
The Application and Prospect of Immune Checkpoints based on PD1
and CTLA4
Ruining Oscar Hang
1,a
, Jinyu Zhou
2,*
, Rui Yuan
3,b
, Huafeng Zheng
4,c
, Xinyuan Li
5,d
and Yuze Guo
6,e
1
The Hun School of Princeton, Princeton, U.S.A.
2
West China School Of Public Health, Sichuan University, Chengdu, 610227, China
3
School of Minerals Processing and Bioengineering, Central South University, Changsha, China
4
Dulwich International High School, Zhuhai, China
5
Victoria Hill School (Partnered with Rosedale Academy), Kunming, China
6
International Center, Jinan Foreign Language School, Jinan, China
c
david.zheng22@stu.dulwich.org,
d
phoebem_lxy@outlook.com,
e
ReginaldGuo@163.com
Keywords: Malignant Tumors, Immunotherapy, Immune Checkpoint Inhibitors, PD-1, CTLA-4ACM Reference Format.
Abstract: Malignant tumors are currently one of the greatest health challenges the world is facing, ranking first among
the various lethal factors that cause death each year. There is a high probability of relapse after treatments
once treated with traditional approaches. Immunotherapy typifies a promising treatment method to increase
survival rates among patients at advanced stages. So far, however, patients can only receive limited benefits
from this novel therapy. In this article, we explore two important immune checkpoints, PD-1 and CTLA-4,
and discuss different factors influencing the functions of the immune checkpoint inhibitors. We also review
the recent developments in the field from the point of view of combinatory therapies. Research proposals
aimed to improve immunotherapies are also included to open new perspectives in enhancing the efficacy and
safety of the treatment of malignant tumors.
1 INTRODUCTION
Chemotherapy, radiation, and surgery are considered
the cornerstones of conventional cancer treatment.
However, it remains difficult for conventional
treatment programs to completely remove tumor cells
largely due to tumors’ ability to grow rapidly, and the
tendency of developing metastasis, and resistance to
radiotherapy and chemotherapy (Hanahan, Weinberg
2011). They also impose lethal effects on normal
cells, greatly harming the patients’ overall health.
Therefore, highly specific treatments with long-
lasting effects have become the primary target in
treating malignant tumors.
The success of immunotherapy is based on both
cancer destruction through the initiation of the host
immune system and the regulation of the cancer-
immune environment (Robert 2020). Scientists have
confirmed that immune checkpoints such as CTLA-4
and PD-1/PD-L1 signaling pathways play an
important role in regulating T cell immune response.
The immune checkpoints blockade can effectively
destroy tumor cells without compromising CTL
function, strengthen the outcome of anti-tumor
immunity. The finding of immune checkpoints
opened up a new path in treating malignant tumors
and greatly promote the development of checkpoint
inhibitor drugs (Freeman 2000). Since 2011, the FDA
has successively approved multiple PD-1/PD-L1
inhibitors for the treatment of various tumors such as
metastatic or unresectable melanoma. Immune
checkpoint inhibitor therapy provides novel and
effectual treatment methods for tumor treatment. It is
believed that more breakthroughs can be obtained in
the near future.
However, the research related to checkpoint
inhibitors is in the early stage, and we have not yet
fully understood its biological characteristics and
related signal pathways. In addition, checkpoint
inhibitors have some potentially serious side effects,
many of which are due to an overactive immune
response, which is related to inflammation of the
intestines, lungs, heart, skin, and other organs.
Approximately half of the advanced patients have no
obvious response to checkpoint inhibitors or no
response at all. Some people live longer without
Oscar Hang, R., Zhou, J., Yuan, R., Zheng, H., Li, X. and Guo, Y.
The Application and Prospect of Immune Checkpoints based on PD1 and CTLA4.
DOI: 10.5220/0011312900003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 901-909
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
901

treatment or live longer before their condition
worsens.
The immune check point-therapy and
combination strategies can provide advanced cancer
patients with superior treatment which can control or
even cure the disease. The specificity, adaptability,
and great capacity allow multiple biomarkers to work
inside the body. Despite the overall survival for
patients have improved, there are limitations and
challenges inherent in immune checkpoints therapy.
