
Screening and Identification of Bacillus amyloliquefaciens from the
Gut of Aquatic Animals
Hedong Lu
1, 2 a
, Chengyuan Gu
1b
, Tao Yan
1c
, Qihan Zhang
1d
, Chengxin Geng
1,* e
and Can Lv
1f
1
School of Life Science and Food Engineering, Huaiyin Institute of Technology, Huaian, China
2
School of Food Science and Technology, Jiangnan University, Wuxi, China
19851781556@163.com
Keywords: Bacillus amyloliquefaciens, Gut, Appraisal, Probiotics.
Abstract: Probiotics have the dual effects of inhibiting pathogenic bacteria and promoting growth, while being green
and environmentally friendly, and will not cause problems such as increased drug resistance, veterinary
drug residue, and meat quality deterioration, making them to be good substitute for antibiotic. In order to
realize healthy and ecological culture and provide good microbial resources for probiotics, this experiment
isolated probiotics from the intestinal tracts of cultured Procambarus clarkii and crab. One strain LPB-5 was
identified according to traditional colony morphological characteristics observation, physiological and
biochemical tests and 16S rDNA sequence analysis. The results showed that the strain had the closest
relationship with Bacillus amyloliquefaciens MPA 1034, the 16S rDNA sequence similarity was 99%, and
the physiological and biochemical reaction spectrum was consistent with the 16S rDNA sequence analysis,
which identified the strain as Bacillus amyloliquefaciens. Procambarus clarkii and crab are important
aquaculture animals. The probiotics isolated from the intestinal tract of procambarus clarkii have their own
biosafety and high efficiency, which lays a foundation for the later development of probiotics application in
aquatic feed.
1 INTRODUCTION
1
There are a very large number of microorganisms in
the intestinal tract, which are generally divided into
neutral bacteria, probiotics and harmful bacteria. In
the long-term evolution of intestinal flora, as the
host adapts to the environment and natural selection,
the microbial community, host and environment
depend on each other to form a system of mutual
restriction, which is always in a state of relative
dynamic balance (Yan, 2017). The relationship
between the host and its intestinal microbes also has
a very important impact on the health of aquaculture
animals (Chaiyapechara, 2012. Liu, 2011). A large
number of studies have found that intestinal flora
a
https://orcid.org/0000-0002-1440-9902
b
https://orcid.org/0000-0002-6723-9572
c
https://orcid.org/0000-0001-8430-018X
d
https://orcid.org/0000-0001-6518-3432
e
https://orcid.org/0000-0002-5830-3106
f
https://orcid.org/0000-0002-0610-8464
can help and promote the body's antagonism to
pathogenic bacteria, enhance the body's immunity
and absorb nutrients (Ravi, 2007. Olmos, 2011).
Using probiotics to replace antibiotics and other
drugs with great side effects to achieve true
ecological aquaculture has become the trend and
research hotspot of the entire aquaculture (Chauhan,
2019). So far, some probiotics have been used as
alternative antibiotics or disease control agents for
the prevention and control of diseases in aquaculture
animals (Das, 2008. Kuebutornye, 2019. Waldroup,
2003). Probiotics inhibit the growth of harmful
bacteria in the gut (Hazel, 2020). The experiment of
Li et al. verified the inhibitory effect of competition
between two kinds of pathogenic bacteria and five
kinds of probiotics on the gastrointestinal mucosa of
fish (Li, 2012). Probiotics can produce non-specific
immune regulatory factors to stimulate the host's
immune function, effectively increase the
concentration of immunoglobulin in the host,
significantly enhance the activity of macrophages
and interferon, and strengthen the host's immunity to
Lu, H., Gu, C., Yan, T., Zhang, Q., Geng, C. and Lv, C.
Screening and Identification of Bacillus amyloliquefaciens from the Gut of Aquatic Animals.
DOI: 10.5220/0011297900003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 855-861
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
855

viruses, bacteria, fungi and parasites
(Elsabagh,2018. Douglas, 2008. Opiyo, 2019).
