
The Application of BH-3 Only Mimetics in Tumor Therapy
Chunzi Jiang
1,a,†
, Zheng Wang
2,b,*,†
and Yang Yang
3,c,*,†
1
School of Engineering and Applied Sciences, State University of New York at Stony Brook, New York, U.S.A.
2
The College of Liberal Arts and Sciences, Arizona State University, Tempe, U.S.A.
3
School of Life Sciences, University of Glasgow, Lanarkshire, Glasgow, U.K.
†
These authors contributed equally
Keywords:
BH-3 Only Protein, BH-3 Only Protein Mimetics, BCL-2 Family, Apoptosis.
Abstract:
Tumors are divided into benign and malignant, and the further development of malignant tumors becomes
cancer. In recent years, the number of deaths from cancer worldwide increasing rapidly. To better fight cancer,
targeted therapy is a more precise method. Among them, BH-3 only protein is a representative protein for
targeted therapy. BH-3 only protein is a BCL-2 family protein with only one BH domain and acts as a pro-
apoptotic protein to regulate cell apoptosis. Different BH-3 only proteins have different binding options with
anti-apoptotic proteins in the BCL-2 family. BH-3 only protein has an excellent performance in inhibiting
cancer cells, and the research of BH-3 only mimics constantly updated. This paper will introduce drug
research on tumor treatment by studying the effects of targeted BH-3only mimics and expanding drugs'
development for its related family proteins.
1 INTRODUCTION
Programmed cell death, or apoptosis, is a unique form
of suicidal cell death accompanied by cell size
reduction and chromatin concentration (Kroemer et
al. 2005). Apoptosis controls the number of cells in
an organism, eliminating harmful or virus-infected
cells. There are mainly three pathways regulating
apoptosis: mitochondria-mediated pathway, death
receptor pathway, and endoplasmic network pathway
(Kerr, Wyllie, Currie 1972). The interactions of the
BCL-2 family control the mitochondrial apoptosis
pathway (Levine, Sinha, Kroemer 2008). Structural
feature similarity is determined by sequence
homology. Hydrophobic slits are formed between the
four (BH1-BH4) domains of protein-protein
interaction, which are involved in the proapoptotic
protein BH3 domain uptake through
heterodimerization. BCL-2 anti-apoptotic proteins
include BCL-2, BCL-W, BCL-XL, MCL-1, and A1
(BCL2A1/ BCL-1), containing four BH homologs
have similar protein 3D structures (Figure 1a)
(Shamas-Din, et al. 2011).
The balance of these interactions determines the
life cycle length of the cells expressing the
corresponding protein. PUMA, BIM, tBID can bind
to all members (Ley, et al. 2005, Qian, Zhang, Zhi
2017). TBID can antagonize the pro-survival
function of BCL-2 and can directly combine with
BAX and BAK to initiate apoptosis (Merino, et al.
2009, Hutt 2015). Except for BH-3, only protein,
which can bind to any pro-survival protein, the
binding of other BH-3 only proteins are selective
(Figure 1b). BH-3 only protein will be selected based
on the above binding to respond to apoptosis signals
so that these BCL-2 anti-apoptotic proteins are
isolated from BAX and BAK (Singh, Letai, Sarosiek
2019).
As of 2018, among the 18 million cases of cancer
globally, the number of deaths is estimated to have
reached 9.6 million, and cancer has become the
world's second prominent cause of death (Copur
2019). During the research process, the excellent
therapeutic effect of targeted drug therapy emerged
as a hot research object in cancer treatment
worldwide. BH-3 mimics anti-tumor drugs are a new
type of anti-tumor drugs targeting BCL-2 family anti-
apoptotic member proteins. This article will
introduce the clinical drug course and progress of
BH-3only mimics developed by the selective binding
of different BH-3 only proteins.
Jiang, C., Wang, Z. and Yang, Y.
The Application of BH-3 Only Mimetics in Tumor Therapy.
