
Restoring mGluR-LTD Failure with the Induction of Arc Protein in
the CA1 Region of APP/PS1 Mice
Yansheng Zheng
1,†
and Lumeng Sun
2,†
1
Dalhousie University, Truro, Nova Scotia, B2N 5E3, Canada
2
Stephen Perse Foundation, Cambridge, CB2 1NA, U.K.
†
They are both first author
Keywords: Alzheimer’s Disease, mGluR-LTD, Arc Protein.
Abstract. Alzheimer’s Disease (AD) has become a severe problem across the world. Besides the major two hypotheses
(β-amyloid protein and tau protein), recent studies have also found that metabotropic glutamate receptor 5-
dependent LTD (mGluR-LTD) failure is also one significant symptom of AD in APP/PS1 mice (AD mice
model). Suppression of the inhibitor of de novo protein synthesis during the mGluR-LTD has successfully
reversed the mGluR-LTD failure. Arc protein is an essential protein that coordinates the mGluR, and the level
of Arc protein in the CA1 region of APP/PS1 mice is reduced. Therefore, an experiment is designed to increase
the Arc level in APP/PS1 exogenously and endogenously to restore the mGluR-LTD by measuring post-
synaptic responses.
1 INTRODUCTION
1.1 Background
Scientists have long been working to find a possible
explanation for how Alzheimer’s Disease (AD) is
developed and methods for curing or mitigating the
AD symptoms. There are several hypotheses about
how it originated. The first one is the Tau and
Amyloid-beta hypothesis. It asserts that tau, which
accounts for the neuron tangles, and amyloid-beta,
which clumps into plaques, act together to cause
synaptic failure, resulting in AD (Förstl, Kurz 1999).
The second one is about vascular problems, which
may lead to a malfunction of the blood barrier. This
failure inhibits glucose from reaching the brain as
well as preventing the clearing away of toxic beta-
amyloid and tau proteins (Förstl, Kurz 1999).
Scientists have also studied microglia cells, a form of
glial cells, as they fail to remove waste and beta-
amyloid plaques in AD (Förstl, Kurz 1999). The
initial symptoms of AD are sometimes mistaken as
aging or stress, with the most serious deficit being the
loss of short-term memory, along with other subtle
vestigial deficits in executive functions of
attractiveness, planning, flexibility, and abstract
thinking (Bäckman, Jones, Berger, Laukka, Small
2004, Waldemar, Dubois, Emre, Georges, McKeith,
Rossor, Scheltens, Tariska, Winblad 2007). With the
development of AD, the early stages of AD are
characterized by linguistic difficulties and impaired
executive functions, deteriorating perception
(agnosia), or degenerating execution of movements
(apraxia) other than memory impairments (Förstl,
Kurz 1999). Patients at the middle stages of AD show
degeneration in speech, reading and writing skills;
more importantly, the long-term memory starts to be
impaired and behavioral and neuropsychiatric change
become more prevalent (Förstl, Kurz 1999, Frank
1994). During the final stage of AD, patients
completely lose speech ability, show extreme apathy
and exhaustion, and eventually die of external factors
like infection of pressure ulcers or pneumonia (Förstl,
Kurz 1999, Frank 1994). The occurrence of AD
greatly influences the society, such as the life of
patients’ family and high financial cost (Abreu,
Forlenza, Barros, de. 2005). Moreover, during covid-
19, the number of AD patients has increased by 16
percent, and one-third of the over-85 population is
estimated to suffer from it by 2031((Bäckman, Jones,
Berger, Laukka, Small 2004). As no one has ever
been cured from AD, finding a therapy to avoid
becoming its victim is essential.
Beside β-amyloid hypothesis and tau protein
hypothesis being accepted as 2 major possible causes
Zheng, Y. and Sun, L.
Restoring mGluR-LTD Failure with the Induction of Arc Protein in the CA1 Region of APP/PS1 Mice.
