
The Function of BDNF and Treatment in Neurological Disorder
Yue Dong
1
, Kunjie Liu
2,*
and Yushi Wu
3,*
1
Victoria College, University of Toronto, ON M5S 1K7, Toronto, Canada
2
B.S. in Biology, College of Saint Mount Vincent, 10471, New York, U.S.A.
3
Diablo Valley College, Pleasant Hill, CA 94523, U.S.A.
†
These authors contributed equally
Keywords: BDNF, Neurological Disorder, Nervous System Diseases.
Abstract: Brain-derived neurotrophic factor (BDNF) is one of the most well-researched factors that thas been shown to
regulate mood and stress-coping. There is growing evidence suggesting the dysfunction of the BDNF pathway
is likely to cause several types of mental disorders such as Major Depressive Disorders, Stroke, and
Parkinson's Disease. This review article provides a detailed examination of the function of BDNF and how it
relates to neurological diseases, particularly depression, as well as different studies and theories explaining
the mechanism behind how dysfunction of the BDNF relates to depression. The article also discussed different
types of treatments, including drug treatment and CRISPR, that target these neurological disorders caused by
BDNF dysfunction. Despite the uncertainty in this field, there is still enough evidence suggesting that
dysregulation of BDNF can be a risk factor for many neurological disorders. Studies on this topic will likely
evoke new perspectives on modern treatments.
1 INTRODUCTION
Neurological disorders range from Major Depressive
Disorder (MDD) to stroke, from stroke to Parkinson's
Disease (PD), millions of people worldwide are
currently estimated to suffer from neurological
disorders. This number will be expected to increase
significantly in the following years. The only stroke
kills more than 6 million people each year, accounting
for nearly 11 percent of deaths worldwide. More than
47.5 million people worldwide suffer from dementia,
of which AD is the most common cause, while more
than 50 million people suffer from epilepsy (WHO
2016). Neuroprotective strategies have been
developed to ameliorate brain injury by preserving or
restoring neurological function. Brain-derived
neurotrophic factor (BDNF) is a widely studied
treatment strategy for several neurological diseases.
This growth factor plays a significant role in the
differentiation, maturation, and survival of neurons.
Despite relative success in the laboratory,
administering neurotrophic factors has not produced
the desired results in clinical trials. Therefore, the
neuroscience field will recognize BDNF levels in
patients with major depressive disorder (MDD) as a
potential threat. The pathway by which low levels of
BDNF directly lead to MDD is not clear right now,
but using the hippocampus as a carrier for the
relationship between the low level of BDNF and
MDD may explain the direct cause-effect
relationship. Otherwise, there have been significant
advances in SSRIs, MAOIs, and CRISPR
technologies to overcome these technical limitations
in clinical trials. However, most neurological diseases
show not only disorders of BDNF but also damage to
its downstream effects-and. Its relevance as a
pathological mechanism needs to be emphasized.
This review paper attempts to consider and
understand the direct and indirect role of BDNF in
neurological diseases, the potential pathways
between BDNF signaling and depression, as well as
the techniques available for clinical trials and the
advantages and disadvantages of treatment options.
This knowledge provides opportunities and new ideas
to guide the design of feasible and effective tools and
approaches for treating brain diseases.
2 NEUROLOGICAL DISORDERS
The most significant clinical feature of the decline in
BDNF signaling is the occurrence of
254
Dong, Y., Liu, K. and Wu, Y.
The Function of BDNF and Treatment in Neurological Disorder.
DOI: 10.5220/0011269100003438
In Proceedings of the 1st International Conference on Health Big Data and Intelligent Healthcare (ICHIH 2022), pages 254-263
ISBN: 978-989-758-596-8
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

neurodegenerative motor disorders. Otherwise,
ranging from Major Depressive Disorder (MDD) to
Stroke, from Stroke to Parkinson Disease (PD), one
of the symptoms of these neurological diseases is the
occurrence of neurodegenerative motor disorders to
varying degrees. At the same time, BDNF, as an
important transmission and balance medium in the
brain, has great research value in many clinical and
neuroscientific experiments.
2.1 Depression
BDNF has some effects on the development of
depression in some ways. Much research has shown
that BDNF is closely related to depression, and the
presence of BDNF mediates the occurrence of
neuronal and synaptic plasticity. In the biological
model of stress, BDNF levels were reduced in both
the cortex and the hippocampus. In other words, after
death, BDNF levels in the brain tissue were
significantly lower in those who took antidepressants.
In contrast, BDNF expression in the hippocampus
was higher in those who did not take antidepressants.
Further research suggests that antidepressant-
dependent BDNF levels may prevent or reduce
hippocampal changes in human samples (Chen 2001).
More recently, Cattaneo et al. (2013) demonstrated
separation between predictors and targets of
antidepressant response. The antidepressant response
was associated with changes in the gene BDNF, but
the gene did not predict specific changes in
physiological indicators of antidepressant and
physiological response. As a result, each biomarker
needs further study to elucidate its role in predicting
antidepressant response. Among the genetic markers,
the brain-derived neurotrophic factor (BDNF) gene
plays a key role in neurodevelopment and therapeutic
response to antidepressants (Ibarguen-Vargas 2009),
so antidepressants are associated with increased
expression of BDNF in animals brains and human
serum (Warner-Schmidt 2006). After the disease
becomes chronic, the level of BDNF in the brain is
upregulated (Nibuya 1995). Antidepressant therapy
results in G-protein-mediated intracellular factor
phosphorylation and stimulates BDNF release
(Watanabe 2010). Therefore, BDNF secretion and
intercellular transport are associated with single
nucleotide polymorphisms (SNPS) in the BDNF
gene, which leads to valine-to-methionine
replacement (Val66Met) (Egan 2003). BDNF
expressed as a precursor proBDNF consists of the n-
terminal and c-terminal precursors of mature BDNF.
The substitution of Val66Met in the anterior domain
induces the transfer of secondary structures to replace
the surrounding region (Anastasia 2013). Neurons
secrete both the mature BDNF protein and the
premitotic domain. Interestingly, compared with the
inactive Val66 predomain, secreted predomain
containing Met replacement promoted the growth
cone retraction of cultured hippocampal neurons
(Anastasia 2013). Meanwhile, two recent meta-
analyses have shown that Met alleles respond better
to antidepressants (Kato 2010). Considering that
BDNF mediates the response to antidepressants
(D’Sa 2002), polymorphisms modifying BDNF gene
expression or different intracellular signaling
pathways may play an important role in the treatment
and pharmacological response to antidepressants.
