
Determination of ICARIIN in Traditional Chinese Medicine
Preparation by HPLC
Ying Wang
1
, Haitao Gong
1
, Cuifang Gao
1
, Yun Zhou
1
, Yonghe Wang
2
and Yanqun Wang
1,*
1
Shandong Drug and Food Vocational College, Zibo, Shandong, 255011, China
2
Jinan Zhangqiu District Hospital of TCM, Jinan, Shandong, 250200, China
Keywords: HPLC, Icariin.
Abstract: A method for the determination of ICARIIN in traditional Chinese medicine by High-paerformance liquid
chromatography was established, in which octadecyl Silane Bonded Silica Gel was used as Filler, acetonitrile-
water (30:70) as mobile phase, the detection wavelength was 270 nm, the flow rate was 1μl /min, the injection
volume was 10μl. Column Temperature is 40℃. The average recovery was 99.97%. LINEAR
CORRELATION COEFFICIENT r=0.9999, precision and repeatability RSD were 0.35% and 0.70%
respectively.
1 INTRODUCTION
The traditional Chinese medicine preparation
Wenshen Huazhuo Capsule (formerly known as
Jiangzhi Capsule) is a well-known traditional
Chinese medicine prescription in Zhangqiu District
Traditional Chinese Medicine Hospital. It is based on
many years of clinical practice experience and a
combination of traditional Chinese medicine theories.
The prescription embodies the guiding ideology of
holistic concept and differentiation and treatment of
traditional Chinese medicine. It has the effects of
warming the kidney and resolving turbidity,
promoting blood circulation and channeling. It can be
used for hyperlipidemia caused by deficiency of
kidney yang and blood turbidity and blood stasis. The
curative effect is remarkable. The prescription
contains medicinal flavors such as roasted
Epimedium, Hawthorn, Mistletoe, Polygonatum
odoratum, which have the effects of lowering blood
fat and nourishing the kidney and removing blood
stasis. In order to give full play to the advantages of
traditional Chinese medicine in lowering lipids and
facilitating the consumption of patients, the
preparations for medical institutions have been made,
and the preparation of traditional Chinese medicine
preparations by medical institutions using traditional
techniques has been completed. While exploring and
improving its process and formulating process
standards, a method for determining the content of
icariin is established as the main control index of the
internal control agent standard. This article uses
HPLC to determine its content.
2 INSTRUMENT AND TEST
MEDICINE
Instrument: Shimadzu SPD-20AT high performance
liquid chromatograph; Ajilent C18 column
250×4.6mm 5μm
Icariin reference substance: the batch number is
110737-201516, the content is 94.2%, and it is
purchased from the China Institute for Food and Drug
Control.
Reagents: German Merck chromatographic pure
acetonitrile, analytical pure ethanol, Wahaha pure
water.
3 METHODS AND RESULTS
3.1 Chromatographic Conditions and
System Applicability Test
It can use octadecylsilane-bonded silica gel as filler;
use acetonitrile-water (30:70) as mobile phase; SPD-
20A ultraviolet detector. Detection wavelength is
270nm, flow rate: 1.0ml/min; injection volume: 10μl;
872
Wang, Y., Gong, H., Gao, C., Zhou, Y., Wang, Y. and Wang, Y.
Determination of ICARIIN in Traditional Chinese Medicine Preparation by HPLC.
DOI: 10.5220/0011311800003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 872-877
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
Column temperature: 40°C. The number of
theoretical plates is calculated based on the peak of
icariin as 12143, RSD=0.30%, and tailing factor
T=1.030.
3.2 Solution Preparation
Preparation of reference substance solution:
accurately weigh an appropriate amount of icariin
reference substance, dissolve it with methanol and
dilute to the mark, shake it well, and make a solution
containing about 35μg/ml of icariin.