DOI: 10.5220/0011294500003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 753-759
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
753

of Alzheimer’s disease. Yang and co-workers found
that in APP/PS1 mice (AD mice model), mGluR-
LTD (Long-term Depression) failure occurs, and
assumed this phenomenon as a potential cause of AD
(Yang, Zhou, Zimmermann, Cavener, Klann, Ma
2016). Therefore, they tried to restore mGluR-LTD to
mitigate the progress of AD in APP/PS1 mice. PERK
is one of the four kinases of elF2 (eukaryotic initiative
factor 2), and it is triggered to phosphorylate eIF2 on
the α subunit which inhibits general synthesis of
protein and impairs memory if the reduction in
protein synthesis is persistent (Ma, Klann 2014,
Trinh, Klann 2013, Wek, Jiang, Anthony 2006, Wek,
Cavener, 2007). Yang and co-workers
pharmacologically and genetically repressed the
expression of PERK (Yang, Zhou, Zimmermann,
Cavener, Klann, Ma 2016), in order to restore the de
novo protein synthesis during the mGluR-LTD, and
they successfully reversed the failure of mGluR-LTD
in APP/PS1 mice. Another research by Yang and co-
workers demonstrated that via pharmacological and
genetic suppression of eEf2K (a kinase for elongation
factor 2) (Yang, Zhou, Ryazanov, Ma. 2021), the
hippocampal mGluR-LTD impairments in APP/PS1
mice had been alleviated.
1.2 Project Design
It has been known that mGluR-LTD failure occurred
in APP/PS1 mice (Yang, Zhou, Zimmermann,
Cavener, Klann, Ma 2016), and unregulated LTD is
associated with dementia and AD (Wilkerson, Julia,
Albanesi, Huber 2018). Based on this, it is assumed
that if mGluR-LTD failure in APP/PS1 mice is
reversed, the mice’s AD symptoms will be improved.
Both these 2 studies focus on the restoration of the
de novo protein synthesis, which is involved in
process of mGluR-LTD, to alleviate the impairments
of mGluR-LTD. Therefore, since Arc protein
synthesis is included in de novo protein synthesis
during mGluR-LTD, this project aims to investigate
whether a great amount of Arc expression will
reverse mGluR-LTD failure, in order to mitigate AD
symptoms.
2 EXPERIMENTAL APPROACH
Whether the induction of a great amount of Arc
protein will reverse mGluR-LTD impairments in the
APP/PS1 mice.
3 METHODS AND MATERIALS
3.1 Mice
All mice were kept in a barrier rearing system
committed to transgenic mice, which accords with the
standards and policies of the US Department of
Agriculture’s Animal Welfare Information Center
and the NIH Guide for Care and Use of Laboratory
Animals. A 12-hour-light/dark cycle is maintained in
the system, with a regular feeding and cage-cleaning
schedule. male and female mice are equally selected
for this research. APP/PS1 transgenic mice (APPswe
+PSEN1/Δ9) and wild type mice were purchased.
FMR1 hippocampus conditional knock-out (FMR1
cKO) were bred, and the method will be described
below. The genotype of all mice was verified using
PCR. Mice around 12-15 months old were used for
this experiment.
3.2 Hippocampal Slice Preparation and
Electrophysiology
The hippocampi were removed from the brains of
mice of 12-15 months, then vibratome was used to
make hippocampal slices at 400 μm(Hu, Serrano,
Oury, Klann 2006). Slices were kept at room
temperature and submerged in artificial CSF (ACSF),
which contains the following (in mM:125 NaCl, 2.5
KCl, 1.25 NaH2PO4, 25 NaHCO3, 25 D-glucose, 2
CaCl2, and 1 MgCl2), and incubated for at least 2 h
to be removed for electrophysiology.