Emerging data and observation suggest that we need
to better understand the reason why there are certain
cancer types that refuse to respond and find the sealed
answer. Motivated by these developments, we now
revisit the critical mechanisms and recent findings
that associated with immune checkpoint therapy,
consider the limitation as well as challenges appear
during the clinical application, and expand upon the
functional roles to find possible approaches to tackle
the corresponding problems of immunotherapy.
2 APPLICATIONS AND
IMPROVEMENTS
2.1 Combination Therapy of Novel
Oncolytic Adenovirus with PD-L1
Inhibitors Resulted in Strengthened
Anti-cancer Effect
Malignant melanoma (MM) is a type of malignant
tumor derived from melanocytes. Early-stage
malignant melanoma is often treated by surgical
resection and a longer survival can be obtained.
However, in the middle and late stages, surgical
resection alone is not good for patients with
melanoma. Chemotherapy, radiotherapy, and
targeted therapy are the main therapies for melanoma,
but the efficacy is still not optimistic due to relapse
and drug resistance.
The emergence of PD-L1 antibodies has
completely altered the treatment strategy for
advanced and metastatic melanoma. However, it has
been reported 40%–60% of melanoma patients do not
gain any notable recovery, and a great portion of
recipients relapses within two years of the treatment.
The low efficiency of the immune checkpoint
inhibitors is largely due to their low response rate and
potent immunosuppressive effect that creates a "cold"
immune tumor microenvironment (TME) (Imbert et
al. 2020). Recent researches have shown that through
infecting the tumor lesion with engineered Oncolytic
adenoviruses, the resulting inflammatory response
can trigger the release of a series of immunoglobulins
and signaling molecules including proinflammatory
cytokines and an influx of NK cells, T cells, and
antigen-presenting cells (APC), consequently
generating the desired “hot” tumor microenvironment
(LaRocca, Warner 2018). Garofalo and Bertinato
treat mice models with melanoma tumors using the
anti-PD-1 antibody combined with a newly designed
oncolytic adenovirus containing modified AdV-D24-
inducible costimulator ligand AdV-D24-ICOSL-
CD40L that only targets and replicates in cancer cells
by intratumor injection (Garofalo et al. 2021),
resulting in a strengthened anti-cancer ability and
immunogenic cell death in vitro and a significant
decrease in tumor volume while ensuring a 100%
survival rate in vivo. The findings demonstrate that
oncolytic adenovirus-expressing potent immune
modulators can drive the systemic efficacy of PD-1
blockade, enhancing anti-cancer effectiveness and
survival (see Figure 1).
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
902
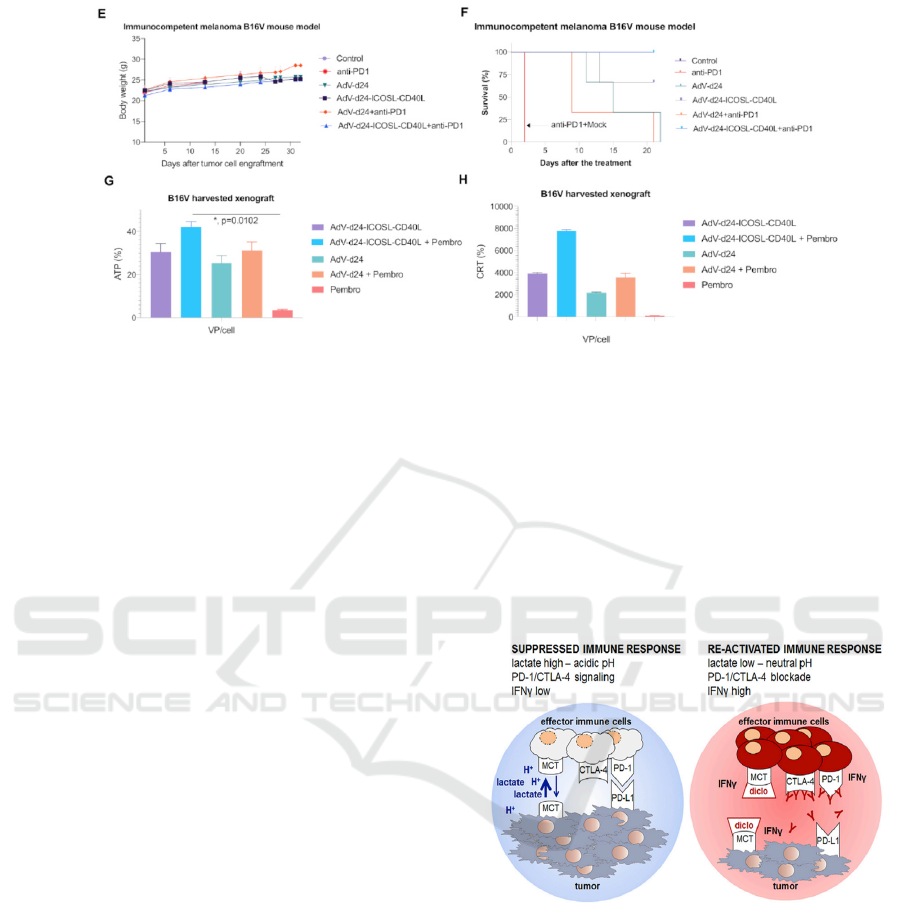
Fig 1. (Garofalo et al. 2021) (A) Tumor volume (mm3) measured through the study. The treatment was performed once per