Abarike ED et al. showed that Nile tilapia can
increase the activity of relevant enzymes in their
bodies and increase their own immunity by
consuming a commercial probiotic called BS (a
mixture of Bacillus subtilis and Bacillus
licheniensis) (Abarike, 2018). Probiotics can secrete
various digestive enzymes (amylase, protease,
lipase, etc.) in the body of aquaculture animals,
promote the absorption and transformation of
nutrients in the feed of aquaculture animals, and
improve feed digestibility, which may play a role in
animal nutrition and feed utilization rate (Das, 2019.
Hai, 2009. Balcáza, 2006). Probiotics can also
improve the constitution and meat quality of aquatic
animals, and improve their tolerance to aquatic
environment (Valente,2016. Wang, 2020). Studies
have shown that after feeding Western King prawns
with probiotics, the water content of the shrimp body
decreases, while the protein content increases, and
their constitution and meat quality are significantly
improved (Hai, 2009).
In this study, procambarus clarkii and crab were
used as experiment subjects and their intestinal
microorganisms were selected, separated and
identified. The isolated probiotics have great
potential in developing probiotics for aquaculture
feed due to their biosafety and high efficiency.
2 MATERIALS AND METHODS
2.1 Test Raw Materials and Media
Five alive procambarus clarkii and five crabs, which
were purchased from dajiang Aquatic Market in
Huai 'an, were used as raw materials.
Nutrient broth medium (per liter): 10 g peptone,
3 g beef extract, 5 g sodium chloride, pH was
adjusted to 7.2-7.4. Nutrient broth agar medium: 17
g agar was added on the basis of the above. It was
sterilized at 121℃ for 20 min in a high pressure
steam sterilizer.
Soluble starch medium (per liter): 10 g peptone,
5 g sodium chloride, 5 g beef extract, 2 g soluble
starch. It was sterilized at 115℃ for 30 min in a high
pressure steam sterilizer.
Gelatin liquefication medium (per liter): 10 g
peptone, 3 g beef extract, 5g sodium chloride, 12g
gelatin; the pH value was adjusted to 7.0 and
sterilize at 115℃ for 30 min in a high-pressure
steam sterilization pot.
Sugar fermentation medium (per liter): 5 g
peptone, 5 g tryptone, 5 g sodium chloride, 5 mL
Tween-80, 1.4 mL 1.6% Bromocresol violet
solution, 10 g sugar, 6 g agar. It was sterilized at
115℃ for 30 min in a high pressure steam sterilizer.
2.2 Incubation and Isolation of the
Strain
Procambarus clarkii and crabs were washed with
sterile water and the body surfaces were disinfected
with 75% ethanol. The intestinal tracts were
dissected in a super clean table under sterile
conditions. They were placed in test tubes with 10
mL sterilized normal saline, shaken and mixed, and
left for 10 min. The supernatant was inoculated in
the nutrient broth medium and placed in a constant
temperature and oscillation incubator for 24 h at
37℃ and 200 rpm. The cultured bacteria solution
was placed in a water bath at 80℃ for 15 min to kill
the non-spore cells, and then inoculated again into
the nutrient broth medium for enrichment and
cultured for 24 h. Appropriate gradient dilution was
performed on the bacterial solution, which was
coated in nutrient broth agar medium, and the
colonies with growth advantage were selected for
repeated lineation separation until pure strains were
obtained.
2.3 Physiological and Biochemical
Identification of Strain
The isolated strains were selected and inoculated on
the nutrient broth agar medium and placed in a
constant temperature incubator at 37℃. Colony
morphological characteristics were observed after 12
h culture, and the bacteria were selected for gram
staining test, contact enzyme test, starch hydrolysis
test and other physiological and biochemical tests.