DOI: 10.5220/0011295300003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 791-796
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
791
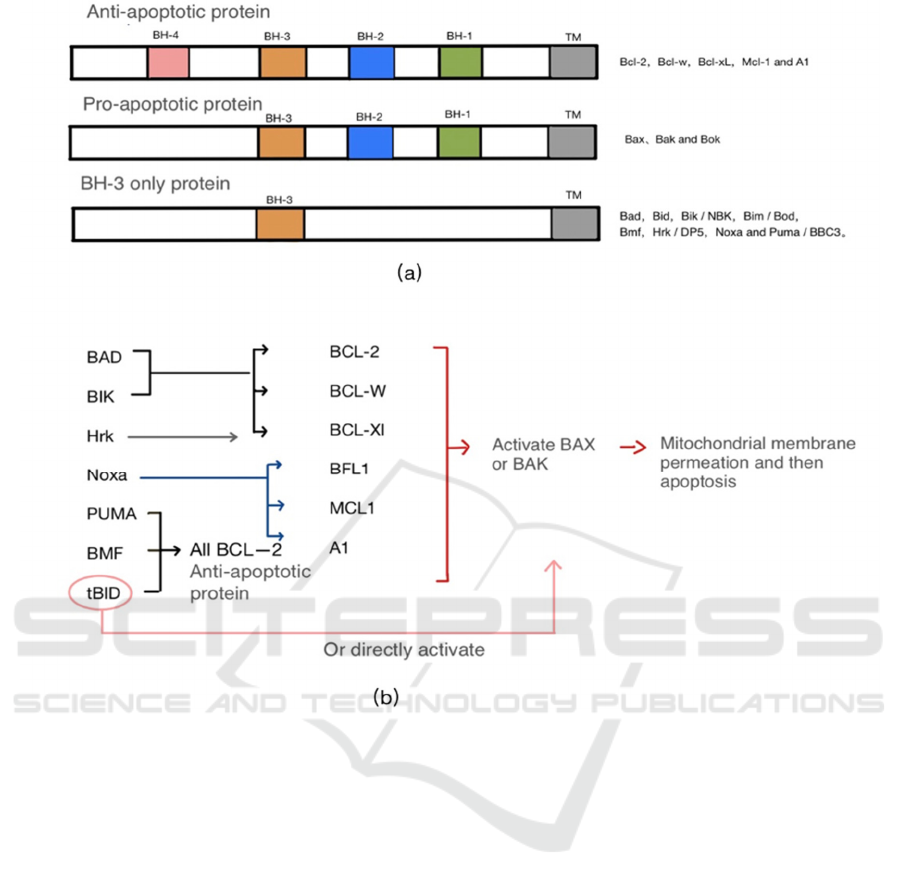
Figure 1: BCL-2 family protein structure and selective binding.
2 BH-3 ONLY MIMETICS
Cancer cells are different from normal cells in that
they have three major characteristics: infinite
proliferation, transformability, and easy metastasis.
These characteristics bring great difficulties to the
treatment of cancer. Since discovering the apoptotic
properties and three-position structure of BCL-2 anti-
apoptotic family proteins, research on its chemical
resistance and protein inhibitors has continued.
Targeted cancer treatment is the current mainstream
idea of clinical treatment of cancer to improve the
cure rate and the survival time of patients.
Overexpression of anti-apoptotic BCL-2 family
proteins can drive cancer cell proliferation or
resistance to chemotherapy drugs. The combination
of BH-3 only protein and the anti-apoptotic BCL-2
family protein leads to the release and activation of
Bak and Bax, which leads to cell apoptosis.
Therefore, BH3 mimics have great prospects in
developing drugs targeting the anti-apoptotic BCL-2
protein (Baell, Huang 2002).
2.1
Target BCL-2 Protein2.2 Target
MCL-1 Protein
2.1.1 S55746
Its selective characteristics indicate that it does not
significantly bind to MCL-1, BFL-1 (BCL2A1/A1),
and has a poor affinity for BCL-XL. S55746 can be
taken orally and is not harmful to BCL-X1-dependent
cells such as platelets. The combination of S55746
and ABT-199 is different. S55746 occupies the
S1/2/3 region and forms a hydrogen bond with the
carboxyl group of the A149 skeleton in the S2 residue
(Casara, et al. 2018), forming a highly specific
binding. ABT-199 occupies more protein surface
area, including S2/3/4/5 (Souers, et al. 2013).