day on days 1–6. The mice were treated according to the scheme (Table 1) with viruses (i.t.) and anti PD-1 antibody (i.v.) (B)
At the end of the study, mice were sacrificed and tumors harvested for weight assessment. (C, D) Tumor volume measurement
on days 6 and 20, respectively. (E) Body weight measurements throughout the study. (F) Survival profile was calculated by
Kaplan–Meier test. (H) Evaluation of CRT exposure after the treatment with oncolytic adenoviruses AdV-24-ICOSL-CD40L
and AdV-D24, and in combination with anti PD-1. CRT exposure was measured in the end of the study (after mice sacrifice)
with anti-calreticulin antibody staining and subsequent flow cytometry analysis (Beckman-Coulter Cytomics FC500). (G)
Assessment of ATP release after the treatment
However, there still contains two major concerns,
the potential risk of causing strong immune side-
effects and the lack of efficacy on tumors that are
undergoing metastasis.
Nanoparticle as a kind of delivery vector is one of
the good strategies to solve these issues (Kosmides
2017). Rather than the intratumor injection, a
nanoparticle delivery system that combines blockade
of PD-1 with the engineer oncolytic adenovirus AdV-
D24-ICOSL-CD40L can be hypothesized in order to
investigate the efficacy on tumor cells in vivo.
Optimistically, this nanoparticle delivery system may
increase the targeting and efficacy of oncolytic
viruses and anti-PD-1 antibodies, while reducing the
immune response of nontarget organs.
2.2 Restricting Glycolysis Preserves T
Cell Effector Functions and
Augments Checkpoint Therapy
(Renner et al. 2019)
The glycolytic activity of tumor cells is enhanced,
and lactic acid is accumulated and acidified in the
tumor. T and NK cells absorb lactic acid and impair
effector functions. But diclofenac can reduce the
secretion of lactic acid by inhibiting the lactate
transporters MCT1 and MCT4 and enhance T cell
function (figure2). So, inhibition of glycolysis
improves treatment at checkpoints. In addition, the
reduction of lactic acid by diclofenac has nothing to
do with changes in glycolysis-related proteins and
MCT expression profiles. And MCT inhibition does
not impair the in vitro function of T cells.
Figure 2: Explains a negative correlation between
glycolytic activity in tumors and response to check point
therapy.
However, the study mentioned above only has an
experimental group and does research on several
kinds of objects at the same time. Then a new clinical
trial is designed. The purpose is to study whether
reducing lactic acid secretion can improve T cell-
mediated killing of melanoma cells. Patients over 18
years of age, with good nutritional status, and tumor
stages in stage I and stage II will be included. And
patients with other tumors, such as pancreatic cancer,
gastric cancer, and patients undergoing anti-PD-1
therapy will be excluded.