2.3.1 Gram Stain Test
Gram dyeing test generally includes four steps:
initial dyeing, medium dyeing, decolorization and
redyeing. The specific operation methods are as
follows: 1) Take strains, smear them and fix them on
the slide. 2) Stain with crystal violet of ammonium
oxalate for 1.5min and rinse with fine water. (3)
Cover the coated surface with iodine solution and
dye it for about 1min. Rinse with fine water and
absorb the water with absorbent paper. 4) Add a few
drops of 95% alcohol, and gently shake for
decolorization, after 20 seconds, wash with water to
absorb the water. (5) After dyeing with sand yellow
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
856

solution for 1min, rinse with distilled water. Dry and
examine under microscope.
2.3.2 Contact Enzyme Test
The single colonies were inoculated on the medium
for 24 h, and then inoculated on the glass with 3%
hydrogen peroxide by inoculation ring. If there were
bubbles, it was positive; otherwise, it was negative.
2.3.3 Starch Hydrolysis Test
Single colonies were inoculated on soluble starch
medium and incubated at 37℃ for 48 h. Then the
iodine solution was dropped on the petri dish. If the
colonies formed a transparent circle around them,
they were positive; if they remained blue and black,
they were negative.
2.3.4 Gelatin Liquefaction Test
The strains were punctured and inoculated into the
test tubes filled with gelatin liquefactional medium,
and the uninoculated test tubes were used as blank
control, and cultured in 37℃ incubator for 24h.
Then the test tube was placed in cold water, and the
phenomenon was observed after the medium in the
blank control group solidified. If the experimental
test tube liquefaction, it was positive, otherwise it
was negative.
2.3.5 Sugar Fermentation Experiment
The strains cultured for 24 h were punctured and
inoculated into a test tube containing sugar
fermentation medium, and then inverted small glass
tubes were added into the test tube. Each sugar was
divided into three parallel groups. The uninoculated
group was cultured in 30℃ incubator for one week.
The phenomenon was observed and recorded, if acid
and ga were produced (from purple to yellow), it
was positive and no change was negative.
2.4 Identification of Strains by
Molecular Biology
Using the kit method, the bacterial DNA was
extracted according to the operation steps of the
bacterial genome extraction kit of Sangon
Biotechnology Company, and then the bacterial 16S
rDNA universal primers 27F/1492R (Table 1) were
used for amplification. Each sample consisted of 50
μL solution containing DNA template, forward
primers, reverse primers, sterile water (Table 2). The
5 μL PCR product was tested by 1% agarose gel
electrophoresis, and the purified PCR sample was
sent to Shanghai Biotechnology Co., LTD for
sequencing. According to sequences returned by
sequencing companies, phylogenetic trees were
constructed using MEGA 5.0 software.
Amplification procedure: 1) pre-denaturation at
94℃ for 3 min; Denaturation at 94℃ for 1 min; 2)
Annealing at 55℃ for 1 min; 3) Extended at 72℃
for 2 min for 25 cycles; 4) Extend at 72℃ for 6 min;
5) Store at 4 ℃.
Table 1: Universal primers for bacterial 16S rDNA.
Prime Genetic sequence
27F 5’-AGA GTT TGA TCC TGG CTC AG-3’
1492R 5’-GGT TAC CTT GTT ACG ACT T-3’
Table 2: PCR amplification system.
Com
p
ositio Volume /
μ
L
Ta
q
Master Mix 25
Primer 1 1
Primer 2 1
DNA template 2
Sterile wate
r
21
3 RESULTS AND ANALYSIS
3.1 Screening Results
After being treated at 80℃ for 15 min, a total of 5
strains were inoculated on the medium, and the
dominant strain LPB-5 was selected as the
experimental strain. The strain grew round colonies
with creamy white opacity, neat edges, smooth and
moist surface on nutrient broth agar medium (FIG.
1). The microscopic results of gram staining are
shown in Figure 2. The sand yellow staining solution
cannot stain the bacteria red, so the bacteria is gram-
positive.
Figure 1: Colony morphology of the strain.