Therefore, the imbalance BDNF decreasing in the
brain may help clarify the relationship between
neuroplasticity and the pathophysiology of
depression.
2.2 Stroke
Many studies have found that the level of BDNF will
decrease with the deterioration of stroke. Otherwise,
cerebral dysfunction is common in stroke patients,
and BDNF is important for post-stroke recovery.
Stroke is the fifth leading cause of death in the United
States. Stroke patients often show a decline in
neurological function of the brain, accompanied by
certain symptoms of movement disorders. A previous
study has shown that several therapeutic interventions
can enhance functional recovery after stroke, such as
exercise and rehabilitation. These treatments lead to
beneficial effects of BDNF and brain plasticity, such
as improved learning, memory, and motor function
and increased expression of related proteins to a
certain extent (Ploughman 2005). To date, a clinical
study has shown an increase in the number of Treg
cells that produce BDNF after stroke, suggesting that
Treg cells may be able to deliver BDNF to the site of
injury to provide neuroprotection after stroke (Chan
2015). Similarly, strategies to increase BDNF have
been widely used in rats with middle cerebral artery
occlusion (MCAO).
During stroke rehabilitation, BDNF levels in the
nervous system have enhanced the neuroplasticity
processes involved in motor relearning. The reduction
of BDNF levels in the brain completely negates the
recovery of skilled movement
(Ploughman 2009).
Therefore, the beneficial effects of brain-derived
neurotrophic factors in the central nervous system
may contribute to post-stroke recovery.
While rapid up-regulation of neurotrophic factor
expression in the penumbra has been observed for
several days (Madinier 2013), permanent reduction of
The Function of BDNF and Treatment in Neurological Disorder
255

BDNF has been observed in animal models of
ischemic stroke (Ferrer 2001). However, BDNF has
never been measured in the postmortem brain of a
stroke patient, although the slight increase in
circulating neuronutrient levels observed that stroke
might reflect intracerebral levels (Chan 2015). The
enhancement of BDNF levels after stroke is mainly
associated with peripheral neurons and microglia,
which has been considered a brain compensatory
mechanism to prevent excessive neuronal death
(Bejot 2011, Kokaia 1995). Several studies have
concluded that BDNF is not involved in functional
recovery after stroke (Zhou 2000). The most likely
explanation for this result is that BDNF fails to trigger
appropriate neurotrophic signals after stroke due to a
pathological imbalance of the TrkB receptor subtype.
In fact, TrkB-FL levels declined sharply in the infarct
core and penumbra, whereas TRKB-T1 levels were
upregulated in human ischemic stroke and ischemic
animal models (Hirata 2011). These changes are the
result of three separate mechanisms induced by
excitotoxicity (Vidaurre 2012).
2.3 PD (Parkinson Disorder)
At the same time, there is increasingly much evidence
that the loss of the BDNF signaling pathway or the
reduction of BDNF contributes to the pathogenesis of
some major diseases and disorders, such as AD and
PD (Li 2020). PD is a neurodegenerative disease that
can be associated with non-motor symptoms, such as
cognitive deficits and changes in BDNF levels. Lack
of BDNF signaling is the most common
neurodegenerative motor disorder. PD is
characterized by progressive loss of dopaminergic
neurons in the substantia nigra pars densa (SNpc),
coupled with accumulation defects in intracellular α
synuclein inclusions (known as Lewy bodies and
Lewy neurites). Postmortem studies of PD patients
showed that BDNF mRNA and protein were
decreased in the susceptible region SNpc and in the
striatum receiving neurotrophic support from SN
(Parain 1999, Altar 1998). However, the loss of the
BDNF survival signal increases the susceptibility of
SN dopaminergic neurons to cytotoxic damage and
may contribute to the development of PD (Ding 2011,
Hung 1996). In fact, inhibition of BDNF expression
leads to selective loss of SNpc dopaminergic neurons
in older animals and exacerbates motor dysfunction
(Boger 2011). In Parkinson's disease, the expression
level of BDNF was so low in the neurons that may be
facing the biggest risk of injury. It may even trigger
an ontology degradation, diseases associated with the
intensity and duration, and the severity of the
symptoms of Parkinson's disease (Costa 2015). A
study shows even neurodegenerative diseases, if, at a
more advanced stage, neurons with low BDNF
expression caused irreversible damage (Gyárfás
2010). In recent years, clinical studies have suggested
that treatment with anti-Parkinson's drugs may
increase BDNF levels (Scalzo 2010). At the same
time, exercise therapy can trigger a few plasticity-
related events in the brain of PD patients, including
cortical motor excitation and changes in BDNF levels
(Hirsch 2016). In general, BDNF may be a potential
biomarker for assessing cognitive changes in
Parkinson's disease and other neurologic syndromes
associated with cognitive decline (Costa 2015).
3 MECHANISM BEHIND BDNF
PATHWAY
The functional pathway of the BDNF gene is the key
to understanding how it relates to numerous different
neurological diseases. The BDNF gene is first
translated into a precursor proBDNF which is about
32kDa. ProBDNF can undergo an intracellular
cleavage by a furin-like convertase to produce mature
BDNF (~13kDa) and a BDNF pro-peptide (~17kDa)
(Yang 2017). Both proBDNF and mature BDNF
(mBDNF) act on hippocampal neuroplasticity by
binding to different receptors. While proBDNF
preferentially binds to a low-affinity neurotrophin
receptor (p75NTR), mBDNF has a higher affinity to
a tropomyosin-related kinase B (TrkB) receptor
(Castrén 2017). Interestingly, the two receptors show
different effects on neural development. The
activation of p75NTR by proBDNF promotes
apoptosis or neurons, synaptic pruning, as well as
NMDAR-dependent long-term depression (LPD) (Lu
2005). However, the binding of TrkB receptors can
promote neural survival, synaptogenesis, and
neuroplasticity. Many lines of evidence suggest that
mBDNF, together with protease plasmin, are highly
involved in the late-phase long-term potentiation (L-
LTP) in the hippocampus (Lu 2005). Considering its
properties and function in neural signalling and
development, BDNF has been shown to be related to
the pathology of depression and many neurological
disorders.