Preparation of test solution: take about 0.5g of this
product, accurately weigh it, place it in a stoppered
conical flask, accurately add 25ml of dilute ethanol,
close the stopper, weigh it, and ultrasonically treat it
(power 300W, frequency 40kHz) For 30 minutes, it
should let it cool, weigh it again, make up the lost
weight with dilute ethanol, shake well, filter, and take
the additional filtrate to get it.
Preparation of negative control solution: use the
same preparation process to prepare a depleted
negative control sample, and prepare a negative
control solution according to the preparation method
of the test solution.
Preparation of sample recovery test solution: take
0.25g of the test product with the measured content,
accurately weigh it, place it in a stoppered conical
flask, precisely add 15ml of icariin reference
substance solution, and add dilute ethanol 10ml,
dense plug, weigh it, ultrasonic treatment (power
300W, frequency 40kHz) for 30 minutes, let it cool,
then weigh it, use dilute ethanol to make up the lost
weight, shake well, filter, and get it. Six copies should
be prepared in parallel.
Preparation of linear test solution: weigh
accurately 10 mg of icariin reference substance
(content: 94.2%), put it in a 50ml measuring flask,
add methanol to dissolve and dilute to the mark,
shake well, as a reference substance stock solution.
Precisely draw the reference substance stock solution
to prepare a solution containing about 0.20μg,
0.30μg, 0.40μg, 0.50μg, 0.60μg of icariin.
3.3 Determination of the Wavelength is
Detected
By consulting the literature (Li, Chen, Zhang, Wang,
Wang 2020) and the 2020 edition of the Chinese
Pharmacopoeia (National Pharmacopoeia
Commission. 2020) the detection method of icariin in
preparations, the wavelength is 270 nm. Therefore,
the detection method chooses 270nm as the detection
wavelength.
3.4 Methodological Investigation
3.4.1 Specificity
Taking 10μl of icariin reference solution, negative
control solution, and test solution respectively, and it
can be injected into liquid chromatograph for
determination. The chromatograms are shown in
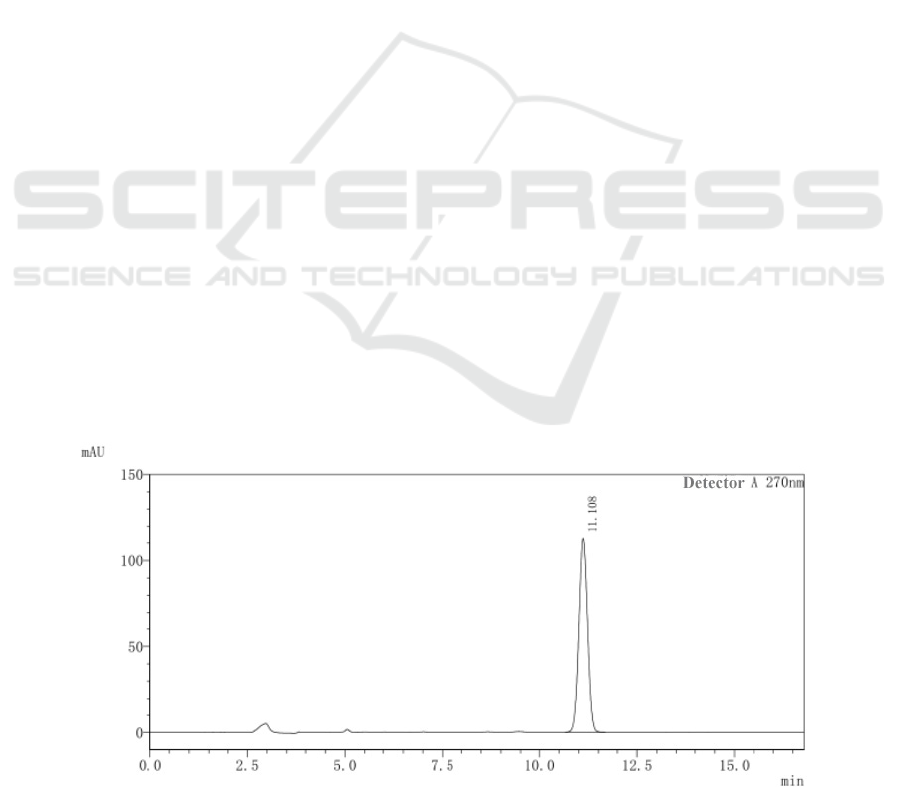
figure 1, figure 2, and figure 3.
