-4 and PD-1 for
treating metastatic melanoma, including CTLA-4
blockers ipilimumab, PD-1 blockers nivolumab, and
PD-1 blockers pembrolizumab (Rotte, 2019). The
two antibodies ipilimumab and nivolumab are
approved on December 22, 2014, by Food and Drug
Administration (FDA) for treating metastatic
melanoma (Hazarika et al., 2017). Anti-CTLA-4 and
anti-PD-1 antibodies can generate durable response
and their adverse events are manageable. However,
these advantages can’t be demonstrated when
utilizing either CTLA-4 blockers or PD-1 blockers
as monotherapies. Patients with metastatic
melanoma are rarely respond to monotherapy (Rotte,
2019). In this case, combination therapies become
significance because they activate anti-tumor
response, increase response rates, and provide rapid
and considerable tumor regression. There are many
clinical studies focused on combination therapies for
treating melanoma. For instance, when phase I
studies received the combination of nivolumab and
ipilimumab, they displayed that tumor regression
appeared in approximately 50% patients with
metastatic melanoma, and 85% of them still survive
after 1-year treatment (Koppolu, n.d.).
4.1 CTLA-4 Inhibitor Plus PD-1
Inhibitor for Metastatic Uveal
Melanoma and Mucosal Melanoma
The addition of CTLA-4 inhibitor and PD-1
inhibitor can also be useful in treating subtypes of
melanoma. For example, it can be impressive for
metastatic uveal melanoma. Uveal melanoma arises
from melanocytes in the iris. It is very rare, and only
five of one million people getting this type of
melanoma each year (Afzal et al., 2018).
Traditionally, metastatic uveal melanoma (MUM)
was treated with chemotherapy alone, but most
patients had poor response or no response to it. In a
systematic review, 841 patients from 40 different
reports were investigated. The overall response rate
(ORR) is only 4.6%, and there were 22 studies that
demonstrated no response to chemotherapy (Buder
0
5
10
15
20
25
30
35
40
The median OS The median PFS
Months
Nivolumab (N = 316) Ipilimumab (N = 315)
Combination Therapies of Metastatic Melanoma and Melanoma Brain Metastases
689
et al., 2013). Compared to employing chemotherapy
alone, the efficacy of combining nivolumab and
ipilimumab on treating MUM is greater based on the
data listed in the prospective phase II GEM1402 trial
(NCT02626962). The ORR was 12%, the median
progression free survival (PFS) was 3.3 months, and
the median OS was 12.7 months (Piulats Rodriguez
et al., 2018). Heppt et al. also analyzed the effect of
utilizing both PD-1 inhibitors only and the
combination of ipilimumab and PD-1 inhibitors in a
clinical trial with 96 MUM patients. For PD-1
inhibitor monotherapy, pembrolizumab was given in
54 patients (25 females and 29 males), and
nivolumab was in 32 patients (13 females and 19
males). The ORR was 4.7% with four patients
demonstrated a PR, and there was no complete
response observed. The median PFS was 3.1 months
in patients with pembrolizumab and 2.8 months with
nivolumab. The median OS was 14.0 months for
pembrolizumab and 10.0 months for nivolumab.
Combining ipilimumab and PD-1 inhibitors leads to
higher ORR compared to monotherapy. Fifteen
patients were investigated, and twelve patients
developed response. There was still no complete
response and only two PRs. The ORR was 16.7%,
which is much larger than the 4.7% ORR in
monotherapy (Heppt et al., 2017).