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
256

3.1 BDNF and Depression
In 1995, researchers conducted the very first study on
the relationship between stress level and BDNF on an
animal model, which found that constant stress could
significantly reduce the BDNF mRNA level in the
hippocampus (Smith 1995). This finding was later
tested on depression patients as well. In a study done
by Karege and his colleagues, they measured the level
of BDNF and neurotrophin-3 in both suicide and non-
suicide victims. The result showed that the level of
BDNF was significantly low in both hippocampus
and ventral prefrontal cortex of suicide victims
compared to non-suicide victims (Karege 2004).
These studies all suggested that low levels of BDNF
could be a potential risk factor for developing
depression. Both studies found that the hippocampus
was affected also suggested that the hippocampus
might be the key link between BDNF and depression.
The pathway of how the low level of BDNF leads
to depression is still unclear. However, some theories
may be able to explain the mechanism behind it. As
mentioned before, mBDNF is crucial to the
generation of new synapses and neural development,
especially in the hippocampus. A decrease in the
mBDNF level can induce less neurogenesis in the
hippocampus. This will lead to decreased
hippocampal volume and cognitive ability since the
hippocampus controls emotions and memory. The
study also supported the study, which found patients
with depression generally have 4-5% smaller
hippocampus than the control group (Yu 2010).
Evidence has also shown that the hippocampus plays
an important role in regulating the stress-coping
mechanism hypothalamic-pituitary-adrenal (HPA)
axis and amygdala. Thus, the dysfunction in the
hippocampus can lead to difficulties in the stress
response (Yu 2010). This could lead to an increase in
the risk of major depressive disorder in patients.
Despite the direct interaction with the
neuroplasticity, dysfunction in the secretion of
mBDNF can also have a deterioration influence on
the serotonin pathway in the hippocampus. In the
study of Ren-Patterson et al., they conducted an
experiment on mice models with double mutant
SERT -/- and BDNF +/- genes. The result suggested
that the double mutant significantly reduces serotonin
in the hippocampus of 37% and hypothalamus of 43%
even compared to the single SERT -/- mutant mice.
There was also evidence indicating that BDNF plays
a crucial role in the differentiation of serotonergic
neurons. This all suggests that a low level of BDNF
can have deleterious effects on the serotonin pathway.
Depression was long known can be improved by
alleviating the level of serotonin in the synapses. This
relationship between BDNF and the serotonin
pathway also helps to explain how the deficiency in
BDNF can be related to depression.
3.2 Polymorphism of Bdnf Gene
Several single-point mutations have been found to be
associated with the low level of mBDNF in the brains
in the past few years. For instance, plenty of research
has been done on a valine to methionine substitution
mutation at codon 66, which is likely to cause
decreased secretion of mBDNF (Anastasia 2013).
According to the study by Anastasia et al. in 2013,
BDNF proteins with this mutation have less stable
secondary and tertiary structures of the prodomain,
which mediate the intracellular trafficking and active
secretion of mBDNF. This Val66Met variant
decreases the binding efficiency and therefore lowers
the amount of mBDNF available in the hippocampus
(Anastasia 2013). This theory was also supported by
some other studies, which found that patients with the
Met66 allele showed abnormal activation in the
bilateral hippocampus (Egan 2003). Mice models
with this mutation showed similar anxiety behaviors
as humans (Bath 2012). Although the mutation was
thought to be associated with many mental illnesses
such as schizophrenia and bipolar disorder, the
strength of this association is still debatable according
to current studies (Castrén 2017).
3.3 BDNF and Antidepressants
Right now, multiple different classes of
antidepressants have proven to upregulate the release
of BDNF. In a study by Nibuya et al. (1995), they
found the administration of tranylcypromine
increased the level of BDNF mRNA to about 100%
in the hippocampus (Nibuya 1995). In fact, many
types of serotonin reuptake inhibitors (SSRIs),
monoamine oxidase inhibitors (MAOIs), and even
some atypical antidepressants have been shown to
alleviate the level of BDNF in brains (Duman 2006).
Moreover, chronic treatment of antidepressants can
also block the downregulation of BDNF caused by
stress (Nibuya 1995). However, not all types of
antidepressants have similar effects. For example,
there is still some debate over if fluoxetine can alter
the expression of BDNF in any part of the brain
(Duman 2006). While one search argued that it could
increase the level of BDNF in the hippocampus
(Dwivedi 2009). Dias et al. suggested that there is no
significant difference made by fluoxetine (Duman
2006).
The Function of BDNF and Treatment in Neurological Disorder
257
Furthermore, BDNF itself can also have an
antidepressant effect on depression patients. In the
study done by Shirayama et al., they performed a
direct infusion of BDNF in the hippocampus of rat
brains and observed a decrease in depressive
symptoms. The effect could last as long as 10 days. It
is also claimed that such effect was also observed in
the treatment with other antidepressants like tricyclic
antidepressants (TCA) or SSRIs (Dias 2003).
However, this effect has only been tested on rats
using different types of behavioral tests such as
forced swim test and learned passive avoidance test
(Dias 2003). There is no evidence that BDNF will
have a similar antidepressant effect on humans, but it
suggested a potential path of a new treatment for
depression using the BDNF pathway.
4 CURRENT TREATMENT
The current treatment of depression is mainly
psychological and pharmacological. Antidepressants
can have various physiological effects on neuronal,
glial, and astrocyte functions and their interactions,
which in turn lead to alterations in signaling between
neuronal networks, ultimately regulating mood,
thinking, and stress response. Currently, there are
three main types of pharmacological treatments:
SSRIs, MAOIs, and TCAs. In recent years, with the
development of CRISPR technology, the groundwork
has also been laid for new strategies to treat
depression.
4.1 Pharmacology of Antidepressants
4.1.1 SSRIs
Selective serotonin reuptake inhibitors (SSRIs) are
usually the drug of choice for depression because they
are relatively safe and have fewer side effects than
most other types of antidepressants (Shirayama
2002). According to Olfson and Marcus in 2009
(Santarsieri 2015), nearly 67% of people taking
antidepressants in the United States were treated with
SSRIs. SSRIs work by increasing serotonin levels in
the brain. Serotonin is a messenger chemical that
transmits signals between nerve cells in the brain and
has a beneficial effect on mood, emotion, and sleep.