Screening and Identification of Bacillus amyloliquefaciens from the Gut of Aquatic Animals
857
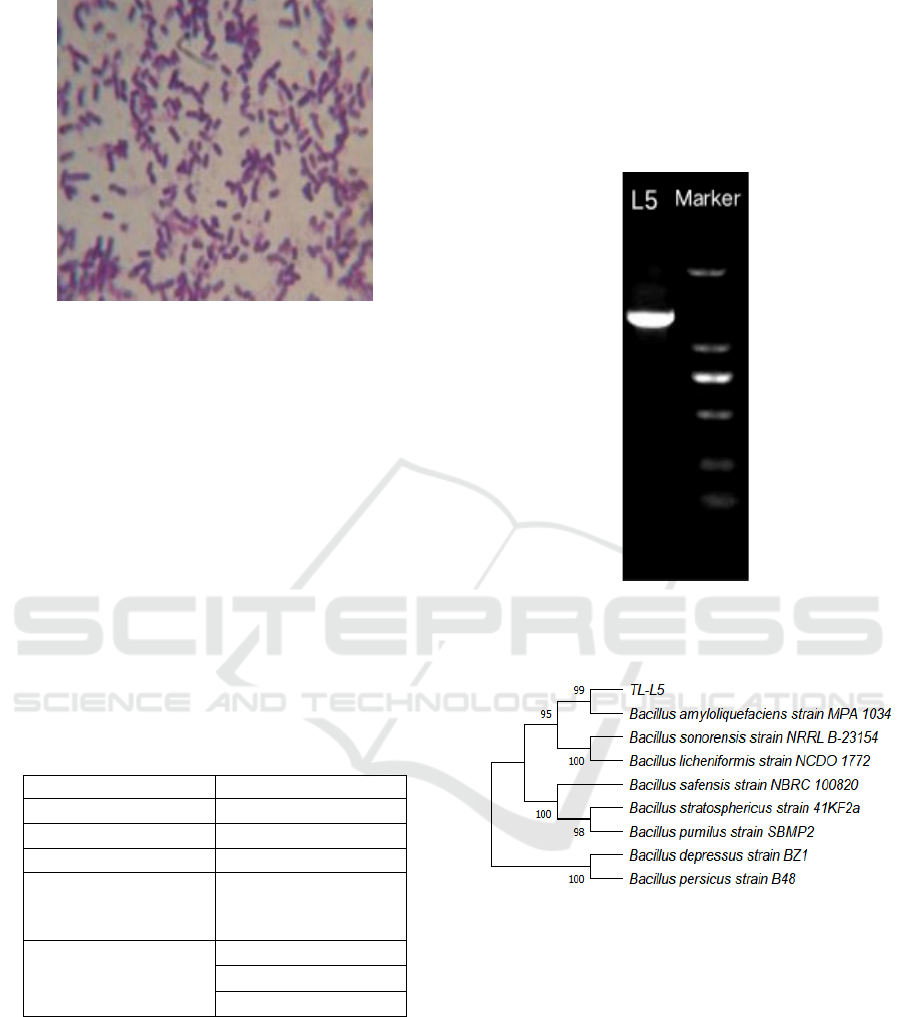
Figure 2: Microscopic diagram of gram stain of strain
(Dye according to the four steps of initial dyeing, medium
dyeing, decolorization and redyeing, and then observe
under the 1000x oil microscope).
3.2 Physiological and Biochemical
Identification
The physiological and biochemical identification of
strain LPB-5 (contact enzyme test, starch hydrolysis
test, gelatin liquefication test, Glucose fermentation
test, sucrose fermentation test, maltose fermentation
test) were carried out, and the results were shown in
Table 3. According to Berger's Manual of
Systematic Bacteriology, the strain could be
preliminarily identified as Bacillus.
Table 3: Physiological and biochemical characteristics of
LPB-5("+" means a positive result, "-" means a negative
result.).
Project Result
Gram stain test +
Contact enzyme test
-
Starch hydrolysis test
-
Gelatin liquefaction
test
+
Sterile water
(Glucose)+
(Sucrose)+
(Maltose)+
3.3 Molecular Identification
The PCR purified product of this strain was sent to
Shanghai Shenggong Bioengineering Technology
Service Co., LTD for sequencing. The agarose gel
electrophoresis of this strain was shown in Figure 3.