The Application and Prospect of Immune Checkpoints based on PD1 and CTLA4
903

100 melanoma patients from five medical centers
will be divided into a control group and an
experimental group. The experimental group will be
treated with diclofenac, while the control group will
be treated with an equal dose of normal saline. The
researchers will follow up 100 patients for six months
and test the anti-tumor immunity of cells in two
groups. Compared with control cells, more lactic acid
and stronger anti-tumor immunity are expected to be
detected in the experimental group. Because reducing
lactic acid secretion by diclofenac may enhance the T
cell-mediated killing effect in melanoma cells.
Continue to add lactic acid to the experimental group
to a high concentration, which will reverse the
positive effect of diclofenac, resulting in suppression
of anti-tumor immunity as T cells may die when the
concentration of lactic acid increases. Overall,
reducing the tumor efflux of lactate can enhance the
immune response to checkpoint suppression.
2.3 Caffeine-Enhanced Anti-tumor
Activity of Anti PD-1 Monoclonal
Antibody
In this study, the authors evaluated the anti-tumor
activity of caffeine and anti-PD1 mAb combination
therapy against B16F10 melanoma tumors (Tej,
Neogi, Nayak 2019). They found that combination
therapy showed a decrease in the infiltration of
CD4+CD25+ T regulatory cells, an increase in
infiltration of CD4 T lymphocytes, CD8 T
lymphocytes, intra-tumoral TNF-α, and IFN-γ levels.
Experiments have confirmed that the combination of
anti-PD1 mAb and caffeine for tumor treatment
produced good results due to the blocking of the a2a
receptor and PD1.
The immunosuppressive environment in the
tumor microenvironment (TME) has always been an
obstacle to immune checkpoint inhibitors, of which
the CD39-CD73 adenosine pathway is an important
participant in the immunosuppressive environment.
The immunostimulating molecule ATP is converted
to AMP through CD39, AMP is converted to
immunosuppressive adenosine through CD73. Once
adenosine combines with its receptors, it will
promote tumor immune escape. To sum up, there are
two primary aspects of immunosuppressive
adenosine: one is targeting CD73 and/or CD39 to
inhibit the production of adenosine, and the other is
targeting the A2a receptors to block adenosine
signaling (Leone, Emens 2018). Antibodies that
target CD73 or CD39 block adenosine production and
relieve immunosuppression, and they can also be
used as both a single drug treatment and a synergistic
anti-tumor effect in combination with immune
checkpoint inhibitors. Referring to the previous
article (Tej, Neogi, Nayak 2019), some experiments
were designed in this paper to study the effects on
tumors by comparing caffeine as an antagonist of
A2aR and TTX-030 (BMS-986179) as an anti-CD39
antibody (anti-CD73 antibody) combine with anti-
PD1 monoclonal antibodies, respectively. The
following design experiments help to further explore
the effectiveness of combination therapies.
(The design ideas of this experiment refer to the
previous article (Tej, Neogi, Nayak 2019).)
Mice were randomly divided into
(A) Anti-PD1 mAb + caffeine group: receive a
combination of injection of anti-PD1 mAb and
caffeine in drinking water
(B) Anti-PD1 mAb + TTX-030(BMS-986179)
group: receive an injection of anti-PD1 mAb and
TTX-030(BMS-986179)
(C) Control group: receive injections of control Ig
After 6 weeks of treatment, calculate tumor
growth rates of individual mice from each group
through dividing tumor size. The efficacy of different
combination therapies is then evaluated in the
following areas as shown in Figure 3.
Figure 3: Evaluation of combination therapy in different aspects.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
904
2.4 MHC Protein Confer Differential
Sensitivity to CTLA-4 and PD-1
Blockade in Untreated Metastatic
Melanoma
Although immune checkpoint blockades (ICB) are
treatments for cancer, the mechanism to cure the
immune system but not directly treating cancer and
the antitumor immune response is highly dependent
on the T-cell cognition of surface-expressed antigen
which is the major histocompatibility complex
(MHC), therefore the reduction of, or the loss
proteins associated with MHC protein can potentially
be a mechanism which tumor escape antitumor
response and induce resistance to the antibodies
during ICB course.