It can be seen from the chromatogram that at the
same position as the reference substance retention
time 11.10min, there is no corresponding
chromatographic peak in the negative control
chromatogram. There is a corresponding
chromatographic peak in the chromatogram of the
test product. Therefore, the depleted negative control
and the solvent do not interfere with the
determination.
Figure 1: HPLC chromatogram of icariin reference substance.
Determination of ICARIIN in Traditional Chinese Medicine Preparation by HPLC
873
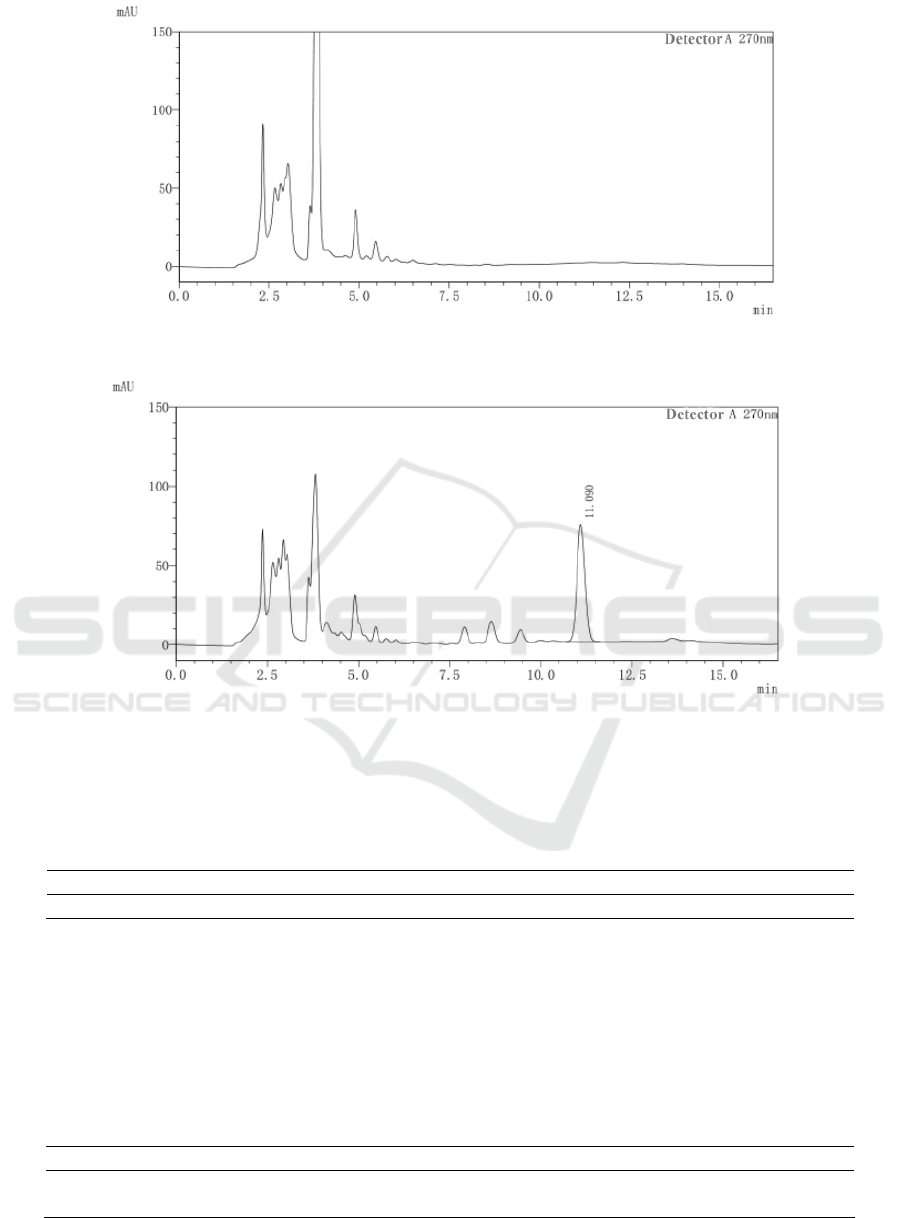
Figure 2: HPLC chromatogram of negative control.