According to the 16S rDNA gene sequence returned
by the company, Blast comparison was performed
with the nucleic acid sequence database in GenBank,
and the phylogenetic tree was constructed by
neighbor-joining method with MEGA 5.0 software
(FIG. 4). Strain LPB-5 was in the same branch with
Bacillus amyloliquefaciens MPA 1034, and had the
highest homology with the closest evolutionary
distance and the similarity was 99%. Therefore, the
strain was identified as Bacillus amyloliquefaciens.
Figure 3:16S rDNA electrophoresis results (Condition:
Voltage =120V, time =30min).
Figure 4: Phylogenetic tree of strain LPB-5(LPB-5 had the
closest relationship with Bacillus amyloliticus MPA 1034,
and the similarity was 99%.).
4 DISCUSSIONS
In this study, nutrient broth, a common medium, was
used to isolate strains from the intestines of healthy
cultured procambarus clarkii and crabs. It was
identified as Bacillus amyloliticus by morphological
observation, physiological and biochemical tests and
16S rDNA sequence analysis. In strain
identification, if a single application of molecular
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
858

biology of the strains identified is likely to affect the
accuracy of the appraisal result, eventually lead to
the wrong conclusion, so the paper also combines
the bacteria morphology observation and
physiological and biochemical test evaluation, to
ensure the accuracy of the appraisal results and
academic.
In order to avoid the problems caused by the use
of antibiotics such as the enhancement of drug
resistance, veterinary drug residues, and the
deterioration of meat quality, and to realize healthy
ecological aquaculture, the aquaculture industry has
an increasingly strong demand for good alternatives
to antibiotic feed (Watts, 2017.Didier, 2020).
Probiotics can improve feed digestibility, promote
growth, inhibit pathogens, improve immunity, and
improve water quality, etc., and are considered as
the most potential alternatives (Wang, 2020.El
Saadony Mohamed T, 2021). In a report in 1893,
Kozasa used spores of Bacillus Toyota as feed
additives for yellowtail fish and found that the
growth rate of yellowtail fish was improved, which
was the first application of probiotics in practice and
proved the beneficial effect of probiotics in
aquaculture (Cruz, 2012).
In recent years, the aquaculture industry has
started to add some probiotics to the feed, and
practice has proved that it is feasible to use
probiotics to prevent and control the diseases of
cultured fish and shrimp, and the use of probiotics in
aquaculture can significantly reduce the need for
antibiotics (Opiyo, 2019. Abarike, 2018). Sun et al.
exposed the fertilized zebrafish larvae to
perfluorinated butanesulfonic acid (PFBS) and
applied the probiotic lactobacillus rhamnosus during
this period (Sun, 2021). The results showed that
probiotics supplement effectively inhibited the
growth retardment caused by PFBS, Probiotics can
secrete various digestive enzymes (amylase,
protease, lipase, etc.) in the body of aquaculture
animals, promoting the absorption and
transformation of nutrients in the feed of aquaculture
animals, improving feed digestibility, and promoting
individual growth (Das, 2019. Hai, 2009). After
entering the digestive tract of the host, probiotics
mainly prevent the growth of pathogens on the
intestinal surface by producing antibacterial
substances, regulating intestinal pH and competing
with harmful bacteria for adsorption sites
(Kuebutornye, 2019. Kuebutornye, 2020.
Chabrillon, 2005). Studies have shown that
maintaining a high level of probiotics in fish ponds
can not only reduce the accumulation of dissolved
organic carbon and granular organic carbon during
the growing season, but also balance the production
of phytoplankton (Cruz, 2012. Kazun, 2019).