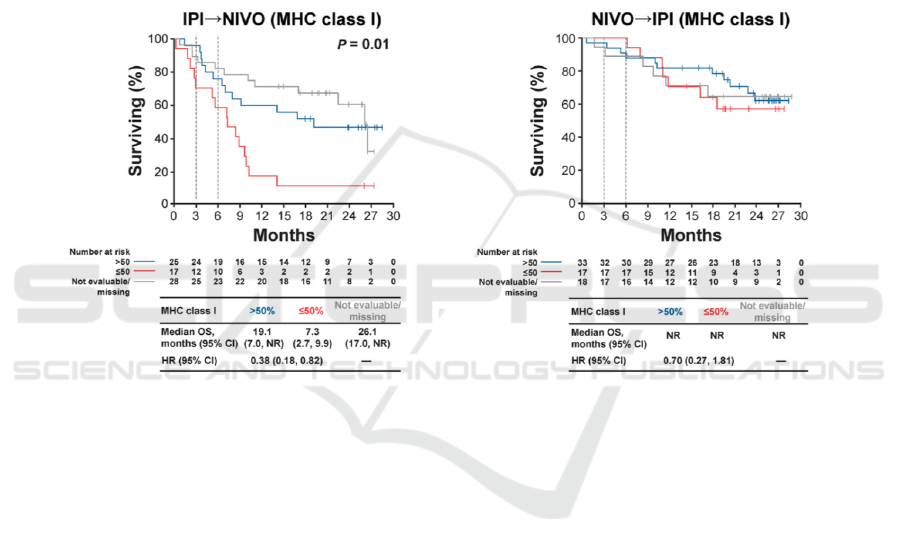
When they examined the overall survival, low
average tumor MHC class I expression (smaller and
equal to 50%) (S. J. Rodig D.G 2018) was
characterized with lower overall survival for the
patients that started with blockade IPI; better overall
survival for the patients that started with NIVO
attributed to unaffected tumor MHC class II
expression. The authors concluded the anti-CTLA-4
response primarily relied on the melanoma surface
MHC class I expression. In comparison, the primary
response to anti-PD-1 is associated with existing
interferon-gamma-mediated
Figure 4: Shows the difference in survival for two group which started with different check-point inhibitors, along with the
MHC class I surface expression.
There are limitations inherent in this
investigation, the association and responses to single
blockade IPI and NIVO lack of experimental results
to confirm the observation, etc.
According to the main findings of the articles, the
downregulation of the major histocompatibility
complex (MHC) is a mechanism of evading
antitumor immune response after the ICB, critically,
the primary resistance to CTLA-4 blockade was
attributed to the reduced melanomaMHC class I
expression. Down-regulation of MHC protein surface
expression is common in melanoma (seliger 2000)
before any approaches have been applied. Whereas
the primary response to anti-PD-1 blockade is
associated with pre-existing interferon-gamma
mediated immune activation.
There are hypotheses stated that interferon-
gamma is a profound molecule in manipulating the
MHC class I surface expression (Delgado, Ganea
2000, B. Seliger S.H et al 1997). the down-regulation
of MHC class I expression is due to the lack of
essential components in the expression pathway.
interferon-gamma is a significant factor there in terms
of modulating the transduction pathway and induce
the production of the new proteasome to replace the
old one or renovate it (B. Seliger S.H et al 1997).
2.5 Compensatory Upregulation of
PD-1, LAG-3, And CTLA-4 Limits
the Efficacy of Single-agent
Checkpoint Blockade in Metastatic
Ovarian Cancer
It is not clear whether the multiple receptors work
together in the process of specific immunity can serve
a better result for combining the ligands on the cancer
cell to the surface of T cells. Therefore, the co-inhabit
method is worth researching.