The potency of the combination of ipilimumab
and nivolumab is also compelling in treating patients
with mucosal melanoma (MM). It is a rare and
aggressive disease with insufficient prognosis. Only
1.5 per million people every year are diagnosed with
MM. The common sites for MM are head and neck
(41%). The cutting of surgery is the primary method
for head and neck MMs, and radiotherapy is always
applied for local control after the surgery (Ascierto
et al., 2017). Even though immune checkpoint
inhibitors have become a promising option for MMs,
the clinical use for it still remains inadequate (Li et
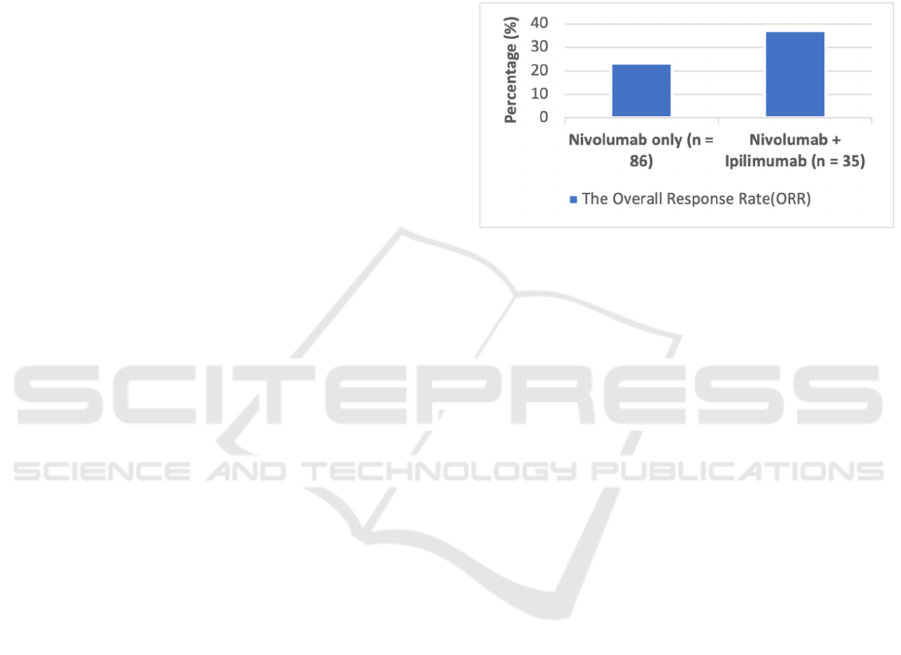
al., 2020). In a pooled analysis, Sandra et al.
reported the competence of nivolumab alone and the
mix with ipilimumab. In clinical studies, 889
patients were received only nivolumab. Among
them, 86 (10%) were mucosal melanoma patients
and 665 (75%) had cutaneous melanoma. For the
addition of nivolumab and ipilimumab, there were
35 mucosal melanoma patients and 326 patients with
cutaneous melanoma. The outcome illustrated that
the median PFS was 3.0 months, and Figure 4
showed that the ORR was 23.3% for MM patients
who accepted nivolumab monotherapy. Combining
nivolumab and ipilimumab manifested a higher
median PFS in patients that was 5.9 months. As
Figure 4 displayed, the ORR was also boosted to
37.1% (D’Angelo et al., 2017). The performance of
the combo seems to be more superior than that of the
agent using alone. Another case report by Fujimura
et al. confirmed the efficiency of this combination
method again. The patient was an 81-year-old
Japanese woman who had anti-PD-1 Ab-resistant
recurrent malignant melanoma of nasal cavity. They
found out that denosumab could enhance the anti-
tumor effects of incorporating nivolumab and
ipilimumab and successfully resolve the advanced
anti-PD-1 Ab-resistant mucosal melanoma by
blending denosumab, nivolumab, and ipilimumab as
a second-line therapy (Fujimura et al., 2020).
Figure 4: The Overall Response Rate in Mucosal
Melanoma Patients Treated with Nivolumab Monotherapy
vs. Nivolumab and Ipilimumab Combination Therapy.
5 COMBINED TREATMENTS
FOR MELANOMA BRAIN
METASTASES
Approximately 20% patients with cancer will
develop brain metastases. Melanoma brain
metastases (MBM) is one of the major ones (7% -
16%) (Berghoff et al., 2016). Brain metastases occur
when tumor cells disperse from a primary tumor
through the blood to the brain microvasculature. The
microenvironment of the brain microvasculature
bolsters the growth of tumors. Among different
ethnic groups, African Americans who are 50 to 59-
year-old with melanoma tended to have larger
probability getting brain metastases. For various
subsets of brain metastases, people with melanoma
have the highest likelihood of obtaining
leptomeningeal metastases, the one in the lining of
brain or spine (Achrol et al., 2019).