Most of the probiotics that make up the
probiotics used in aquaculture come from the
digestive tracts of mammals, or soil and seawater,
etc. The probiotics used in aquaculture were
originally commercial probiotics designed for land
animals. In the development of probiotics for
aquaculture, the probiotics in the intestinal tract of
aquaculture animals are good raw materials, because
they are not only biosafe, but also can effectively
improve the survival rate and colonization rate of
probiotics in the intestinal tract (Ramlucken, 2020).
In this study, only one strain with this characteristic
was screened, but the development of relevant
probiotic preparation products still needs to consider
various factors such as hemolysis, antibiotic
resistance, compliance analysis, pH and bile salt
tolerance. (Balcázar, 2008.). In addition, in some
studies, to test the safety of probiotics, probiotics
were injected directly into fish intraperitoneally to
study mortality without inducing severe pathogenic
symptoms (El-Rhman, 2009.Aly, 2008). In any case,
safety is always the most important, so further
experiments are needed to verify the addition of this
strain as a probiotic preparation to aquatic feed.
5 CONCLUSIONS
In this study, a probiotic strain with quantity
advantage was screened and isolated from the
intestinal tract of procamblus clarkii and crab which
were purchased from dajiang Aquatic Market of
Huai 'an city. The strain was identified by
observation of colony morphology, physiological
and biochemical tests and 16S rDNA sequence
analysis. The results showed that the strain had the
closest relationship with Bacillus amyloliquefaciens
MPA 1034. The 16S rDNA sequence similarity was
99%, and the physiological and biochemical reaction
spectrum was consistent with the 16S rDNA
sequence analysis. The biosafety and high efficiency
of the strain in aquaculture feed can improve the
excellent microbial resources for further
development of probiotics and other products.
ACKNOWLEDGEMENTS
This work was supported by the National Natural
Science Foundation of China (31801524), and
Screening and Identification of Bacillus amyloliquefaciens from the Gut of Aquatic Animals
859

Natural Science Foundation of Jiangsu Province
(BK20170461, BK20181063).
REFERENCES
Abarike, E. D, Cai, J, Lu, Y, Yu, H, Chen, L, Jian, J,
Tang, J, Jun, L. and Kuebutornye, F. K. A (2018).
Effects of a commercial probiotic BS containing
Bacillus subtilis and Bacillus licheniformis on growth,
immune response and disease resistance in Nile
tilapia, Oreochromis niloticus J Fish and Shellfish
Immunology, 82.
Aly, S. M, Ahmed, Y. A.-G, Ghareeb, A. A.-A. and
Mohamed, M. F(2008). Studies on Bacillus subtilis
and Lactobacillus acidophilus, as potential probiotics,
on the immune response and resistance of Tilapia
nilotica (Oreochromis niloticus) to challenge
infections J Fish and Shellfish Immunology, 25, 1.
Aly, S. M, Abd-El-Rahman, A. M, John, G. and
Mohamed, M. F (2008). Characterization of Some
Bacteria Isolated from Oreochromis niloticus and their
Potential Use as Probiotics %J Aquaculture, 277, 1-2.
Aly, S. M, Mohamed, M. F. and John, G (2008). Effect of
probiotics on the survival, growth and challenge
infection in Tilapia nilotica (Oreochromis niloticus) J
Aquaculture Research, 39, 6.
Balcázar, J. L, Blas, I. d, Ruiz-Zarzuela, I, Cunningham,
D, Vendrell, D. and Múzquiz, J. L (2006). The role of
probiotics in aquaculture J Veterinary Microbiology,
114, 3.
Balcázar, J. L, Vendrell, D, Blas, I. d, Ruiz-Zarzuela, I,
and Girones, O (2008). Characterization of probiotic
properties of lactic acid bacteria isolated from
intestinal microbiota of fish J Aquaculture, 278, 1.
Chabrillón, M, Rico, R. M, Arijo, S, Díaz‐Rosales, P,
Balebona, M. C. and Moriñigo, M. A (2005).
Interactions of microorganisms isolated from gilthead
sea bream, Sparus aurata L, on Vibrio harveyi, a
pathogen of farmed Senegalese sole, Solea
senegalensis (Kaup) J Journal of Fish Diseases, 28, 9.