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
792

2.1.2 Phenothiazine
Tumor cells have developed a variety of strategies to
achieve proliferative advantages. Researchers are
developing chemicals to impede the interaction
between the pro-apoptotic protein, thereby
mimicking the mechanism of action between the pro-
apoptotic protein and the BH-3 domain (Degterev, et
al. 2001). Other drugs in the article, such as ABT-737
and ABT-263, have appeared and used for many
years. To increase the treatment of tumors, the
interaction between phenothiazine and biofilm is due
to the amphiphilic nature of the molecule. The
thiazide core is relatively hydrophobic (Philot, et al.
2016, Homem-de-Mello, Mennucci, Tomasi, et al.
2005). Therefore, phenothiazine drugs have a great
potential for the anti-apoptotic protein BCL-2
inhibitory effect and appear in the actual treatment
process as drugs for the treatment of tumors.
2.1.3 Gossypol /ApoG2
Gossypol is a polyphenol extracted from the
Campagnaceae plant (Flack, et al. 1993). Gossypol is
helpful in human clinical trials of stage ii cancer.
Gossypol is a well-known toxic compound. However,
Apogossypolone (ApoG2) can be obtained by
removing two aldehyde groups (Arnold, et al., 2008).
Moreover, in clinical trials, due to the existence of
two reactive aldehyde groups, which are related to
side effects of gossypol, such as vomiting and
diarrhea, the design and synthesis of ApoG2
eliminates the reactive groups, minimizing side
effects, and ApoG2 has higher stability and efficacy
than its parent compound.
Follicular lymphoma (FL) is the fifth most
diagnosed cancer (Rogers 2005). ApoG2 was found
to be used in the treatment of follicular lymphoma.
Lymphoma (FL is very effective, it inhibits cell
growth by lysing the cell lymphoma cell line (WSU-
FSCCL). The cell growth inhibition rate is 50%. In
nasopharyngeal carcinoma cells, ApoG2 completely
blocks the anti-apoptotic function of BCL-2 family
proteins (McDermott, Dutt, Watkinson 2001).
ApoG2 has three types. Researchers have shown
through research that ApoG2 may be a new BCL -2
family protein inhibitor. By targeting these proteins,
it may become a promising drug for the treatment of
nasopharyngeal carcinoma.
2.2 Target MCL-1 Protein
2.2.1 S63845
As a BH-3 mimic, S63845 can bind to MCL1 with
high affinity and specificity. S63845 can effectively
kill MCL1-dependent cancer cells, although as a
single drug, it can effectively act on multiple
myeloma, leukemia, and lymphoma cells (Kotschy, et
al. 2016). S63845 binds to MCL-1 more efficiently
and specifically. The binding affinity of S63845
synthetic MCL-1 inhibitor for MCL-1 is 20 times that
of A-1210477, and the effect of killing MCL1-
dependent H929 multiple myeloma cells is 1000
times that of A-1210477 (Merino, et al. 2017, Li, Z.,
He, and Look 2019). The combination of S63845 and
ABT-737, ABT-263, ABT-199, which have a low
binding capacity to BCL-1, can improve the
therapeutic efficacy (Merino, et al. 2017).
Experiments have shown that the combined
administration of S63845 and ABT-199 can more
effectively induce human T-lymphocytic leukemia
(T-ALL) cells (Li, Z., He, and Look 2019).
2.2.2 S64315/MIK665
S64315 is simulant S63845, and the effect is better
than S68345 (Hird and Tron 2019). Compared with
S63845, there is no clinical trial (Szlavik, et al. 2020).
Acute myeloid leukemia (AML), myelodysplastic
syndrome (MDS) (NCT03672695 and NCT
02979366), and multiple myeloma (MM)
(NCT02992483), the first phase of MDS and MM
trials just ended in March 2021.
2.2.3 VU661013
VU661013 is a derivative of indole-2-carboxylic
acid, which can reduce the expansion of AML cell
lines (Ramsey, et al. 2018). VU661013 can also make
ER+ breast cancer cells apoptosis and will not up-
regulate BCL-2 or BCL-XL in ER+ breast cancer
cells during treatment (Williams, et al. 2019), which
is better than S68345.