Serotonin is present in the form of vesicles and is
transmitted from the presynaptic neuron to the
receptor of the postsynaptic neuron via the 5-HT
transporter. After transmitting the information,
serotonin can be reabsorbed by nerve cells, called
"reuptake". Inhibitors of serotonin reuptake trigger
the reactivation of adolescent-like neuroplasticity. By
acting on the 5-HT transporter, the SSRI can increase
the concentration of 5-hydroxytryptamine in the
synaptic gap by inhibiting the reuptake of the
neurotransmitter 5-hydroxytryptamine by the
synaptic reuptake pump, leaving more serotonin
available to transmit further information between
nearby nerve cells (Olfson 2009). A 2012 Cambridge
University investigation of Short-term SSRI
treatment that normalises amygdala hyperactivity in
depressed patients also showed that short-term SSRI
treatment in depressed patients could remedy
amygdala overreaction to negative emotional stimuli
prior to clinical improvement in the depressed mood
(Cottone 2020). The amygdala is a key center for the
fear formation and expression and a key structure for
the automatic processing of negative facial
expressions. In patients with depression, the
organization and function of the amygdala are altered.
There is enhanced amygdala activation in processing
negative emotional faces in depressed patients, both
in conscious and unconscious conditions. The
regulation of emotions can be influenced clinically by
modulating the function of the amygdala to guide the
treatment of related disorders (Godlewska 2012).
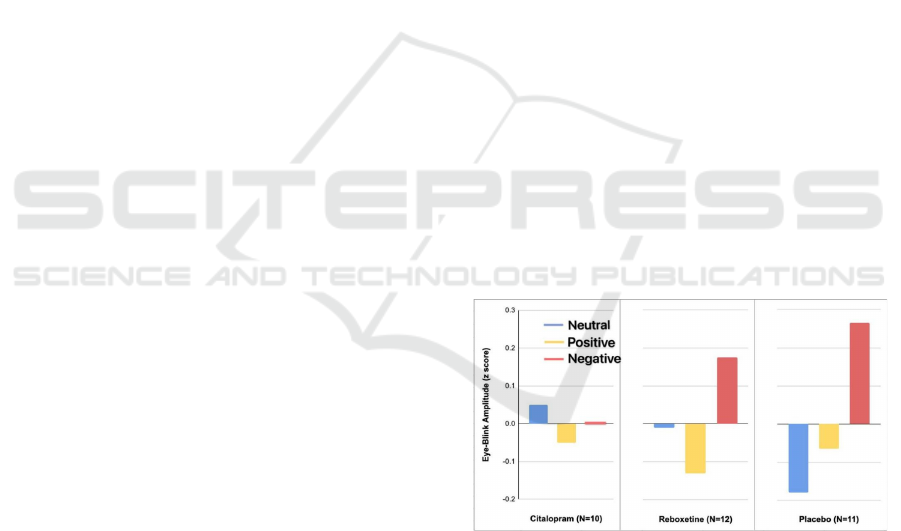
Indeed, in a behavioral study by Harmer et al. (Zhong
2012), we recently found that a single dose of
reboxetine in depressed patients reversed negative
emotion bias in facial expression recognition and
emotional memory in the absence of any change in
subjective mood.
* Values represent mean eye blinks (z transformed) during
the presentation of positive, negative, and neutral pictures.
There was a significant interaction between group and
stimulus type (F=2.7, df=3, 60, p=0.04).
Figure 1. Emotion-Potentiated Eye-Blink Startle Response
in 33 Healthy Volunteers After 1 Week of Randomly
Assigned Double-Blind Intervention with Citalopram,
Reboxetine, or Placeboa (Zhong 2012)
4.1.2 MAOIs
MAOIs are one of the most effective antidepressants
and have long been used as antidepressants. Although
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
258

MAOIs are particularly effective for atypical
depression and treatment-resistant depression,
MAOIs are not supported as first-line treatments due
to safety and tolerability concerns and the need for
dietary restrictions (Harmer 2004). By inhibiting
monoamine oxidase, MAOIs allow more of these
neurotransmitters to be retained in the brain, thereby
enhancing mood by improving communication
between brain cells. MAOIs are divided into two
main categories, non-selective and selective. Non-
selective MAOIs inhibit monoamine oxidase A and
monoamine oxidase B. As a result, serotonin,
norepinephrine, and dopamine levels are increased.
They are also known as irreversible MAOIs because
they bind irreversibly to the enzymes, permanently
blocking their function. Once these enzymes are
inhibited, the monoamine neurotransmitters are
loaded into preexisting vesicles. This way, when the
next action potential reaches the presynaptic
membrane, more neurotransmitters are released into
the synaptic cleft, thus relieving the symptoms of
depression (Tang 2014). In contrast, selective MAO-
B inhibitors have a high affinity for DA,
phenethylamine, benzylamine and selegiline,
pargyline (Thase 2012). Their inhibitory effects are
mainly found in the brain's glial cells and can lead to
an increase in dopamine (DA) levels in patients.
4.2 CRISPR Technology
4.2.1 The Concept of CRISPR
CRISPR technology is a simple yet powerful
genome-editing tool that allows researchers to easily
alter DNA sequences and modify gene function, an
adaptive immune system used by microorganisms to
defend against invading viruses by recording and
targeting their DNA sequences. It can be reused in
living cells to edit the genomes of mammals and other
organisms (Xiao). CRISPR technology was adapted
from the natural defense mechanisms of bacteria and
archaea (Lander 2016). These organisms use
CRISPR-derived RNA and Cas proteins to cut off and
destroy the DNA of foreign invaders to thwart attacks
by viruses and other foreign agents. CRISPR-Cas9
stands for clusters of regularly spaced short
palindromic repeats and CRISPR-associated protein
9. CRISPR is a specialized DNA fragment, and the
protein Cas9 is an enzyme that acts like "molecular
scissors" that cut two DNA strands at specific
locations in the genome and then add or remove DNA
fragments. A piece of RNA called guide RNA
(gRNA) consists of a small piece of pre-designed
RNA sequence (approximately 20 bases long) located
within a longer RNA scaffold (Aparna 2018),
designed to find and bind to a specific sequence in
DNA. The scaffold partially binds to the DNA, and
the RNA bases of the guide RNA are complementary
to the bases of the target DNA sequence in the
genome to ensure that the Cas9 enzyme cleaves at the
correct position in the genome (Aparna 2018). Cas9
follows the guide RNA to the same position in the
DNA sequence and cleaves on both DNA strands. In
this way, the DNA repair mechanism can be used to
alter one or more genes in the genome of the cell of
interest, acting more directly on the gene to alleviate
depression at a faster rate.