The Application and Prospect of Immune Checkpoints based on PD1 and CTLA4
905
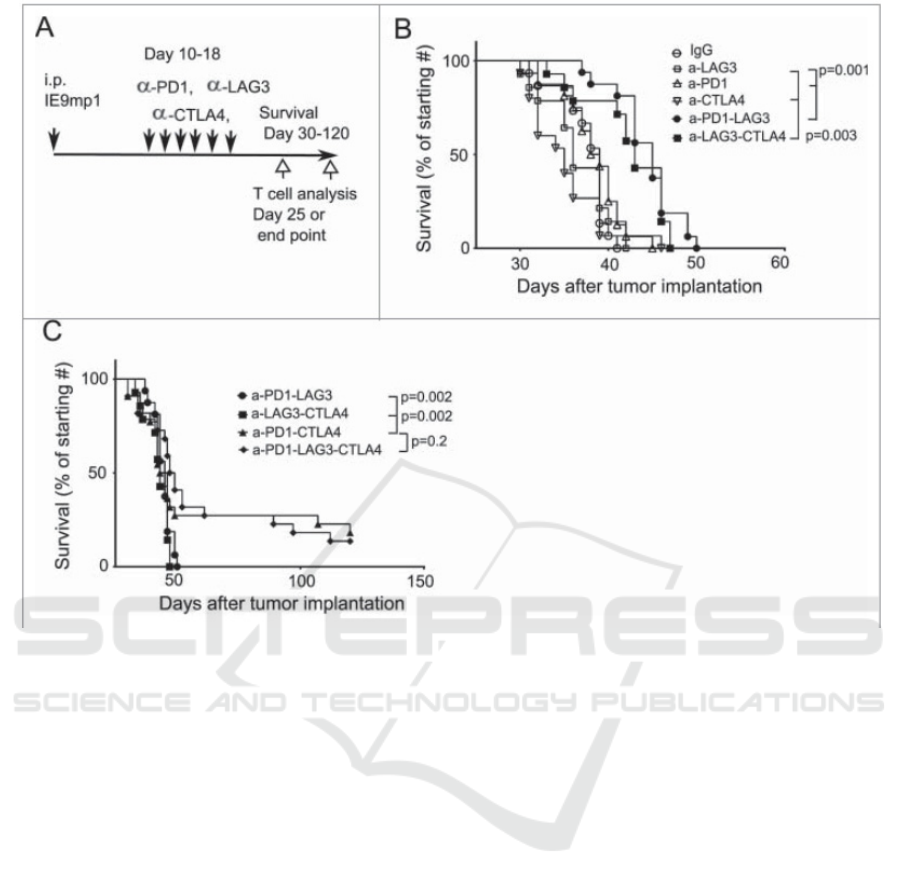
Figure 5: Shows the different survival rate of mice with different receptors.
According to figure 4, researchers have selected
three receptors, PD-1, LAG-3, and CTLA-4, which
are the immune checkpoints of T cells to regulate the
replication of metastatic ovarian cancer cells (Huang
et al 2016). The researchers test the survival rate of
mice with PD-1, PD-1 and LAG-3, PD-1 and CTLA-
4 blocking group, and triple blocking group. The
experiment result shows that the mice(C57BL/6) that
have two or three inhabitation pathways are slightly
more effective in dealing with the repelling of cancer
cells. The PD-1KO mice have little cytotoxic
materials when they have three inhabit pathways
compare to the normal mice.
Researchers established an ovarian tumor model
for collecting the data. Also, they splited TALs
(Tumor-associated lymphocytes) and TILs (Tumor-
infiltrating lymphocytes) from lymphocytes to
analyze. By reviewing the result, they knew that
several receptors are responsible for inhibiting
metastatic ovarian cancer, and the blocking of a
single receptor causes the upregulation of the co-
inhibitory system. The mice combining four
antigens can secret more cytotoxic materials to attack
normal cells than the mice with triple checkpoint
pathways. The mice with LAG-3, CTLA-4, and PD-
1 blockage or the dual inhibition pathways mice are
more effective to block the replication of metastatic
ovarian cancer than single pathway since knocking
out of PD-1 or LAG-3 can enhance the ability of the
other receptors to combine with the antigen.