Figure 3: The HPLC chromatogram of the test sample.
3.4.2 Precision
It can take 10μl of the e solution and inject it into the
high-performance liquid chromatograph, and inject 5
times continuously to measure the peak area. The
results are shown in table 1.
Table 1: Precision test results.
Peak area 1081871 1079740 1079205 1071382 1076269
RSD (%) 0.35
The test results show that the instrument precision
meets the requirements.
3.4.3 Repetitiveness
It should take 10μl of the test solution, inject it into
the high performance liquid chromatograph, repeat
the determination 6 times, and calculate the content
by the external standard method. The results are
shown in table 2.
Table 2: Results of the reproducible findings.
Conten
t
(%) 0.1847 0.1879 0.1845 0.1852 0.1856 0.1867
Mean conten
t
(%) 0.1858
RSD (%) 0.70
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
874
The test results show that the reproducibility of
the measurement method meets the requirements.
3.4.4 Sample Recovery Rate
The next step is to take 10μl of the sample recovery
test solution, inject it into the high performance liquid
chromatograph, determine, and calculate the content
by external standard method. The results are shown
in table 3.
Table 3: Results of sample recovery rate.
No.
Sampling
amount
(g)
Content in the
sample
(μg)
Control
addition (μg)
Measurements
(μg)
Rate of
recovery
(%)
Average
recovery rate
(%)
RSD
(%)
1 0.2780 0.2061 0.2125 0.4184 99.91
99.97 0.06
2 0.2762 0.2047
0.2125
0.4170 99.91
3 0.2747 0.2036
0.2125
0.4161 100.00
4 0.2768 0.2052 0.2125 0.4176 99.95
5 0.2727 0.2021 0.2125 0.4147 100.05
6 0.2735 0.2027 0.2125 0.4152 100.00
The test results show that the recovery rate of the
measurement method meets the regulations.
3.4.5 Linear and Range
The next is to take 10 μl each of the linear test
solution and inject it into the high performance liquid
chromatograph, measure the peak area respectively,
and calculate the linear correlation. The results are
shown in table 4.
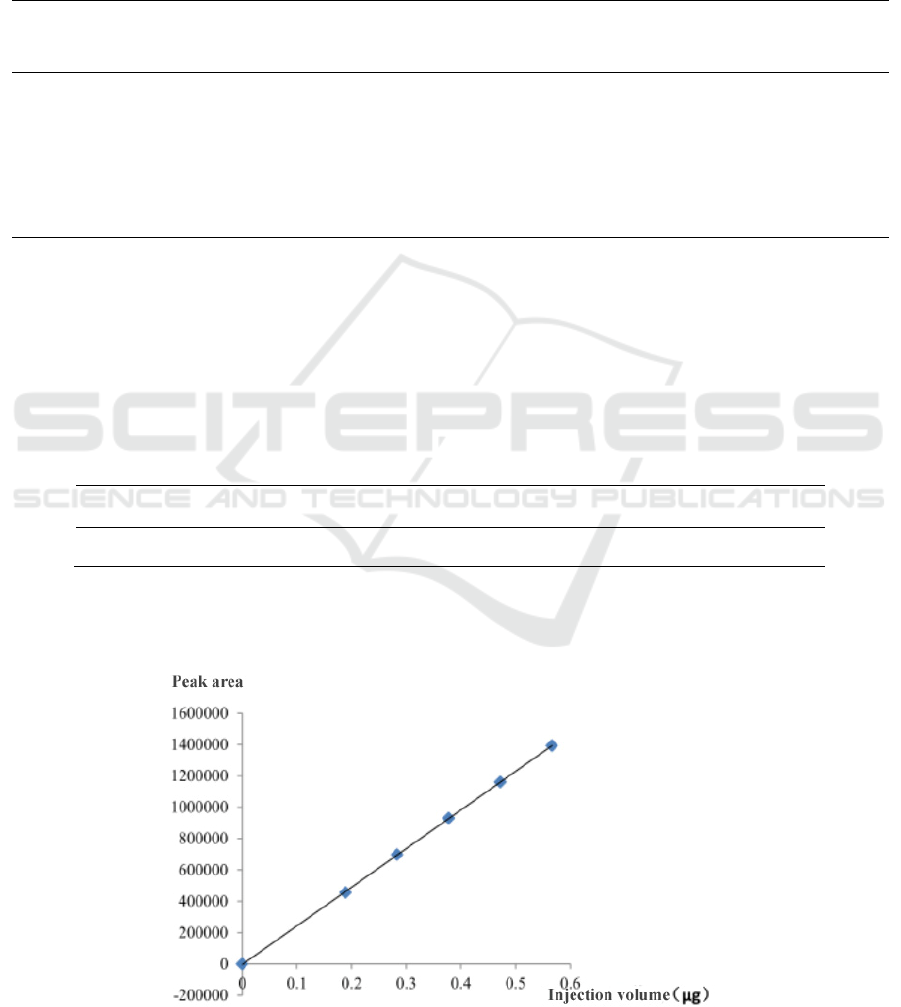
Table 4: Results of the linear relationship investigation.
Sample size (μg) 0.1888 0.2832 0.3776 0.4720 0.5664