4.2.2 Applications of CRISPR
Although antidepressants are frequently used to treat
depression, at least 50% of patients respond that
antidepressants do not actually work. Moreover, the
clinical response occurs only after weeks to months
of treatment and is only effective with long-term
adherence to antidepressant therapy (Sander 2014). In
recent years, researchers are trying a new approach
based on CRISPR technology to alleviate depression
in addition to medication. Before the depression,
CRISPR technology has been widely used in other
fields. For example, in bladder cancer gene therapy,
treatment strategies have been ineffective because
certain genes, while actively expressed in tumor cells
in one organ, may also be expressed at relatively
active levels in normal cells in other different tissues.
Nowadays, researchers have cleverly used CRISPR
gene-editing technology and the principles of logical
pathways to solve these problems, providing new
ideas for studying tumor gene therapy. Researchers
established an editing system for targeted editing of
E6 and E7 genes based on CRISPR technology and
transfected the system into cervical cancer cell lines
infected with HPV-16 positive virus, and experiments
with SiHa cells also provide new ideas in the
treatment of cervical cancer and other HPV-related
cancers as a new therapeutic strategy (Masi 2011).
4.3 New Possible for CRISPR
A study at Pennsylvania State University indicated
that enhancing the activity of a subclass of neuronal
cells that produce the neurotransmitter gamma-
aminobutyric acid (GABA) and elevating GABA
neurotransmitter levels had antidepressant effects in
mice modeled with depression (Liu 2015). GABA is
part of the pathogenesis of anxiety and depression and
is responsible for many of the symptoms of these
disorders. GABA dysfunction is a major culprit of
The Function of BDNF and Treatment in Neurological Disorder
259

depression, and depressed patients often have reduced
GABA concentrations in the brain (State 2016).
Researchers at Pennsylvania State University
increased GABA signaling by disabling GABA
receptors in a specific group of neurons suspected of
being involved in major depression (Liu 2015). Under
normal conditions, this group of neurons, called
SST+ interneurons (growth inhibitor positive -
GABAergic interneurons), produce GABA, which
reduces the activity of other surrounding neurons. In
contrast, most peripheral neurons release the
neurotransmitter glutamate. When researchers
selectively disabled GABA receptors in SST+
interneurons, these cells could no longer receive
deceleration signals, so they over-released GABA,
which further slowed the glutamate-producing
neurons' activity (Liu 2015). As a result, rats
receiving this treatment behaved as if they were
taking antidepressants in a series of behavioral tests.
However, a study by Hyunjung Oh et al. in 2019
showed that GABA was the main determinant, as
manipulation of BDNF signaling resulted in an initial
deficit in GABA, not glutamate (Oh 2019). The basic
anxiolytic-antidepressant representing a GABA-A
positive modulator is very promising (Kalueff 2007).
The GABA-A type receptor is involved in cell
signaling through direct interaction with GABA.
There are also two subtypes of GABA-A receptors:
one containing the δ subunit δ and the other
containing the γ2 subunit.
4.3.1 Functional Abnormalities Associated
with Mutations
Dr. Steven Mennerick, Professor of Psychiatry at
Washington School of Medicine, induced point
mutations in the gene encoding the GABA-A δ
subunit in the mouse hippocampus using CRISPR-
Cas9 technology, making the GABA A δ subunit
resistant to picrotoxin resistant, thereby blocking the
chloride channel to inhibit receptor activity of the
chemical. Mice were then bred with these bitter toxin-
resistant GABA-A δ receptors. After dissecting and
sectioning the hippocampus of developing mice, they
stimulated neuronal cells in the presence of bitter
toxins to observe specific responses of δ subunits (Oh
2019). These experiments revealed the contribution
of the GABA-A δ subunit to short-term phase
synaptic responses after electrical stimulation.
Meanwhile, ASDS resulted in a range of emotional
and cognitive changes in adult animals, including
reduced social interest, increased anxiety-like
behavior, and impaired cognitive switch function,
accompanied by attenuated mPFC GABAergic
inhibitory synaptic transmission and a significant
reduction in the average frequency, but not amplitude,
of spontaneous inhibitory postsynaptic current
(sIPSC) delivery (CAS 2020). Further testing of
GABAergic signaling molecules by the Chinese
Academy of Sciences last year revealed that the
mRNA expression level of the mPFC GABA
synthase GAD65 was also significantly reduced.
These suggest that adolescent stress leads to sustained
suppression of GABAergic function in this brain
region. The decrease in BDNF due to ASDS is mainly
associated with reduced levels of BDNF IV promoter
transcripts. The results also suggest that mPFC
GABAergic synaptic transmission may be a
downstream mediating pathway for the depression-
like behavioral comorbid cognitive impairment and
BDNF signaling impairment caused by ASS. These
results suggest that GABAergic synaptic
transmission downstream of mPFC BDNF signaling
is primarily involved in the onset of depressive
comorbid cognitive impairment due to social stress in
adolescence, rather than depressive co-morbid
anxiety-like behaviors (CAS 2020). This study
provides a potential target for future rapid treatment
of cognitive dysfunction in depressed patients.
Given that the findings they generated have only
been relevant to mice, we must be cautious about
interpreting these results. That said, this study will
hopefully lay the groundwork for future studies on the
role of different cell types and mechanisms,
diversifying our approach to the study of depression
(Gardner 2018).