Treatments for brain metastases include surgery
if it is in a surgically accessible region, whole brain
radiotherapy (WBRT) for multiple symptomatic
brain metastases, stereotactic radiosurgery (SRS)
treating metastatic lesions without damaging the
surrounding brain tissues, systemic therapy that
conjugates with local therapy and provides greater
intracranial control, cytotoxic therapy, targeted
therapy, and immunotherapy (Rishi & Yu, 2020).
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
690

5.1 Nivolumab Plus Ipilimumab
Treatment
Within immunotherapy, the combination of
nivolumab and ipilimumab presented an essential
efficaciousness in patients who had melanoma brain
metastases. According to a phase II study
(NCT02320058) with 94 melanoma patients who
had at least one brain metastasis (tumor diameter 0.5
to 3 cm). The trial indicated that the intracranial
clinical benefit rate (CBR) was 57%, the complete
response was 26%, and partial response was 30%.
This study affirmed that adding nivolumab and
ipilimumab was impressive for melanoma patients
who had untreated brain metastases (Combined
Nivolumab and Ipilimumab in Melanoma Metastatic
to the Brain | NEJM, n.d.). Recently, this phase II
study (NCT02320058) updated their data for
asymptomatic patients. The intracranial CBR was
58.4% for 101 asymptomatic patients, and the PFS
and OS were still investigating. Due to the reliable
clinical benefits in asymptomatic MBM patients,
nivolumab plus ipilimumab was considered as the
first-line therapy (Tawbi et al., 2021).
5.2 Radiotherapy Plus PD-1 or
CTLA-4 Blockers
Moreover, except for conjoining the immune
checkpoint inhibitors, stereotactic radiosurgery
(SRS) along with nivolumab or ipilimumab can also
serve as a way for patients with MBM. Giuseppe et
al. examined the adequacy of concurrent SRS plus
nivolumab or ipilimumab for patients with untreated
MBM. Eighty patients with 326 MBM were engaged
in the study. There were 45 patients gained the SRS
and ipilimumab, and 35 patients got SRS and
nivolumab. The median follow-up was 15 months.
Among the 45 patients with SRS and ipilimumab, 32
of them (71%) obtained an intracranial progression
event while 20 patients (57%) in the SRS and
nivolumab group got such advancement. The median
OS was 22.0 months for the SRS and nivolumab
group and 14.7 months in patients with SRS and
ipilimumab. Besides, the 12-month and 24-month
survival probabilities were 78% and 42% for the
SRS and nivolumab patients. Compared to that, the
SRS and ipilimumab group had lower rate, which
were 68% and 20% individually. Both groups
exhibited valid intracranial activities, and the combo
of SRS and nivolumab had more excellent
intracranial control (Minniti et al., 2019).
6 CONCLUSIONS
Combination therapies of immune checkpoint
inhibitors have the bright future for treating
metastatic melanoma and melanoma brain
metastases. As I mentioned before, immune
checkpoint inhibitors, anti-PD-1 and anti-CTLA-4
inhibitors target the checkpoint proteins and turn on
the immune response against the melanoma cells.
However, no matter checkpoint inhibitors are used
as individual therapy or combing with one another,
all of them can generate immune-related adverse
events (irAEs), which can be life-threatening. For
ICIs that combines with another existing therapy,
this may cause severe immune related adverse
events happen more frequently. For instance,
therapies that involve anti-CTLA-4 inhibitors have
higher incidence of irAEs occurring in the
gastrointestinal tract, renal system, and endocrine
system. Most irAEs of anti-PD-1 therapies appear at
endocrine system, gastrointestinal tract,
musculoskeletal system, and hepatobiliary system.
Thus, it is necessary for future research to identify
predictive biomarkers for irAEs which can not only
help patients with metastatic melanoma and select
the therapy that has optimal benefits, but also avoid
the toxicities. There’s also a need for next generation
research to investigate and evaluate the drugs that
can improve the response to ICIs, such as microbiota
modifiers, drugs targeting co-inhibitory receptors,
and oncolytic viruses.
REFERENCES
Achrol, A. S., Rennert, R. C., Anders, C., Soffietti, R.,
Ahluwalia, M. S., Nayak, L., Peters, S., Arvold, N. D.,
Harsh, G. R., Steeg, P. S., & Chang, S. D. (2019).