2.3 Target BCL-XL Protein
2.3.1 A-1155463/A-1331852
BCL-XL is an anti-apoptotic protein located in
mitochondria and one of the key factors of cell
apoptosis. Malignant pleural mesothelioma (MPM)
has been tested with a series of BH-3 mimics, and
BCL-XL is the main pro-survival protein. Malignant
pleural mesothelioma (MPM) is one of the cancers
The Application of BH-3 Only Mimetics in Tumor Therapy
793

with the lowest survival rate. This experiment
discovers that BCL-XL is a feasible breakthrough in
treating malignant pleural mesothelioma (MPM)
(Arulananda, et al. 2020). A-1331852 is a potent and
selective BCL-XL inhibitor that can be taken orally.
Using structure-based drug design to redesign the
BCL-XL inhibitor A-1155463 reported earlier is also
a further discovery of BCL-XL inhibitors. The
inhibitor is a small molecule (Wang, et al. 2020).
Research on A-1155463 found that it has a huge
effect on BCL-XL-dependent tumors, and it also
retains the cell system of BCL-2 and MCL-1. The
Epstein-Barr virus (EBV) related T cell and natural
killer (NK) cell malignancies, A-1331852-induced
apoptosis ENKTL cell line SNK6 established a
xenograft model provides evidence that A-1331852
treatment may be effective It is beneficial in vivo
(Arulananda, et al. 2020). After treatment with A-
1331852, it can continue to induce apoptosis to
achieve the therapeutic effect.
2.4 Target Multiple Proteins
2.4.1 ABT-737
ABT-737, as a kind of BH-3 only mimic, can bind to
Bcl-2, BCL-W, and BCL-XL (Shin, et al. 2015).
ABT-737 leads cancer cells to apoptotic, but it is
harmful to common cells (Oltersdorf, et al. 2005).
Although it just can be combined with the MCL-1 that
involves many apoptosis pathways, ABT-737 still has
excellent prospects in clinical. Overexpression of
MCL-1 and A1 will weaken the sensitivity of cells to
drugs. If MCL-1 is inactivated, overexpression of
BCL-2 will not reduce the cytotoxic activity of ABT-
737 for cancer cells, but overexpression of BCL-CL
will relatively reduce the efficacy of the drug. Mcl-1
is an unstable protein, and the half-life of Mcl-1
mRNA and MCL-1 protein is very short (Anderson,
et al. 2016). Seliciclib, cyclin-dependent kinase
inhibitor and protein synthesis inhibitor
cyclohexylamine (CHX), can reduce MCL-1 levels
and significantly increase the sensitivity of cells to
ABT-737 (F.van 2006). This report shows that the
combination of inactivated MCL-1 and ABT-737 is
promising for clinical treatment. It has been reported
that ABT-737 is particularly sensitive to acute
myeloid leukemia stem cells. Phenformin can
increase cell sensitivity more on this basis, and ABT-
737 combined with phenformin can be more suitable
for targeting hematological malignancies (Velez, et
al. 2016).
2.4.2 ABT-263
ABT-737 is the most successful and potential BH3
mimic compound developed by Abbott. In recent
years, this mimic has played an indispensable role in
regulating apoptosis therapy. However, ABT-737
also has restrictions. That is, it cannot be taken orally.
The full name of ABT-263 is navitoclax (Tse, et al.
2008). But it is different from ABT-737. For
chemotherapeutics that use drugs to adjust cell
apoptosis, cell apoptosis resistance caused by
inducing factors is a key hindrance to cancer control.
To study this problem, a phase I experiment was
conducted in 2007 (Gandhi, et al. 2011). These
problems reflect the need for improvement of ABT-
263. In the records of Gernot et al. in 2015, wogonin,
apigenin, chrysin, etc., can reduce the efficacy of
enhancing ABT-263 and thus reduce the dosage of
drugs (Polier, et al. 2015). In an experiment on ABT-
263 in 2020, ABT-263 affected bone changes and cell
damage to a certain degree in aged mice (Sharma, et
al. 2020). The experiment is based on the in vitro oral
administration of several groups of old mice. The use
of isolation and contrast culture of bone marrow
stromal cells from ABT-263 or carrier-treated mice
obtained experimental results.