Chaiyapechara, S, Rungrassamee, W, Suriyachay, I,
Kuncharin, Y, Klanchui, A, Karoonuthaisiri, N. and
Jiravanichpaisal, P (2012). Bacterial Community
Associated with the Intestinal Tract of P. monodon in
Commercial Farms J Microbial Ecology, 63, 4.
Chauhan, A. and Singh, R (2019). Probiotics in
aquaculture: a promising emerging alternative
approach J Springer Netherlands, 77, 2.
Cruz, P. M, Ibáñez, A. L, Hermosillo, O. A. M, Saad, H.
C. R, Kingsley, D. H. and Mitsumori, M (2012). Use
of Probiotics in Aquaculture J ISRN Microbiology
Das, S, Ward, L. R. and Burke, C. Prospects of using
marine actinobacteria as probiotics in aquaculture %J
Applied Microbiology and Biotechnology, 81, 3
(2008).
Didier, W, S, J. P, Jane, P. E, Max, T, Shannon, M,
Stephan, H, Anaïs, L, Irene, L, Tiscar, G, G, H. P. J,
Carolee, C, Melanie, C, Gunilla, S. S, V, M. C, H, S.
A. J, Barbara, W, Karl, P, Annegret, S. and Didier, P
(2020). Evidence for action: a One Health learning
platform on interventions to tackle antimicrobial
resistance J The Lancet Infectious Diseases,
prepublish.
Douglas, Linda C. and Sanders Mary E (2008). Probiotics
and prebiotics in dietetics practice. J Journal of the
American Dietetic Association, 108, 3.
Elsabagh, M, Mohamed, R, Moustafa, E. M, Hamza, A,
Farrag, F, Decamp, O, Dawood, M. A. O. and
Eltholth, M (2018). Assessing the impact of Bacillus
strains mixture probiotic on water quality, growth
performance, blood profile and intestinal morphology
of Nile tilapia, Oreochromis niloticus J Aquaculture
Nutrition, 24, 6.
El-Rhman, A. M. A, Khattab, Y. A. E. and Shalaby, A. M
(2009). E. Micrococcus luteus and Pseudomonas
species as probiotics for promoting the growth
performance and health of Nile tilapia, Oreochromis
niloticus J Fish and Shellfish Immunology, 27, 2.
El Saadony Mohamed T, Alagawany Mahmoud and Patra
A.K.The functionality of probiotics in aquaculture: An
overview J Fish & Shellfish Immunology, prepublis .
Hai, N. V, Buller, N. and Fotedar, R (2009). The use of
customised probiotics in the cultivation of western
king prawns (Penaeus latisulcatus Kishinouye, 1896) J
Fish and Shellfish Immunology, 27, 2.
Hai, N. V. and Fotedar, R (2009). Comparison of the
effects of the prebiotics (Bio-Mos ® and β-1,3-D-
glucan) and the customised probiotics (Pseudomonas
synxantha and P. aeruginosa) on the culture of juvenile
western king prawns (Penaeus latisulcatus Kishinouye,
1896) J Aquaculture, 289, 3.
Hazel, K, Ben, T, Anke, L, David, B. and R, T. C (2020).
Probiotics and competitive exclusion of pathogens in
shrimp aquaculture J Reviews in Aquaculture, 13, 1.
Kazun, B. and Kazun, K (2019). Using probiotics in
freshwater larviculture J Fisheries & Aquatic Life, 27,
3.
Kuebutornye Felix K A, Delwin, A. E. and Yishan,
L(2019). A review on the application of Bacillus as
probiotics in aquaculture. J Fish & shellfish
immunology.
Kuebutornye, F. K. A, Abarike, E. D, Lu, Y, Hlordzi, V,
Sakyi, M. E, Afriyie, G, Wang, Z, Li, Y. and Xie, C. X
(2020). Mechanisms and the role of probiotic Bacillus
in mitigating fish pathogens in aquaculture J Fish
Physiology and Biochemistry, 46, 15.