For electrophysiology, slices were transferred to
recording chambers (preheated at 32°C) which were
superfused with oxygenated ACSF. Extracellular
field and whole-cell patch-clamp recording in CA1
stratum radiatum using pipettes are performed
(Schmeisser et al 2012). fEPSPs (field Excitatory
PostSynaptic Potentials) and fEPSP slopes are
recorded using the same pipettes. Long-term
potentiation will be induced by a single tetanus of 100
pulses at 100 Hz (Malenka, Bear 2004). For long-
term depression, CA1 is isolated by a microcut set at
the edge of CA2/CA3 before recording synaptic
response, and LTD is induced by 15 min paired-pulse
stimulation at 1 Hz with 50 ms between single pulses
in the presence of 1ml gabazine (Weiler et al 1997).
For NMDA/AMPA ratio, NMDA and AMPA are
separately recorded by compound EPSC (Excitatory
PostSynaptic Currents) of -60mv and +40mv, and ten
consecutive EPSCs for each holding potential will be
averaged.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
754
3.3 Drug Treatment
DHPG (Abcam, Cambridge, MA) will be prepared
within a week and diluted before the experiment.
3.4 Endogenous Application
The Cre-LoxP system will be used to breed
conditional knock-out mice suitable for endogenous
expression of Arc protein. The translation of Arc
protein is strongly related to a gene named FMR1.
FMR1 gene codes Fragile X Retardation Protein
(FMRP), which serves as a negative regulator of
translation and binds to the mRNA (Weiler et al
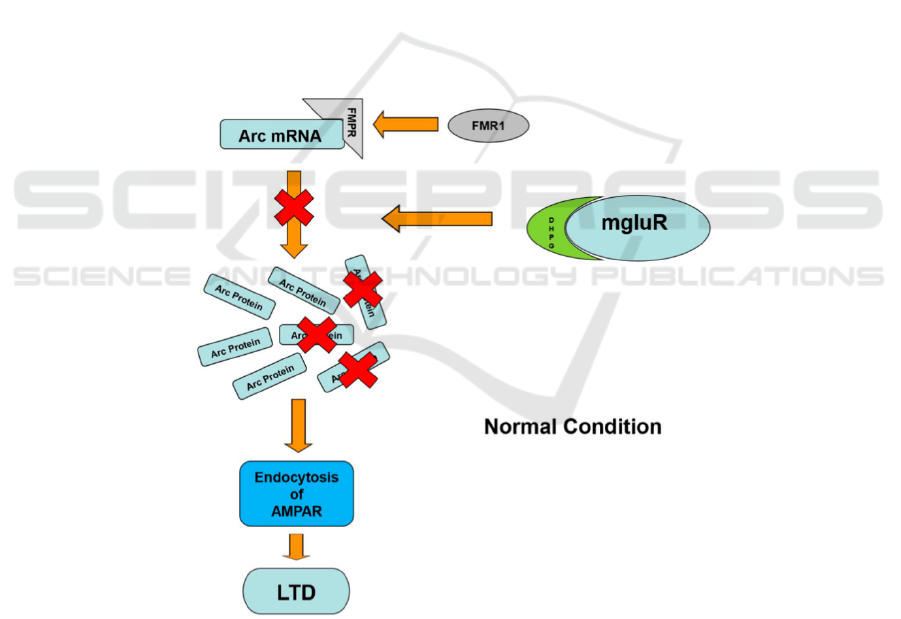
1997). As shown in Figure 1, The Arc protein
translation is induced by the mGluR activated by
DHPG (mGluR agonist). As the FMRP binding to the
mRNA. FMRP inhibits the process of de novo protein
synthesis, so that the amount of protein translated is
maintained at a normal level, as well as the intensity
of mGluR-LTD (Hou, et al 2006, Laggerbauer 2001).
Therefore, the FMR1 gene will be conditionally
knocked out in the CA1 region of APP/PS1 mice, so
that the Arc protein level in that region will increase,
and the mGluR-LTD is likely to be restored. shows
the mechanism of FMR1 inhibiting the translation of
Arc protein. The occurrence of mGluR-LTD is
closely related to the FMRP inhibition.