2.4.3 ABT-199
Following ABT-737 and ABT-263, there have been
new advances in BCL-2 inhibitors. ABT-199 is a new
type of small molecule inhibitor. Although the first
two have targeted treatment characteristics, the
selectivity of ABT-199 is special. The treatment of
tumors by BH-3 mimics is achieved by adding drugs
to the metabolic mechanism. ABT-199 can resist
tumors and reduce the damage to certain cells during
treatment (Davids and Letai 2013). As mentioned
above, ABT-263, the inhibitor, can be taken orally
and cause damage to platelets. Souers and colleagues
redesigned reverse engineering in which ABT-199
selectively killed BCL-2 cells without destroying
BCL-XL cells. Proved that ABT-199 is a highly
effective and selective inhibitor. In other
developments, ABT-199 has also made new
breakthroughs (Jakubowska, et al. 2019). It will not
destroy the steady state of certain ions in the internal
environment. ABT-199 has a slight effect on the
content of ions in the solute and does not significantly
change the ion steady state in PAC. It is superior to
earlier BCL-2 inhibitors. Therefore, the side effects
of ABT-199 when used in the treatment of leukemia
are relatively low. In recent years, it has been
clinically discovered that a deacetylase inhibitor
chidamide (CS055) combined with ABT-199
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
794

treatment can ensure cell viability and enhance ABT-
199 activity (Chen, et al. 2020, Lucantoni, et al.
2018). In breast cancer treatment, selective inhibitors
of BCL-2 and BCL-Xl slow down the synthesis of
ATP and lethal cancer cells (Szlavik, et al. 2020).
However, the overexpression of the drug in certain
lymphoid
malignancies is caused by drug resistance.
Through the whole genome screening of human acute
myeloid leukemia (AML), it is known that the
mitochondrial structure causes a sensitive response to
ABT-199. In the end, Kristina et al. proved that
mitochondrial chaperone protein (CLPB) directly
interacts with the main regulator of mitochondrial
dynamics (OPA1) (Chen, et al. 2019). It is possible
that evasion of ABT-199 resistance can be achieved
by targeting mitochondria.
3 CONCLUSIONS
BH-3 protein plays an indispensable role in cell
apoptosis by selectively binding to BCL-2 family
anti-apoptotic proteins and inducing apoptosis. ABT-
737, ABT-263, and ABT-199 promote the
oligoylation of BAX and BAK and, eventually, the
apoptosis of cancer cells. They have shown efficacy
in some cancer cases and play an indispensable role
in regulating apoptosis therapy. S63845, S64315, and
VU661013 are all bH-3 mimics that inhibit BCL-1
and reduce the amplification of AML cell lines. A-
1331852 is a practical, oral selective inhibitor of
BCL-XL. ApoG2 study provides new ideas for
nasopharyngeal carcinoma, respectively. Each of
these drugs has its advantages but is not sufficient on
its own because of the complexity of cancer and
apoptotic procedures. Clinical studies have shown
that combinations of drugs that impede BCL-2 and
McL-1 proteins can improve treatment outcomes,
such as the combination of S63845 and ABT-199,
which can prolong the life of patients. This suggests
that BH-3 mimics still have a promising application
in cancer treatment.
REFERENCES
Arnold, A.A., et al., Preclinical studies of Apogossypolone:
a new nonpeptidic pan small-molecule inhibitor of Bcl-
2, Bcl-XL and Mcl-1 proteins in Follicular Small
Cleaved Cell Lymphoma model. Mol Cancer, 2008. 7:
p. 20.
Arulananda, S., et al., BCL-XL is an actionable target for
treatment of malignant pleural mesothelioma. Cell
Death Discov, 2020. 6(1): p. 114.
Anderson, G.R., et al., PIK3CA mutations enable targeting
of a breast tumor dependency through mTOR-mediated
MCL-1 translation. Sci Transl Med, 2016. 8(369): p.
369ra175.