Liu, H, Wang, L, Liu, M, Wang, B, Jiang, K, Ma, S. and
Li, Q (2011). The intestinal microbial diversity in
Chinese shrimp (Fenneropenaeus chinensis) as
determined by PCR–DGGE and clone library analyses
J Aquaculture, 317, 1.
Li, S, Sun, L, Wu, H, Hu, Z, Liu, W, Li, Y. and Wen, X
((2012)). The intestinal microbial diversity in mud
crab (S cylla paramamosain) as determined by PCR ‐
DGGE and clone library analysis J Journal of Applied
Microbiology, 113, 6.
Olmos, J, Ochoa, L, Paniagua-Michel, J. and Contreras, R
(2011). Functional Feed Assessment on Litopenaeus
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
860

vannamei Using 100% Fish Meal Replacement by
Soybean Meal, High Levels of Complex
Carbohydrates and Bacillus Probiotic Strains J Marine
Drugs, 9, 6 .
Opiyo, Jumbe, Ngugi and Charo-Karisa (2019). Dietary
administration of probiotics modulates non-specific
immunity and gut microbiota of Nile tilapia
(Oreochromis niloticus) cultured in low input ponds J
International Journal of Veterinary Science and
Medicine, 7, 1.
Ramlucken, U, Roets, Y, Ramchuran, S. O, Moonsamy,
G, Rensburg, C. J. v, Thantsha, M. S. and Lalloo,
R(2020). Isolation, selection and evaluation of
Bacillus spp. as potential multi-mode probiotics for
poultry:Full Papers J The Journal of General and
Applied Microbiology, 66, 4.
Ravi, A. V, Musthafa, K. S, Jegathammbal, G, Kathiresan,
K. and Pandian, S. K (2007). Screening and evaluation
of probiotics as a biocontrol agent against pathogenic
Vibrios in marine aquaculture J Letters in Applied
Microbiology, 45, 2.
Sun, B, Liu, M, Tang, L, Hu, C, Huang, Z, Zhou, X. and
Chen, L (2021). Probiotic supplementation mitigates
the developmental toxicity of
perfluorobutanesulfonate in zebrafish larvae. The
Science of the total environment, 799, 149458.
Valente, L. M. P, Araújo, M, Batista, S, Peixoto, M. J,
Sousa-Pinto, I, Brotas, V, Cunha, L. M. and Rema, P
(2016). Carotenoid deposition, flesh quality and
immunological response of Nile tilapia fed increasing
levels of IMTA-cultivated Ulva spp. J Springer
Netherlands, 28, 1.
Waldroup, P. W, Oviedo-Rondon, E. O. and Fritts, C.
A(2003). Comparison of Bio-Mos<sup>®</sup> and
Antibiotic Feeding Programs in Broiler Diets
Containing Copper Sulfate J International Journal of
Poultry Science, 2, 1 .
Wang C, Chuprom J, Wang Y (2020). Beneficial bacteria
for aquaculture: nutrition, bacteriostasis and
immunoregulation. J Journal of applied microbiology,
128, 1.
Wang, X, Zhou, C. F, Cheng, J. R, Cao, S. F, Yang, H. X.
and Zhao, J. R(2021). Aquamicrobium zhengzhouense
sp. nov, a Bacterium Isolated from Farmland Soil
Applied with Amino Acid Fertilizer. Current
microbiology.
Watts, J. E. M, Schreier, H. J, Lanska, L, Hale, M. S,
Place, A, Jagus, R. and Long, P (2017). The Rising
Tide of Antimicrobial Resistance in Aquaculture:
Sources, Sinks and Solutions J Marine Drugs, 15, 6.
Yan, Q, Gast, C. J. v. d. and Yu, Y (2017). Bacterial
community assembly and turnover within the
intestines of developing zebrafish. J PLoS ONE, 7, 1.
Screening and Identification of Bacillus amyloliquefaciens from the Gut of Aquatic Animals
861