First, after finding a tissue specific promoter in
the CA1 region of APP/PS1 mice, and then a Cre-
recombinase gene will be introduced downstream
after the promoter (Ray, Fagan, Brunicardi 2000).
Following the treatment, the FMR1 gene in CA1
region of APP/PS1 will be genetically modified with
2 sets of LoxP sites. As shown in Figure 2, each set
of LoxP sites contains 2 loxP sites flanking the
FMR1, and one set of Loxp site will react with the
Cre-recombinase, so that the FMR1 gene can be
inverted and shut down. Afterwards, those 2 types of
genetically modified mice (APP/PS1 Cre mice and
APP/PS1 lox P) will mate with each other. After
mating, their offspring will be APP/PS1/FMR1_
(CA1 region conditional knock-out) mice that can be
used for further treatments.
Figure 1: Mechanism of how FMR1 regulates the interaction between Arc mRNA and mGluR-LTD.
Restoring mGluR-LTD Failure with the Induction of Arc Protein in the CA1 Region of APP/PS1 Mice
755
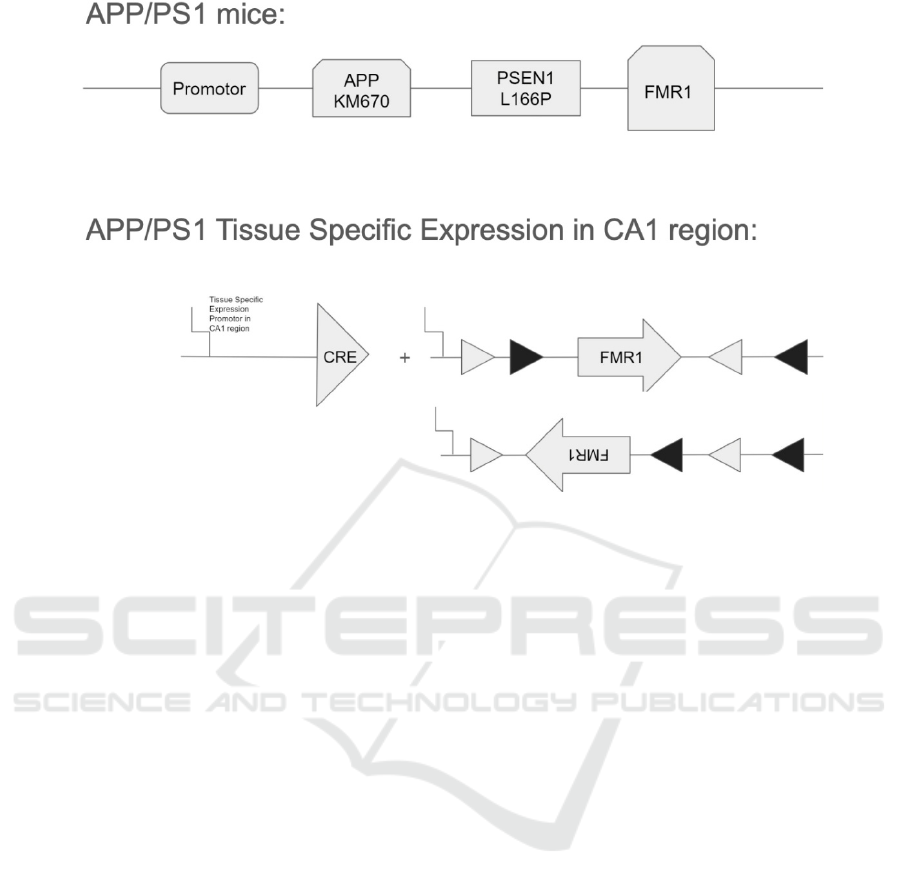
Figure 2: A sketch of how FMR1 conditional knock-out in CA1 region of APP/PS1 mice.