Huang et al. provide evidence suggesting that the
block of LAG-3 and CTLA-4 can increase the
survival rate of PD-1KO mice. Similarly, the block of
PD-1 and CTLA-4 can enhance the survival rate of
LAG-3KO mice significantly (Huang et al 2016). A
single checkpoint pathway is not efficient compare to
dual checkpoint pathways. However, more than three
receptors' mice do not serve an excellent response to
the wild-type mice. Researchers should test the
relation between survival rate and cytotoxic materials
for finding the regulation. Researchers can analyze
300 mice in the research and divide them into three
groups. The first group of mice (PD-1KO) have
LAG-3, CTLA-4, and IgG; the second group will
contain the same receptors while they have no
treatment; the third group (PD-1KO) will be knocked
out the other receptors and only left CTLA-4. After
separate the groups, the researchers follow up these
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
906
mice for 6 months to compare the overall survival
with three different groups, which can find out
whether more than three receptors can enhance the
secret of cytotoxic materials.
2.6 Immune Microenvironment
Modulation Unmasks Therapeutic
Benefit of Radiotherapy and
Checkpoint Inhibition
Although the clinical effect of ICIs in the treatment
of solid tumors is exciting, many patients haven’t
achieved a sustained response. One of the main
barriers to treatment is the immunosuppressive tumor
immune microenvironment (TIME). Therefore,
researchers made a hypothesis of which the
combination of targeted radiotherapy and time-
dependent immunomodulation was able to improve
the ICI response rate of solid tumors. To test their
hypothesis, head and neck tumor was used on
C57BL/6J male mice that are 8-10 weeks of age to
explore the tumor characteristics limiting the efficacy
of immune checkpoint inhibitors in solid tumors and
to develop combination therapy strategies to
maximize their advantages. After the tumor became
established, mice received combinatory treatments
involving immune checkpoint inhibitions, tumor-
directed radiation, and immunomodulation of
cyclophosphamide (CTX) and L-n6-(1-iminoethyl)-
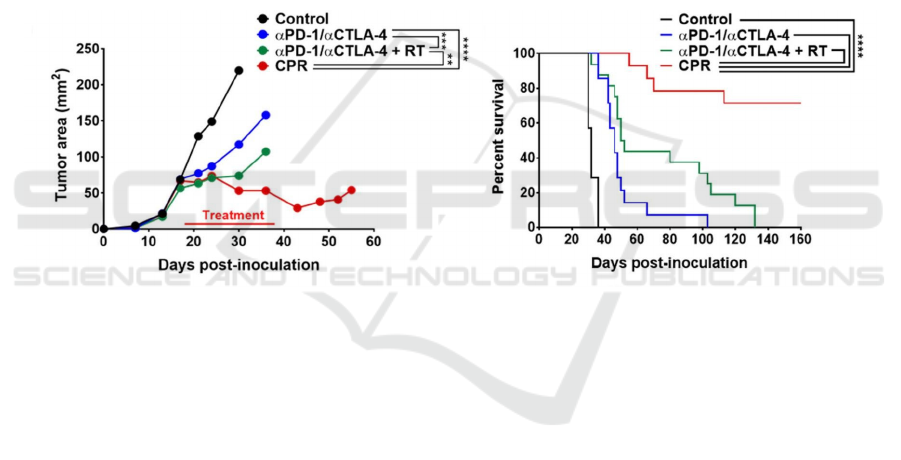
lysine (L-NIL). The result is that they found that
modulation of TIME using CTX and L-NIL,
combined with two checkpoint inhibitors and
radiotherapy, had better effects than these treatments
alone. It resulted in more than 70% of established
mEER tumor rejection, doubling the median survival
rate of the B16 melanoma model (Newton J.M et al.
2019).
Figure 6: (Newton J.M et al. 2019) The average tumor area and percent survival of mice before the first euthanasia under
different treatment combinations (CTX/L-NIL immunomodulation combined with α PD-1/ α CTLA-4 and tumor directed
radiation are collectively called the “CPR” regimen).
The previous study (Newton J.M et al. 2019) has
shown that regulating the immune microenvironment
can release the efficacies of ICIs and radiotherapy to
activate immune rejection in the therapy of refractory
tumors. However, it only made experiments on head
and neck cancer. Therefore, the purpose of the new
experiment is to see if the same regimen works on
lung cancer and gastric cancer, since these two
cancers are solid tumor which will respond to ICIs
used well, and they are very common. This study will
also use C57BL/6 male mice aged 8-10 weeks. After
tumors become established, treatments will start.