Brain metastases. Nature Reviews Disease Primers,
5(1), 1–26. https://doi.org/10.1038/s41572-018-0055-y
Afzal, M. Z., Mabaera, R., & Shirai, K. (2018). Metastatic
uveal melanoma showing durable response to anti-
CTLA-4 and anti-PD-1 combination therapy after
experiencing progression on anti-PD-1 therapy alone.
Journal for Immunotherapy of Cancer, 6, 13.
https://doi.org/10.1186/s40425-018-0322-1
Ascierto, P. A., Accorona, R., Botti, G., Farina, D.,
Fossati, P., Gatta, G., Gogas, H., Lombardi, D.,
Maroldi, R., Nicolai, P., Ravanelli, M., & Vanella, V.
(2017). Mucosal melanoma of the head and neck.
Critical Reviews in Oncology/Hematology, 112, 136–
152. https://doi.org/10.1016/j.critrevonc.2017.01.019
Berghoff, A. S., Schur, S., Füreder, L. M., Gatterbauer, B.,
Dieckmann, K., Widhalm, G., Hainfellner, J.,
Zielinski, C. C., Birner, P., Bartsch, R., & Preusser, M.
(2016). Descriptive statistical analysis of a real life
cohort of 2419 patients with brain metastases of solid
Combination Therapies of Metastatic Melanoma and Melanoma Brain Metastases
691

cancers. ESMO Open, 1(2), e000024.
https://doi.org/10.1136/esmoopen-2015-000024
Buder, K., Gesierich, A., Gelbrich, G., & Goebeler, M.
(2013). Systemic treatment of metastatic uveal
melanoma: Review of literature and future
perspectives. Cancer Medicine, 2(5), 674–686.
https://doi.org/10.1002/cam4.133
Combined Nivolumab and Ipilimumab in Melanoma
Metastatic to the Brain | NEJM. (n.d.). Retrieved
October 1, 2021, from
https://www.nejm.org/doi/10.1056/NEJMoa1805453
Commissioner, O. of the. (2021, September 22). U.S.
Food and Drug Administration. FDA; FDA.
https://www.fda.gov/home
D’Angelo, S. P., Larkin, J., Sosman, J. A., Lebbé, C.,
Brady, B., Neyns, B., Schmidt, H., Hassel, J. C., Hodi,
F. S., Lorigan, P., Savage, K. J., Miller, W. H., Mohr,
P., Marquez-Rodas, I., Charles, J., Kaatz, M., Sznol,
M., Weber, J. S., Shoushtari, A. N., … Wolchok, J. D.
(2017). Efficacy and Safety of Nivolumab Alone or in
Combination With Ipilimumab in Patients With
Mucosal Melanoma: A Pooled Analysis. Journal of
Clinical Oncology, 35(2), 226–235.
https://doi.org/10.1200/JCO.2016.67.9258
Davis, L. E., Shalin, S. C., & Tackett, A. J. (2019).
Current state of melanoma diagnosis and treatment.
Cancer Biology & Therapy, 20(11), 1366–1379.
https://doi.org/10.1080/15384047.2019.1640032
DiCaprio, M. R., Abousayed, M. M., & Kambam, M. L.
R. (2020). Orthopaedic Manifestations of Melanoma
and Their Management. JAAOS - Journal of the
American Academy of Orthopaedic Surgeons, 28(13),
e540. https://doi.org/10.5435/JAAOS-D-18-00757
Fujimura, T., Kambayashi, Y., Ohuchi, K., Amagai, R.,
Sato, Y., Tanita, K., Hashimoto, A., & Aiba, S.
(2020). Successful Treatment of a Patient with anti-
PD1 Antibody-Resistant Advanced Mucosal
Melanoma with Nivolumab, Ipilimumab plus
Denosumab Combination Therapy. Case Reports in
Oncology, 13(1), 271–275.