3.5 Exogenous Application
3.5.1 Peptide
A type of peptide called the Arg9 peptide will be
used, which is the most efficient peptide (Ray, Fagan,
Brunicardi 2000). The peptide gets into the cell in a
non-endocytosis transmembrane process. This
eliminates the need of entering the cell by
endocytosis of proteins, which will send the
endosome to the lysosome for degradation. However,
if it’s a non-endocytosis transmembrane process, it
means that the peptide will first attach to the cell
membrane and make a hole on the membrane. The
hole allows the peptide to go into the cell. However,
scientists are still investigating the detailed process of
how peptide gets into the cell as the peptide can repair
the hole so swiftly that scientists can’t observe it
(Jiawan, 2015).
3.5.2 Create Fusion Protein
The Arg9 peptide and arc protein will be fused
together to get the Arg9-Arc protein gene through
genetic engineering.
3.5.3 Construct Prokaryotic Expression
Plasmid
After the fusion protein gene is obtained, it will be
loaded into the plasmid for better transfecting cells.
This recombinant plasmid includes both the target
gene and the plasmid vector.
To get the target gene, the primers are designed
first. The primers will allow DNA to be inserted into
the plasmid. The design can be done by using Primer
Blast or Primer Premier 5.0. The 5 primer’s end will
contain the his-tag, which allows for easy purification
and detection of the recombinant protein. The 3
primer’s end will contain the green fluorescence
protein, which is useful for identifying the protein
localization and analyzing the gene expression. Then
the primers will clone the Arg9-Arc fragment, which
is the target gene fragment wanted. Restriction
enzyme digestion is performed to get this clonal
fragment and the cohesive ends of the plasmid vector.
The recombinant plasmid is then obtained via
enzyme-linking of the fragment and the plasmid
vector. This prokaryotic expression plasmid will then
be sent to the sequence analysis company to confirm
that it is constructed successfully.
3.5.4 Transfection of the Plasmid
A. Expression of the recombinant fusion protein
After DNA is loaded into the plasmid, it will be
transfected into Escherichia coli (E. coli). In E. coli,
the recombinant fusion protein will be expressed. As
adding the inducer will increase the amount of
expression, an inducer will be added when the
bacterial growth reaches the exponential phase of
growth. Herein, the amount of the inducer is being
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
756
added and the time it’s going to induce will need to
be taken into consideration. To optimize the
expression conditions, tests will be set up to
investigate under which conditions the protein will
express the most.
Investigation of time for inducing
(1) Take bacterial fluid which has been induced 0
hour (h), 1h, 2h, 3h, 6h, 8h respectively.
(2) Extract lysates and run SDS-PAGE gel.
(3) Stain the protein gel and elute protein.
(4) Observe the protein band that is at the
expected molecular mass of Arg9-Arc fusion protein.
(5) With time of inducing increases, the
concentration of the target band increases, which
means the brightness of the band increases. Observe
after which hour, the brightness stops increasing. This
means that with this hour’s time of inducing, protein
expression amount is maximized.
B. Purification of the recombinant fusion protein
Then the recombinant fusion protein will be purified
by using the His-tag Ni purification system. This
system can result in the protein of high concentration.
The SDS-PAGE gel electrophoresis is the used to
assess the protein purity. If the target protein band at
the expected molecular mass of Arg9-Arc is distinct,
it means that the purity of protein is high, and the
protein is prepared to be sent into the cells.
4 GROUP SET-UP
1. 10 APP/PS1 mice + DHPG vs. 10 Wild type mice
+ DHPG (LTP, LTD and NMDA/AMPA
measurements)
2. 10 APP/PS1 mice + exogenous Arc protein in
dendrites + DHPG (in CA1 Region) vs. 1(LTP, LTD
and NMDA/AMPA measurements)
3. 10 APP/PS1 FMR1 cKO mice + DHPG (in
CA1 region) vs. 1 (LTP, LTD and NMDA/AMPA
measurements)
5 DATA ANALYSIS
Data are presented as mean ± SEM. For comparison
of the 2 groups, if the data are normally distributed,
then a 2-tailed student t-test was used, otherwise, a
Mann-Whitney test was used. For comparison
between multiple groups, if the data are normally
distributed, then an ANOVA was used, followed by
individual post hoc tests when applicable, otherwise,
a Kruskal-Wallis test was used.