Baell, J.B. and D.C. Huang, Prospects for targeting the Bcl-
2 family of proteins to develop novel cytotoxic drugs.
Biochem Pharmacol, 2002. 64(5-6): p. 851-63.
Copur, M.S., State of Cancer Research Around the Globe.
Oncology (Williston Park), 2019. 33(5): p. 181-5.
Casara, P., et al., S55746 is a novel orally active BCL-2
selective and potent inhibitor that impairs
hematological tumor growth. Oncotarget, 2018. 9(28):
p. 20075-20088.
Chen, K., et al., Preclinical evaluation of a regimen
combining chidamide and ABT-199 in acute myeloid
leukemia. Cell Death Dis, 2020. 11(9): p. 778.
Chen, X., et al., Targeting Mitochondrial Structure
Sensitizes Acute Myeloid Leukemia to Venetoclax
Treatment. Cancer Discov, 2019. 9(7): p. 890-909.
Degterev, A., et al., Identification of small-molecule
inhibitors of interaction between the BH3 domain and
Bcl-xL. Nat Cell Biol, 2001. 3(2): p. 173-82.
Davids, M.S. and A. Letai, ABT-199: taking dead aim at
BCL-2. Cancer Cell, 2013. 23(2): p. 139-41.
Flack, M.R., et al., Oral gossypol in the treatment of
metastatic adrenal cancer. J Clin Endocrinol Metab,
1993. 76(4): p. 1019-24.
F.van, M., The BH3 mimetic ABT-737 targets selective
Bcl-2 proteins andefficiently induces apoptosis via
Bak/Bax if Mcl-1 is neutralized. November 13, 2006.
10(5): p. 389-399.
Gandhi, L., et al., Phase I study of Navitoclax (ABT-263),
a novel Bcl-2 family inhibitor, in patients with small-
cell lung cancer and other solid tumors. J Clin Oncol,
2011. 29(7): p. 909-16.
Hutt, K.J., The role of BH3-only proteins in apoptosis
within the ovary. Reproduction, 2015. 149(2): p. R81-
9.
Homem-de-Mello, P., Mennucci, B., Tomasi, J. et al., The
effects of solvation in the theoretical spectra of cationic
dyes.. Theor Chem Acc June 2005. 113: p. 274–280.
Hird, A.W. and A.E. Tron, Recent advances in the
development of Mcl-1 inhibitors for cancer therapy.
Pharmacol Ther, 2019. 198: p. 59-67.
Jakubowska, M.A., et al., ABT-199 (Venetoclax), a BH3-
mimetic Bcl-2 inhibitor, does not cause Ca (2+) -
signalling dysregulation or toxicity in pancreatic acinar
cells. Br J Pharmacol, 2019. 176(22): p. 4402-4415.
Kroemer, G., et al., Classification of cell death:
recommendations of the Nomenclature Committee on
Cell Death. Cell Death Differ, 2005. 12 Suppl 2: p.
1463-7.
Kerr, J.F., A.H. Wyllie, and A.R. Currie, Apoptosis: a basic
biological phenomenon with wide-ranging implications
in tissue kinetics. Br J Cancer, 1972. 26(4): p. 239-57.
Kotschy, A., et al., The MCL1 inhibitor S63845 is tolerable
and effective in diverse cancer models. Nature, 2016.
538 (7626): p. 477-482.
The Application of BH-3 Only Mimetics in Tumor Therapy
795

Levine, B., S. Sinha, and G. Kroemer, Bcl-2 family
members: dual regulators of apoptosis and autophagy.
Autophagy, 2008. 4(5): p. 600-6.
Ley, R., et al., Regulatory phosphorylation of Bim: sorting
out the ERK from the JNK. Cell Death Differ, 2005.
12(8): p. 1008-14.
Li, Z., S. He, and A.T. Look, The MCL1-specific inhibitor
S63845 acts synergistically with venetoclax/ABT-199
to induce apoptosis in T-cell acute lymphoblastic
leukemia cells. Leukemia, 2019. 33(1): p. 262-266.