Peak area 455117 697336 930217 1160026 1393169
Taking the injection volume as the abscissa and
the peak area as the ordinate, linear regression is
performed to obtain the regression equation, y =
2.4649×106x -2980.2, and the correlation coefficient
r=0.9999. It can be seen in figure 5.
Figure 4: Linear regression curves.
Determination of ICARIIN in Traditional Chinese Medicine Preparation by HPLC
875

The results show a good linear relationship
between sample size and peak area between 0.1888
μg~0.5664 μg.
3.4.6 Sample Stability
At 0 hour, 2 hours, 4 hours, 8 hours, and 24 hours, 10
μl of the test solution was taken and injected into the
high performance liquid chromatograph to determine
the peak area of icariin. The results are shown in table
5.
Table 5: Results of the stability test.
Time (h) 0 2 4 8 24
Peak area 945015 946455 949716 941619 945068
RSD (%)
0.31
From the test results, the test product solution is
well stable within 24 hours and can meet the
determination needs.
3.5 Sample Determination
According to the determination method and
conditions, three batches of samples were tested, and
the content was calculated by the external standard
method. The results are shown in table 6.
Table 6: Results of epicanosin content determination.
Lot. 20200812 20200813 20200813
Content (%) 0.1850 0.1853 0.1863
Content (mg)
0.7900 0.7916 0.7948
4 DISCUSSION
4.1 In this paper, octadecylsilane-bonded silica gel is
used as the filler, and acetonitrile-water (30:70) is
used as the mobile phase (Wang 2020). The detection
wavelength is 270nm, the flow rate is 1.0ml/min, the
injection volume is 10μl, and the column temperature
is 40°C. The content of icariin in Jiangzhi capsules
was determined by external standard method. The
method’s specificity, accuracy, precision, linearity,
stability all meet the requirements of methodology
verification.
4.2 Solvent (National Pharmacopoeia
Commission. 2020) with dilute ethanol is
environmentally friendly and cheap.
4.3 Icariin was extracted by ultrasound for 20
minutes, 30 minutes, 40 minutes, and 60 minutes and
the measured results remained unchanged, so 30
minutes of ultrasound was used to extract icariin.