https://doi.org/10.1159/000506327
Hazarika, M., Chuk, M. K., Theoret, M. R., Mushti, S.,
He, K., Weis, S. L., Putman, A. H., Helms, W. S.,
Cao, X., Li, H., Zhao, H., Zhao, L., Welch, J.,
Graham, L., Libeg, M., Sridhara, R., Keegan, P., &
Pazdur, R. (2017). U.S. FDA Approval Summary:
Nivolumab for Treatment of Unresectable or
Metastatic Melanoma Following Progression on
Ipilimumab. Clinical Cancer Research, 23(14), 3484–
3488. https://doi.org/10.1158/1078-0432.CCR-16-
0712
Heppt, M. V., Heinzerling, L., Kähler, K. C., Forschner,
A., Kirchberger, M. C., Loquai, C., Meissner, M.,
Meier, F., Terheyden, P., Schell, B., Herbst, R.,
Göppner, D., Kiecker, F., Rafei-Shamsabadi, D.,
Haferkamp, S., Huber, M. A., Utikal, J., Ziemer, M.,
Bumeder, I., … Berking, C. (2017). Prognostic factors
and outcomes in metastatic uveal melanoma treated
with programmed cell death-1 or combined PD-
1/cytotoxic T-lymphocyte antigen-4 inhibition.
European Journal of Cancer, 82, 56–65.
https://doi.org/10.1016/j.ejca.2017.05.038
Hodi, F. S., Chiarion-Sileni, V., Gonzalez, R., Grob, J.-J.,
Rutkowski, P., Cowey, C. L., Lao, C. D., Schadendorf,
D., Wagstaff, J., Dummer, R., Ferrucci, P. F., Smylie,
M., Hill, A., Hogg, D., Marquez-Rodas, I., Jiang, J.,
Rizzo, J., Larkin, J., & Wolchok, J. D. (2018).
Nivolumab plus ipilimumab or nivolumab alone
versus ipilimumab alone in advanced melanoma
(CheckMate 067): 4-year outcomes of a multicentre,
randomised, phase 3 trial. The Lancet Oncology,
19(11), 1480–1492. https://doi.org/10.1016/S1470-
2045(18)30700-9
Koppolu. (n.d.). Checkpoint immunotherapy by nivolumab
for treatment of metastatic melanoma. Retrieved
September 10, 2021, from
https://www.cancerjournal.net/article.asp?issn=0973-
1482;year=2018;volume=14;issue=6;spage=1167;epag
e=1175;aulast=Koppolu
Larkin, J., Minor, D., D’Angelo, S., Neyns, B., Smylie,
M., Miller, W. H., Gutzmer, R., Linette, G.,
Chmielowski, B., Lao, C. D., Lorigan, P., Grossmann,
K., Hassel, J. C., Sznol, M., Daud, A., Sosman, J.,
Khushalani, N., Schadendorf, D., Hoeller, C., …
Weber, J. (2018). Overall Survival in Patients With
Advanced Melanoma Who Received Nivolumab
Versus Investigator’s Choice Chemotherapy in
CheckMate 037: A Randomized, Controlled, Open-
Label Phase III Trial. Journal of Clinical Oncology,
36(4), 383–390.
https://doi.org/10.1200/JCO.2016.71.8023
Li, J., Kan, H., Zhao, L., Sun, Z., & Bai, C. (2020).
Immune checkpoint inhibitors in advanced or
metastatic mucosal melanoma: A systematic review.
Therapeutic Advances in Medical Oncology, 12,
1758835920922028.
https://doi.org/10.1177/1758835920922028
Melanoma Cells that Pass through Lymph More Likely to
Spread—National Cancer Institute
(nciglobal,ncienterprise). (2020, September 30).
[CgvBlogPost]. https://www.cancer.gov/news-
events/cancer-currents-blog/2020/melanoma-spread-
lymph-nodes-ferroptosis
Melanoma of the Skin—Cancer Stat Facts. (n.d.). SEER.
Retrieved October 10, 2021, from
https://seer.cancer.gov/statfacts/html/melan.html
Melanoma—Statistics. (2012, June 25). Cancer.Net.
https://www.cancer.net/cancer-
types/melanoma/statistics
Minniti, G., Anzellini, D., Reverberi, C., Cappellini, G. C.