5 CONCLUSIONS
BDNF is essential for synaptogenesis, nerve growth,
and LTP in the hippocampus and many other parts of
the brain. More and more evidence suggest that low
levels of BDNF can be a risk factor for neurological
diseases like depression, stroke, and Parkinson's
disease. In the case of depression, the mutation of
Met66Val in the bdnf gene can decrease the secretion
of mBDNF, which has two possible pathways to alter
the function of the brain. Dysfunction of BDNF can
cause a decrease in the hippocampal volume and the
dysregulation in the serotonin pathway, while both
can increase the chance of major depressive disorder
in patients. In addition, it was also found that the
upregulation of BDNF has an antidepressant effect in
depression patients, which leads to new ways to treat
this disease. Currently, SSRIs and MAOIs are two of
the most common antidepressants available in the
markets. Both have shown to be effective in
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
260

increasing the level of neurotransmitters such as
serotonin and epinephrine, which significantly
improve the symptoms of depression but with minor
side effects. CRISPR is one of the modern
technologies that can potentially treat depression by
editing genes such as the bdnf gene. There has been
an example of researchers introducing a point
mutation for the gene of GABA-A δ subunit. With
research like this, hopefully, we will be able to treat
more neurological diseases using CRISPR in the
future.
REFERENCES
Altar, C.A.; DiStefano, P.S. Neurotrophin trafficking by
anterograde transport. Trends Neurosci. 1998, 21, 433–
437
Anastasia A, Deinhardt K, Chao MV, Will NE, Irmady K,
Lee FS, Hempstead BL, Bracken C (2013) Val66Met
polymorphism of BDNF alters prodomain structure to
induce neuronal growth cone retraction. Nat Commun
4:2490. doi:10.1038/ncomms3490
Anastasia, A., Deinhardt, K., Chao, M. V., Will, N. E.,
Irmady, K., Lee, F. S., Hempstead, B. L., & Bracken,
C. (2013). Val66Met polymorphism of BDNF alters
prodomain structure to induce neuronal growth cone
retraction. Nature Communications, 4(1).
https://doi.org/10.1038/ncomms3490
Aparna Vidyasagar (2018). What Is CRISPR? Live Science
Contributor April 20, 2018
Bath, K. G., Chuang, J., Spencer-Segal, J. L., Amso, D.,
Altemus, M., McEwen, B. S., & Lee, F. S. (2012).
Variant Brain-Derived Neurotrophic Factor
(Valine66Methionine) Polymorphism Contributes to
Developmental and Estrous Stage-Specific Expression
of Anxiety-Like Behavior in Female Mice. Biological
Psychiatry, 72(6), 499–504.
https://doi.org/10.1016/j.biopsych.2012.03.032
Bejot, Y.; Prigent-Tessier, A.; Cachia, C.; Giroud, M.;
Mossiat, C.; Bertrand, N.; Garnier, P.; Marie, C. Time-
dependent contribution of non neuronal cells to BDNF
production after ischemic stroke in rats. Neurochem.
Int. 2011, 58, 102–111.
Boger, H.A.; Mannangatti, P.; Samuvel, D.J.; Saylor, A.J.;
Bender, T.S.; McGinty, J.F.; Fortress, A.M.; Zaman,
V.; Huang, P.; Middaugh, L.D.; et al. Effects of brain-
derived neurotrophic factor on dopaminergic function
and motor behavior during aging. Genes Brain Behav.
2011, 10, 186–198.
CAS, Psychology Institute identifies GABAergic synaptic
modulation mechanism of adolescent stress-induced
depressive co-morbid cognitive impairment, Chinese
Academy of Sciences, 2020-12-08
Castrén, E., & Kojima, M. (2017). Brain-derived
neurotrophic factor in mood disorders and
antidepressant treatments. Neurobiology of Disease,
97, 119–126.
https://doi.org/10.1016/j.nbd.2016.07.010
Castrén, E., & Kojima, M. (2017). Brain-derived
neurotrophic factor in mood disorders and
antidepressant treatments. Neurobiology of Disease,
97, 119–126.
https://doi.org/10.1016/j.nbd.2016.07.010
Chan, A.; Yan, J.; Csurhes, P.; Greer, J.; McCombe, P.
“Circulating brain derived neurotrophic factor (BDNF)
and frequency of BDNF positive T cells in peripheral
blood in human ischemic stroke: effect on outcome,”
Journal of Neuroimmunology, vol. 286, pp. 42–47,
2015.
Chan, A.; Yan, J.; Csurhes, P.; Greer, J.; McCombe, P.
Circulating brain derived neurotrophic factor (bdnf)
and frequency of bdnf positive T cells in peripheral
blood in human ischemic stroke: Effect on outcome. J.
Neuroimmunol. 2015, 286, 42–47.
Characteristics of amygdala volume and functional
activation in depression.
Chen. B, Dowlatshahi. D, G. M. MacQueen, J. F. Wang,
and L. T. Young, “Increased hippocampal BDNF
immunoreactivity in subjects treated with
antidepressant medication,” Biological Psychiatry, vol.
50, no. 4, pp. 260–265, 2001.
China Academic Journal Electronic Publishing House,
10.11886/jissn. 1007-3256.2014.06.29.
Chinese Journal of Behavioral Medicine and Brain Science,
June 2012, Vol. 21, No. 6
Costa, A.; Peppe, A.; G. Carlesimo et al. “Brain-derived
neurotrophic factor serum levels correlate with
cognitive performance in Parkinson’s disease patients
with mild cognitive impairment,” Frontiers in
Behavioral Neuroscience, vol. 9, p. 253, 2015.
Cottone, John. “A Better Understanding of SSRI
Antidepressants.” Psychology Today, Sussex
Publishers, 18 May 2020.
D’Sa C, Duman RS (2002) Antidepressants and
neuroplasticity. Bipolar Disord 4:183–194.
doi:10.1034/j.1399-5618.2002.01203.x
Dias, B. “Differential Regulation of Brain Derived
Neurotrophic Factor Transcripts by Antidepressant
Treatments in the Adult Rat Brain.”
Neuropharmacology, vol. 45, no. 4, 2003, pp. 553–
563., doi:10.1016/s0028-3908(03)00198-9.
Ding, Y.X.; Xia, Y.; Jiao, X.Y.; Duan, L.; Yu, J.; Wang, X.;
Chen, L.W. The TrkB-positive dopaminergic neurons
are less sensitive to MPTP insult in the substantia nigra
of adult C57/BL mice. Neurochem. Res. 2011, 36,
1759–1766.