4.4 When the sample dosage is 0.2g, 0.5g, 1.0g,
the measured result of icariin remains unchanged, so
0.5g is selected.
4.5 Hyperlipidemia is a disorder of lipid
metabolism and an important risk factor for
atherosclerosis. It can lead to atherosclerotic diseases
such as coronary heart disease, cerebral infarction
and peripheral vascular disease, as well as fatty liver
and acute pancreatitis other diseases. Hyperlipidemia
and atherosclerosis are common in middle-aged and
elderly people. Chinese medicine believes that when
people are over forty years old, the kidney essence
gradually declines, and the qi function is weakened.
The clearing will change from turbidity, and lipid
cohesion will cause hyperlipidemia, qi deficiency and
blood weakness, turbidity, blockage of blood, and
blood stasis atherosclerosis. The disease is located in
the blood vessels, which is a syndrome of deficiency
and deficiency of the underlying condition,
deficiency of the liver, spleen, and kidney are the
roots, the deficiency of the kidney is the mainstay,
and turbid phlegm and blood stasis are the indicators.
Clinical treatment often starts with kidney deficiency
and blood stasis. The treatment is suitable for
warming the kidney and removing turbidity, and
promoting blood circulation to clear the pulse.
Combination of Chinese medicines in Jiangzhi
Capsule, it can warm the kidney and remove turbidity
without injuring the yin, promote blood circulation
and dredge the collaterals without breaking the blood.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
876

5 CONCLUSION
Hyperlipidemia is a disorder of lipid metabolism and
an important risk factor for atherosclerosis. It can lead
to atherosclerotic diseases such as coronary heart
disease, cerebral infarction and peripheral vascular
disease, as well as fatty liver and acute pancreatitis.
The combination of all the medicines in this
prescription can warm the kidney and dissolve
turbidity without injuring the yin. It can also promote
blood circulation and dredge the collaterals without
breaking the blood. As China enters a well-off society
in an all-round way, the improvement of living
conditions and the acceleration of the pace of life
have brought about changes in the dietary structure,
and the population with hyperlipidemia tends to be
younger. In order to facilitate the patient’s use, the
research team explored well-known traditional
Chinese medicine prescriptions. According to the
Chinese Medicine Law of the People’s Republic of
China, the relevant requirements of medical
institutions apply traditional techniques to configure
traditional Chinese medicine preparations. A
controllable technical process route was established
and the internal control agent standard was
formulated with reference to the Chinese
Pharmacopoeia. The internal control standard of this
preparation, through microscopic identification
items, thin-layer identification items, and HPLC
determination of the effective ingredient content of
the main drugs in the preparation, comprehensively
controls the quality of the preparation, and
successfully completes the preparation of the
preparation work. In this study, the traditional
decoction is changed into capsules, the technological
parameters are formulated, and the quality control
indicators are increased. It has made useful
explorations for the protection and discovery of the
proven prescriptions and the promotion of the
modernization of Chinese medicine.
ACKNOWLEDGMENTS
Fund Project: Shandong Provincial TCM Science and
Technology Development Plan Project 2019-0548
REFERENCES
Li Tingting, Chen Maoqin, Zhang Jing, Wang Qian, Wang
Xueqin, Determination of Icariin Content in Jiuye Wine
by High Performance Liquid Chromatography, China
Pharmaceuticals. 2020. 29(13): 74-76.
Liu Lina, Determination of Icariin in Buqi Yishen Oral
Liquid by High Performance Liquid Chromatography.
Science and Technology Innovation. 2018. (17): 47-48.
Pharmacopoeia of the People’s Republic of China[M].
China Medical Science and Technology Press. National
Pharmacopoeia Commission. 2020.
Wang Rushan, Determination of Icariin in Bushenyanggu
Oral Liquid by HPLC, Chinese National Folk
Medicine. 2020. 29 (13: 36-38).
Determination of ICARIIN in Traditional Chinese Medicine Preparation by HPLC
877