A., Marchetti, L., Bianciardi, F., Bozzao, A., Osti, M.,
Gentile, P. C., & Esposito, V. (2019). Stereotactic
radiosurgery combined with nivolumab or Ipilimumab
for patients with melanoma brain metastases:
Evaluation of brain control and toxicity. Journal for
ImmunoTherapy of Cancer, 7(1), 102.
https://doi.org/10.1186/s40425-019-0588-y
Mishra, H., Mishra, P. K., Ekielski, A., Jaggi, M., Iqbal,
Z., & Talegaonkar, S. (2018). Melanoma treatment:
From conventional to nanotechnology. Journal of
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
692

Cancer Research and Clinical Oncology, 144(12),
2283–2302. https://doi.org/10.1007/s00432-018-2726-
1
O’Reilly, A., & Larkin, J. (2017). The safety of nivolumab
for the treatment of metastatic melanoma. Expert
Opinion on Drug Safety, 16(8), 955–961.
https://doi.org/10.1080/14740338.2017.1351537
Patient education: Melanoma treatment; advanced or
metastatic melanoma (Beyond the Basics)—
UpToDate. (n.d.). Retrieved October 11, 2021, from
https://www.uptodate.com/contents/melanoma-
treatment-advanced-or-metastatic-melanoma-beyond-
the-basics#H1
Peri, C. (n.d.). Metastatic Melanoma. WebMD. Retrieved
October 10, 2021, from
https://www.webmd.com/melanoma-skin-
cancer/metastatic-melanoma
Piulats Rodriguez, J. M., De La Cruz Merino, L.,
Espinosa, E., Alonso Carrión, L., Martin Algarra, S.,
López-Castro, R., Curiel García, M. T., Rodriguez
Abreu, D., Rullan Iriarte, A. J., & Berrocal Jaime, A.
(2018). 1247PD - Phase II multicenter, single arm,
open label study of nivolumab in combination with
ipilimumab in untreated patients with metastatic uveal
melanoma (GEM1402.NCT02626962). Annals of
Oncology, 29, viii443.
https://doi.org/10.1093/annonc/mdy289.003
Rebecca, V. W., Somasundaram, R., & Herlyn, M. (2020).
Pre-clinical modeling of cutaneous melanoma. Nature
Communications, 11(1), 2858.
https://doi.org/10.1038/s41467-020-15546-9
Rishi, A., & Yu, H.-H. M. (2020). Current Treatment of
Melanoma Brain Metastasis. Current Treatment
Options in Oncology, 21(6), 45.
https://doi.org/10.1007/s11864-020-00733-z
Rotte, A. (2019). Combination of CTLA-4 and PD-1
blockers for treatment of cancer. Journal of
Experimental & Clinical Cancer Research : CR, 38,
255. https://doi.org/10.1186/s13046-019-1259-z
Tawbi, H. A., Forsyth, P. A., Hodi, F. S., Lao, C. D.,
Moschos, S. J., Hamid, O., Atkins, M. B., Lewis, K.,
Thomas, R. P., Glaspy, J. A., Jang, S., Algazi, A. P.,
Khushalani, N. I., Postow, M. A., Pavlick, A. C.,
Ernstoff, M. S., Reardon, D. A., Puzanov, I.,
Kudchadkar, R. R., … Margolin, K. A. (2021). Safety
and efficacy of the combination of nivolumab plus
ipilimumab in patients with melanoma and
asymptomatic or symptomatic brain metastases
(CheckMate 204). Neuro-Oncology, noab094.
https://doi.org/10.1093/neuonc/noab094
Weber, J., Mandala, M., Del Vecchio, M., Gogas, H. J.,
Arance, A. M., Cowey, C. L., Dalle, S., Schenker, M.,
Chiarion-Sileni, V., Marquez-Rodas, I., Grob, J.-J.,
Butler, M. O., Middleton, M. R., Maio, M., Atkinson,
V., Queirolo, P., Gonzalez, R., Kudchadkar, R. R.,
Smylie, M., … Ascierto, P. A. (2017, September 10).
Adjuvant Nivolumab versus Ipilimumab in Resected
Stage III or IV Melanoma (world) [Research-article].
Http://Dx.Doi.Org/10.1056/NEJMoa1709030;
Massachusetts Medical Society.
https://doi.org/10.1056/NEJMoa1709030
What Causes Melanoma? | Causes of Melanoma Skin
Cancer. (n.d.). Retrieved October 11, 2021, from
https://www.cancer.org/cancer/melanoma-skin-
cancer/causes-risks-prevention/what-causes.html
Combination Therapies of Metastatic Melanoma and Melanoma Brain Metastases
693