Duman, Ronald S., and Lisa M. Monteggia. “A
Neurotrophic Model for Stress-Related Mood
Disorders.” Biological Psychiatry, vol. 59, no. 12,
2006, pp. 1116–1127.,
doi:10.1016/j.biopsych.2006.02.013
Dwivedi, Yogesh. “Brain-Derived Neurotrophic Factor:
Role in Depression and Suicide.” Neuropsychiatric
Disease and Treatment, 2009, p. 433.,
doi:10.2147/ndt.s5700.
Egan MF, Kojima M, Callicott JH, Goldberg TE,
Kolachana BS, Bertolino A, Zaitsev E, Gold B,
The Function of BDNF and Treatment in Neurological Disorder
261

Goldman D, Dean M, Lu B, Weinberger DR (2003) The
BDNF val66met polymorphism affects activity-
dependent secretion of BDNF and human memory and
hippocampal function. Cell 112(2):257–269.
doi:10.1016/S0092- 8674(03)00035-7
Egan, M. F., Kojima, M., Callicott, J. H., Goldberg, T. E.,
Kolachana, B. S., Bertolino, A., Zaitsev, E., Gold, B.,
Goldman, D., Dean, M., Lu, B., & Weinberger, D. R.
(2003). The BDNF val66met Polymorphism Affects
Activity-Dependent Secretion of BDNF and Human
Memory and Hippocampal Function. Cell, 112(2), 257–
269. https://doi.org/10.1016/s0092-8674(03)00035-7
Ferrer, I.; Krupinski, J.; Goutan, E.; Marti, E.; Ambrosio,
S.; Arenas, E. Brain-derived neurotrophic factor
reduces cortical cell death by ischemia after middle
cerebral artery occlusion in the rat. Acta Neuropathol.
2001, 101, 229–238.
GABAergic neurons may be a new target for
antidepressants. BioDiscovery, 2016-11-10
Gardner, Heidi. (2018) Fighting Depression with CRISPR.
CRISPR NEWS
Godlewska, B. R., Norbury, R., Selvaraj, S., Cowen, P. J.,
& Harmer, C. J. (2012). Short-term SSRI treatment
normalises amygdala hyperactivity in depressed
patients. Psychological medicine, 42(12), 2609–2617.
Gyárfás, T.; Knuuttila, J.; Lindholm P.; Rantamäki, T.;
Castrén, E. “Regulation of brain-derived neurotrophic
factor (BDNF) and cerebral dopamine neurotrophic
factor (CDNF) by anti-parkinsonian drug therapy in
vivo,” Cellular and Molecular Neurobiology, vol. 30,
no. 3, pp. 361–368, 2010.
Harmer, C. J., Shelley, N. C., Cowen, P. J., & Goodwin, G.
M. (2004).
Hirata, K.; Kuge, Y.; Yokota, C.; Harada, A.; Kokame, K.;
Inoue, H.; Kawashima, H.; Hanzawa, H.; Shono, Y.;
Saji, H.; et al. Gene and protein analysis of brain
derived neurotrophic factor expression in relation to
neurological recovery induced by an enriched
environment in a rat stroke model. Neurosci. Lett. 2011,
495, 210–215.
Hirsch, M. A.; Iyer, S. S.; Sanjak M. “Exercise-induced
neuroplasticity in human Parkinson’s disease: what is
the evidence telling us?,” Parkinsonism & Related
Disorders, vol. 22, Suppl 1, pp. S78–S81, 2016.
Hung, H.C.; Lee, E.H. The mesolimbic dopaminergic
pathway is more resistant than the nigrostriatal
dopaminergic pathway to MPTP and MPP+ toxicity:
Role of BDNF gene expression. Brain Res. Mol. Brain
Res. 1996, 41, 14–26.
Ibarguen-Vargas Y, Surget A, Vourc’h P, Leman S, Andres
CR, Gardier AM, Belzung C (2009) Deficit in BDNF
does not increase vulnerability to stress but dampens
antidepressant-like effects in the unpredictable chronic
mild stress. Behav Brain Res 202:245–251. doi:
10.1016/j.bbr.2009.03.040
Increased positive versus negative affective perception and
memory in healthy volunteers following sselective
serotonin and norepinephrine reuptake inhibition. The
American journal of psychiatry, 161(7), 1256–1263.
Kalueff, A. V., & Nutt, D. J. (2007). Role of GABA in
anxiety and depression. Depression and anxiety, 24(7),
495–517.
Karege, Félicien, et al. “Neurotrophin Levels in
Postmortem Brains of Suicide Victims and the Effects
of Antemortem Diagnosis and Psychotropic Drugs.”
Molecular Brain Research, vol. 136, no. 1-2, 2005, pp.
29–37., doi:10.1016/j.molbrainres.2004.12.020.
Kato M, Serretti A (2010) Review and meta-analysis of
antidepressant pharmacogenetic findings in major
depressive disorder. Mol Psychiatry 15:473–500.
doi:10.1038/mp.2008.116
Kokaia, Z.; Zhao, Q.; Kokaia, M.; Elmer, E.; Metsis, M.;
Smith, M.L.; Siesjo, B.K.; Lindvall, O. Regulation of
brain-derived neurotrophic factor gene expression after
transient middle cerebral artery occlusion with and
without brain damage. Exp. Neurol. 1995, 136, 73–88.
Lander E. S. (2016). The Heroes of CRISPR. Cell, 164(1-
2), 18–28.
Li, Xia, et al. “Applications of Acupuncture Therapy in
Modulating the Plasticity of Neurodegenerative
Disease and Depression: Do MicroRNA and
Neurotrophin BDNF Shed Light on the Underlying
Mechanism?” Neural Plasticity, Sept. 2020, pp. 1–17.
EBSCOhost, doi:10.1155/2020/8850653.
Liu, C., Li, Z., & Zhang, Y. (2015). Zhongguo fei ai za zhi
= Chinese journal of lung cancer, 18(9), 571–579.
Lu, B., Pang, P. T., & Woo, N. H. (2005). The yin and yang
of neurotrophin action. Nature Reviews Neuroscience,
6(8), 603–614. https://doi.org/10.1038/nrn1726
M. Ploughman, V. Windle, C. L. MacLellan, N. White, J. J.
Dore, and D. Corbett, “Brain-derived neurotrophic
factor contributes to recovery of skilled reaching after
focal ischemia in rats,” Stroke, vol. 40, no. 4, pp. 1490–
1495, 2009.