6 DISCUSSIONS
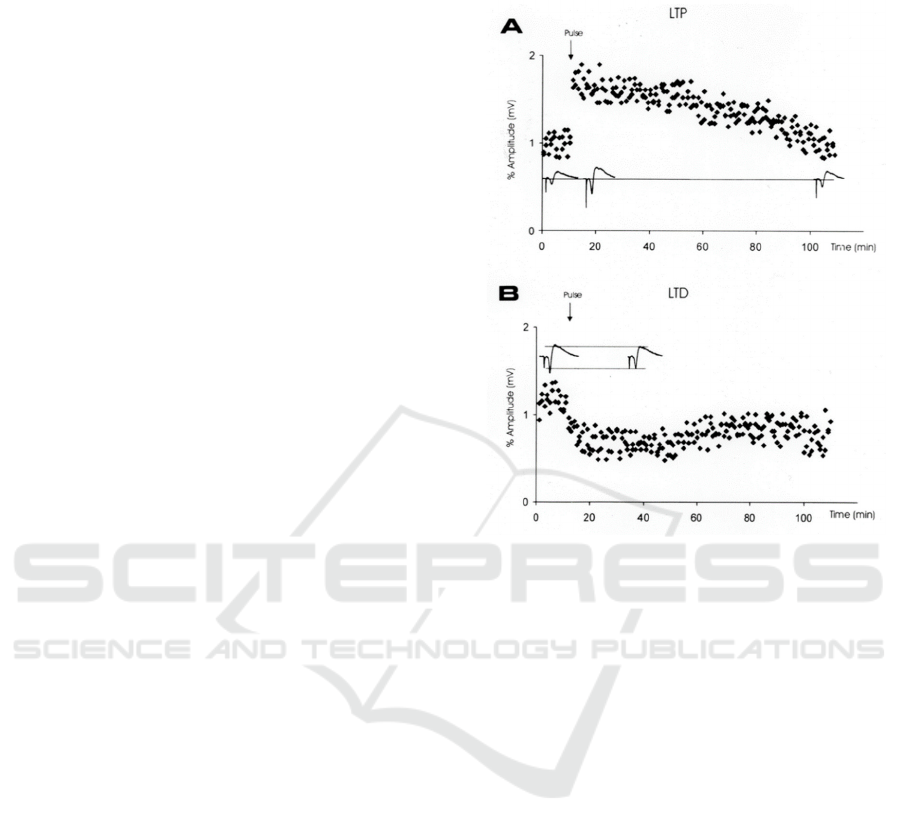
Figure 3: Expected result from electrophysiology showing
LTP and LTD (Leff 2002).
If the results are in accord with the hypothesis, then
the LTP and LTD is likely to be observed in the
recorded diagram similar to Figure 3 below:
For AMPA ratio, a significant reduction of
AMPARs on the synaptic surface will be detected.
Based on these two phenomena, it can be concluded
that the induction of Arc protein reverses the mGluR-
LTD failure, and future study can be performed to
investigate whether the AD symptoms have been
mitigated with the induction of Arc protein.
7 CONCLUSIONS
In the experiment, the mGluR-LTD invalidation in
APP/PS1 mice is reversed through inducing Arc
protein through two approaches and observe whether
the cognitive deficits in the mice have been mitigated.
If the cognitive deficits are improved when mGluR-
LTD failure is reversed, it means that the part of
cognitive abilities is restored.