Lucantoni, F., et al., BCL2 and BCL(X)L selective
inhibitors decrease mitochondrial ATP production in
breast cancer cells and are synthetically lethal when
combined with 2-deoxy-D-glucose. Oncotarget, 2018.
9(40): p. 26046-26063.
Merino, D., et al., The role of BH3-only protein Bim
extends beyond inhibiting Bcl-2-like prosurvival
proteins. J Cell Biol, 2009. 186(3): p. 355-62.
McDermott, A.L., S.N. Dutt, and J.C. Watkinson, The
aetiology of nasopharyngeal carcinoma. Clin
Otolaryngol Allied Sci, 2001. 26(2): p. 82-92.
Merino, D., et al., Synergistic action of the MCL-1 inhibitor
S63845 with current therapies in preclinical models of
triple-negative and HER2-amplified breast cancer. Sci
Transl Med, 2017. 9(401).
Oltersdorf, T., et al., An inhibitor of Bcl-2 family proteins
induces regression of solid tumours. Nature, 2005.
435(7042): p. 677-81.
Philot, E.A., et al., Binding of phenothiazines into allosteric
hydrophobic pocket of human thioredoxin 1. Eur
Biophys J, 2016. 45(3): p. 279-86.
Polier, G., et al., Targeting CDK9 by wogonin and related
natural flavones potentiates the anti-cancer efficacy of
the Bcl-2 family inhibitor ABT-263. Int J Cancer, 2015.
136(3): p. 688-98.
Qian, K., Y. Zhang, and X. Zhi, [Retrospective Study of
Efficacy in BIM Gene Polymorphism on First-line
EGFR-TKIs Treatment for Advanced Lung
Adenocarcinoma]. Zhongguo Fei Ai Za Zhi, 2017.
20(8): p. 543-548.
Rogers, B., Looking at lymphoma and leukemia. Nursing,
2005. 35(7): p. 56-63; quiz 63-4.
Ramsey, H.E., et al., A Novel MCL1 Inhibitor Combined
with Venetoclax Rescues Venetoclax-Resistant Acute
Myelogenous Leukemia. Cancer Discov, 2018. 8(12):
p. 1566-1581.
Shamas-Din, A., et al., BH3-only proteins: Orchestrators of
apoptosis. Biochim Biophys Acta, 2011. 1813(4): p.
508-20.
Singh, R., A. Letai, and K. Sarosiek, Regulation of
apoptosis in health and disease: the balancing act of
BCL-2 family proteins. Nat Rev Mol Cell Biol, 2019.
20(3): p. 175-193.
Souers, A.J., et al., ABT-199, a potent and selective BCL-2
inhibitor, achieves antitumor activity while sparing
platelets. Nat Med, 2013. 19(2): p. 202-8.
Szlavik, Z., et al., Discovery of S64315, a Potent and
Selective Mcl-1 Inhibitor. J Med Chem, 2020. 63(22):
p. 13762-13795.
Shin, J.A., et al., Targeting ERK1/2-bim signaling cascades
by BH3-mimetic ABT-737 as an alternative therapeutic
strategy for oral cancer. Oncotarget, 2015. 6(34): p.
35667-83.
Sharma, A.K., et al., The Senolytic Drug Navitoclax (ABT-
263) Causes Trabecular Bone Loss and Impaired
Osteoprogenitor Function in Aged Mice. Front Cell
Dev Biol, 2020. 8: p. 354.
Tse, C., et al., ABT-263: a potent and orally bioavailable
Bcl-2 family inhibitor. Cancer Res, 2008. 68(9): p.
3421-8.
Velez, J., et al., Biguanides sensitize leukemia cells to
ABT-737-induced apoptosis by inhibiting
mitochondrial electron transport. Oncotarget, 2016.
7(32): p. 51435-51449.
Williams, M.M., et al., Therapeutic inhibition of Mcl-1
blocks cell survival in estrogen receptor-positive breast
cancers. Oncotarget, 2019. 10(52): p. 5389-5402.
Wang, L., et al., Discovery of A-1331852, a First-in-Class,
Potent, and Orally-Bioavailable BCL-XL Inhibitor.
ACS Med Chem Lett, 2020. 11(10): p. 1829-1836.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
796