Madinier, A.; Bertrand, N.; Rodier, M.; Quirie, A.; Mossiat,
C.; Prigent-Tessier, A.; Marie, C.; Garnier, P.
Ipsilateral versus contralateral spontaneous post-stroke
neuroplastic changes: Involvement of BDNF?
Neuroscience 2013, 231, 169–181.
Masi, G., & Brovedani, P. (2011). The hippocampus,
neurotrophic factors and depression: possible
implications for the pharmacotherapy of depression.
CNS drugs, 25(11), 913–931.
Nibuya M, Morinobu S, Duman RS (1995) Regulation of
BDNF and trkB mRNA in rat brain by chronic
electroconvulsive seizure and antidepressant drug
treatments. J Neurosci 15:7539–7547
Nibuya, M, et al. “Regulation of BDNF and TrkB MRNA
in Rat Brain by Chronic Electroconvulsive Seizure and
Antidepressant Drug Treatments.” The Journal of
Neuroscience, vol. 15, no. 11, 1995, pp. 7539–7547.,
doi:10.1523/jneurosci.15-11-07539.1995.
Oh, H., Piantadosi, S. C., Rocco, B. R., Lewis, D. A.,
Watkins, S. C., & Sibille, E. (2019). The Role of
Dendritic Brain-Derived Neurotrophic Factor
Transcripts on Altered Inhibitory Circuitry in
Depression. Biological psychiatry, 85(6), 517–526.
Olfson, M., & Marcus, S. C. (2009). National patterns in
antidepressant medication treatment. Archives of
general psychiatry, 66(8), 848–856.
ICHIH 2022 - International Conference on Health Big Data and Intelligent Healthcare
262

Parain, K.; Murer, M.G.; Yan, Q.; Faucheux, B.; Agid, Y.;
Hirsch, E.; Raisman-Vozari, R. Reduced expression of
brain-derived neurotrophic factor protein in
Parkinson’s disease substantia nigra. Neuroreport 1999,
10, 557–561.
Ploughman M., Granter-Button S., Chernenko G., Tucker
B. A., Mearow K. M., and Corbett D., “Endurance
exercise regimens induce differential effects on brain-
derived neurotrophic factor, synapsin-I and insulin-like
growth factor I after focal ischemia,” Neuroscience,
vol. 136, no. 4, pp. 991– 1001, 2005.
Sander, J. D., & Joung, J. K. (2014). CRISPR-Cas systems
for editing, regulating and targeting genomes. Nature
biotechnology, 32(4), 347–355.
Santarsieri, D., & Schwartz, T. L. (2015). Antidepressant
efficacy and side-effect burden: a quick guide for
clinicians. Drugs in context, 4, 212290.
Scalzo, P.; Kümmer, A.; Bretas, T. L.; Cardoso, F.;
Teixeira, A. L. “Serum levels of brain-derived
neurotrophic factor correlate with motor impairment in
Parkinson’s disease,” Journal of Neurology, vol. 257,
no. 4, pp. 540–545, 2010.
Shirayama, Yukihiko, et al. “Brain-Derived Neurotrophic
Factor Produces Antidepressant Effects in Behavioral
Models of Depression.” The Journal of Neuroscience,
vol. 22, no. 8, 2002, pp. 3251–3261.,
doi:10.1523/jneurosci.22-08-03251.2002.
Smith, MA, et al. “Stress and Glucocorticoids Affect the
Expression of Brain-Derived Neurotrophic Factor and
Neurotrophin-3 MRNAs in the Hippocampus.” The
Journal of Neuroscience, vol. 15, no. 3, 1995, pp. 1768–
1777., doi:10.1523/jneurosci.15-03-01768.1995.
State, Penn. Increasing the activity of the neurotransmitter
GABA in the brains of depressed mice has
antidepressant effects. Molecular Psychiatry,
November 8, 2016.
Tang, Zhuojun (2014). Advances in antidepressant
research.
Thase M. E. (2012). MAOIs and depression treatment
guidelines. The Journal of clinical psychiatry, 73(7),
e24.
Vidaurre, O.G.; Gascon, S.; Deogracias, R.; Sobrado, M.;
Cuadrado, E.; Montaner, J.; Rodriguez-Pena, A.; Diaz-
Guerra, M. Imbalance of neurotrophin receptor
isoforms TrkB-FL/TrkB-T1 induces neuronal death in
excitotoxicity. Cell. Death Dis. 2012, 3, e256.
Warner-Schmidt JL, Duman RS (2006) Hippocampal
neurogenesis: opposing effects of stress and
antidepressant treatment. Hippocampus 16(3):239–
249. doi:10.1002/hipo.20156
Watanabe K, Hashimoto E, Ukai W, Ishii T, Yoshinaga T,
Ono T, Tateno M, Watanabe I, Shirasaka T, Saito S,
Saito T (2010) Effect of antidepressants on brain-
derived neurotrophic factor (BDNF) release from
platelets in the rats. Prog Neuropsychopharmacol Biol
Psychiatry 34(8):1450–1454.
doi:10.1016/j.pnpbp.2010.07.036
WHO(2016); [http://www.who.int/features/qa/55/en/]
Xiao, Yifan, Ling, Justin, Aranda, Alex, Nixon-Shapiro,
Elizabeth, Vasiljević, Filip Monoamine oxidase
inhibitors
Yang, B., Ren, Q., Zhang, J.-c, Chen, Q.-X., & Hashimoto,
K. (2017). Altered expression of BDNF, BDNF pro-
peptide and their precursor proBDNF in brain and liver
tissues from psychiatric disorders: rethinking the brain–
liver axis. Translational Psychiatry, 7(5).
https://doi.org/10.1038/tp.2017.95
Yu, H., & Chen, Z.-yu. (2010). The role of BDNF in
depression on the basis of its location in the neural
circuitry. Acta Pharmacologica Sinica, 32(1), 3–11.
https://doi.org/10.1038/aps.2010.184
Zhao, L.R.; Mattsson, B.; Johansson, B.B. Environmental
influence on brain-derived neurotrophic factor
messenger RNA expression after middle cerebral artery
occlusion in spontaneously hypertensive rats.
Neuroscience 2000, 97, 177–184.
Zhong, Mingtian, Yi, Jinyao, Zhu, Xiongzhao, Yao,
Shujiao (2012)
The Function of BDNF and Treatment in Neurological Disorder
263