Meanwhile, if a significant reversal of mGluR-
LTD invalidation in APP/PS1 mice can be observed,
then the inducement of Arc protein can be a potential
Restoring mGluR-LTD Failure with the Induction of Arc Protein in the CA1 Region of APP/PS1 Mice
757

cure for AD. Although, future investigation of Arc
protein inducing mGluR-LTD failure is still required
to ensure the multi-relationship among Arc protein,
mGluR-LTD and the exact does of Ar protein that
needs to maximize the efficiency.
REFERENCES
Abreu, I. D. de, Forlenza, O. V., & Barros, H. L. de. (2005).
Demência de Alzheimer: Correlação entre memória e
autonomia. Rev. psiquiatr. clín. (São Paulo), 131–136.
Bäckman, L., Jones, S., Berger, A.-K., Laukka, E. J., &
Small, B. J. (2004). Multiple cognitive deficits during
the transition to Alzheimer’s disease. Journal of
Internal Medicine, 256(3), 195–204.
https://doi.org/10.1111/j.1365-
2796.2004.01386.xYang, Wenzhong, Xueyan Zhou,
Helena R. Zimmermann, Douglas R. Cavener, Eric
Klann, and Tao Ma. 2016. “Repression of the EIF2α
Kinase PERK Alleviates MGluR-LTD Impairments in
a Mouse Model of Alzheimer’s Disease.”
Neurobiology of Aging 41 (May): 19–24.
doi:10.1016/j.neurobiolaging.2016.02.005.
Förstl, H., & Kurz, A. (1999). Clinical features of
Alzheimer’s disease. European Archives of Psychiatry
and Clinical Neuroscience, 249(6), 288–290.
https://doi.org/10.1007/s004060050101The bad news:
no one has ever recovered from Alzheimer's |
SeniorLiving.com. (2021). Retrieved 31 May 2021,
from https://www.seniorliving.com/journal/bad-news-
no-one-has-ever-recovered-alzheimers
Frank, E. M. (1994). Effect of Alzheimer’s disease on
communication function. Journal of the South Carolina
Medical Association (1975), 90(9), 417–423.
Hou, L., Antion, M. D., Hu, D., Spencer, C. M., Paylor, R.,
& Klann, E. (2006). Dynamic Translational and
Proteasomal Regulation of Fragile X Mental
Retardation Protein Controls mGluR-Dependent Long-
Term Depression. Neuron, 51(4), 441–454.
https://doi.org/10.1016/j.neuron.2006.07.005
Hu, D., Serrano, F., Oury, T. D., & Klann, E. (2006).
Aging-Dependent Alterations in Synaptic Plasticity and
Memory in Mice That Overexpress Extracellular
Superoxide Dismutase. The Journal of Neuroscience,
26(15), 3933–3941.
https://doi.org/10.1523/JNEUROSCI.5566-05.2006
Jiawan, X. (2015). Penetrating Effect of Arg9-EGFP Green
Fluorescent Protein [Master Thesis]. Hunan University.
Laggerbauer, B., Ostareck, D., Keidel, E.-M., Ostareck-
Lederer, A., & Fischer, U. (2001). Evidence that fragile
X mental retardation protein is a negative regulator of
translation. Human Molecular Genetics, 10(4), 329–
338. https://doi.org/10.1093/hmg/10.4.329
Leff, P., Romo, H., Matus, M., Hernández, A., Calva, J. C.,
Acevedo, R., Torner, C., Gutiérrez, R., & Anton, B.
(2002). Understanding the neurobiological
mechanisms of learning and memory: Memory systems
of the brain, long term potentiation and synaptic
plasticity. Part III B. Salud Mental, 25(4), 78–94.
Ma, T., & Klann, E. (2014). PERK: A novel therapeutic
target for neurodegenerative diseases? Alzheimer’s
Research & Therapy, 6(3), 30.
https://doi.org/10.1186/alzrt260
Malenka, R. C., & Bear, M. F. (2004). LTP and LTD: An
Embarrassment of Riches. Neuron, 44(1), 5–21.
https://doi.org/10.1016/j.neuron.2004.09.012
Ray, M. K., Fagan, S. P., & Brunicardi, F. C. (2000). The
Cre–loxP System: A Versatile Tool for Targeting
Genes in a Cell- and Stage-Specific Manner. Cell
Transplantation, 9(6), 805–815.
https://doi.org/10.1177/096368970000900607
Schmeisser, M. J., Ey, E., Wegener, S., Bockmann, J.,
Stempel, A. V., Kuebler, A., Janssen, A.-L., Udvardi,
P. T., Shiban, E., Spilker, C., Balschun, D., Skryabin,
B. V., Dieck, S. tom, Smalla, K.-H., Montag, D.,
Leblond, C. S., Faure, P., Torquet, N., Le Sourd, A.-M.,
… Boeckers, T. M. (2012). Autistic-like behaviours
and hyperactivity in mice lacking ProSAP1/Shank2.
Nature, 486(7402), 256–260.
https://doi.org/10.1038/nature11015
Trinh, M. A., & Klann, E. (2013). Translational control by
eIF2α kinases in long-lasting synaptic plasticity and
long-term memory. Neurobiology of Learning and
Memory, 105, 93–99.
https://doi.org/10.1016/j.nlm.2013.04.013
Waldemar, G., Dubois, B., Emre, M., Georges, J., McKeith,
I. G., Rossor, M., Scheltens, P., Tariska, P., & Winblad,
B. (2007). Recommendations for the diagnosis and
management of Alzheimer’s disease and other
disorders associated with dementia: EFNS guideline.
European Journal of Neurology, 14(1), e1–e26.
https://doi.org/10.1111/j.1468-1331.2006.01605.x
Weiler, I. J., Irwin, S. A., Klintsova, A. Y., Spencer, C. M.,
Brazelton, A. D., Miyashiro, K., Comery, T. A., Patel,
B., Eberwine, J., & Greenough, W. T. (1997). Fragile
X mental retardation protein is translated near synapses
in response to neurotransmitter activation. Proceedings
of the National Academy of Sciences, 94(10), 5395–
5400. https://doi.org/10.1073/pnas.94.10.5395
Wek, R. C., & Cavener, D. R. (2007). Translational control
and the unfolded protein response. Antioxidants &
Redox Signaling, 9(12), 2357–2371.
https://doi.org/10.1089/ars.2007.1764
Wek, R. C., Jiang, H.-Y., & Anthony, T. G. (2006). Coping
with stress: EIF2 kinases and translational control.
Biochemical Society Transactions, 34(Pt 1), 7–11.
https://doi.org/10.1042/BST20060007
Wilkerson, Julia R., Joseph P. Albanesi, and Kimberly M.
Huber. 2018. “Roles for Arc in Metabotropic
Glutamate Receptor-Dependent LTD and Synapse
Elimination: Implications in Health and Disease.”
Seminars in Cell & Developmental Biology,
Arc/ARg3.1, 77 (May): 51–62.
doi:10.1016/j.semcdb.2017.09.035.
Yang, Wenzhong, Xueyan Zhou, Alexey G. Ryazanov, and
Tao Ma. 2021. “Suppression of the Kinase for
Elongation Factor 2 Alleviates MGluR-LTD
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
758

Impairments in a Mouse Model of Alzheimer’s
Disease.” Neurobiology of Aging 98 (February): 225–
30. doi: 10.1016/j.neurobiolaging.2020.11.016.
Yang, Wenzhong, Xueyan Zhou, Helena R. Zimmermann,
Douglas R. Cavener, Eric Klann, and Tao Ma. 2016.
“Repression of the EIF2α Kinase PERK Alleviates
MGluR-LTD Impairments in a Mouse Model of
Alzheimer’s Disease.” Neurobiology of Aging 41
(May): 19–24.
doi:10.1016/j.neurobiolaging.2016.02.005.
Restoring mGluR-LTD Failure with the Induction of Arc Protein in the CA1 Region of APP/PS1 Mice
759