There will have two sets of experiments, one for lung
cancer, and one for gastric cancer. Each set of the
experiments will have two groups. One group with
modulation of TIME uses combination therapy of
PD-1 and CTLA-4 inhibitors, radiotherapy, and
immunomodulatory drugs: CTX and L-NIL. Another
group is the control experiment without modulation
of TIME, combining dual inhibition and
radiotherapy. All experiments will be replicated for at
least twice, and each experiment will have 5-10
samples in average. For the result, the hypothesis is
that the strategy of combining tumor-targeted
radiation with tumor immune microenvironment
regulation can improve the ICI response rate of lung
and gastric tumors.
3 CONCLUSION
This paper first explores the monoclonal antibody
immunotherapy of PD-1 in tumors. The novel
combination therapy of Oncolytic Adenovirus with
Anti-PD1 inhibitors presents an alternative treatment
therapy in treating Malignant melanoma. In addition,
the inhibition of glycolysis preserved T cell and NK
cell function, and enhanced anti-PD-1 treatment
The Application and Prospect of Immune Checkpoints based on PD1 and CTLA4
907

response is observed. Checkpoint immunity also
plays an important antitumor role in combination
therapy. The combined blockade of the a2a pathway
and pd1 pathway showed more effective anti-tumor
activity than monotherapy. Another study concluded
the primary response to anti-CTLA-4 is rely on the
melanoma surface MHC class II expression and that
anti-PD-1 is associated with existing interferon-
gamma-mediated immune activation. Besides,
knocking out of PD-1 and keeping the other co-
inhibitory workers can enhance the repelling of
metastatic ovarian cancer cells. Finally, a therapy of
combining modulation of tumor immune
microenvironment with dual checkpoint inhibition
(PD-1 and CTLA-4), and radiotherapy has shown a
good effect.
This research is of great significance because the
immune checkpoint is widely studied in medicine. It
is widely used in the treatment of cancer, such as
hepatocellular carcinoma (HCC), urinary system
malignant tumors, breast cancer, recurrent/metastatic
nasopharyngeal cancer and lung cancer. In addition,
the Immune checkpoint plays an important role in
acute pancreatitis (AP). Besides, scientists have done
a lot of research on the interaction between intestinal
flora and immune checkpoint inhibitors.
Although ICIs have played an important role in
cancer treatment and shown great promise in so many
different diseases, much more research on long-term
toxicity and survivorship issues is needed since new
side effects were found (Kottschade 2019). They
were often referred to as immune-related adverse
events (irAEs) or immune-mediated adverse
reactions. Moreover, as these strategies are used in
more and more malignant tumors, more side effects
are constantly observed, including but not limited to
endocrine toxicity, rheumatologic toxicity. These
side effects may be life-threatening, so in the future,
researchers should pay attention to acute toxicity,
long-term toxicity, and other treatments to improve
the life of patients after cure.
Recently, there is a pronounced increase in the
number of articles listed in PubMed that associated
with bispecific antibodies (biAbs) in the
immunotherapy of cancer, apparently, this innovation
has become a crucial part of immunotherapy for the
next generation. However, in cancer immunotherapy,
the competition never stops, another candidate
includes chimeric antigen receptor T cells, NK cell-
engaging biAbs, or macrophage-engaging biAbs.
More importantly, they all have the capacity to prove
they can potentially control, or even cure the
malignant tumor.
REFERENCES
B. Seliger S.H., A. Hohne, R. Zeidler, A. Knuth, C.D.
Gerharz, and C. Huber. (1997). IFN-y-mediated
Coordinated Transcriptional Regulation of the Human
TAP-i and LMP-2 Genes in Human Renal Cell
Carcinoma1 Barbara Seliger,2 Silke J. Clin Cancer
Res.3:573– 8.
Delgado M., Ganea D. (2000). Inhibition of IFN-gamma-
induced janus kinase-1-STAT1 activation in
macrophages by vasoactive intestinal peptide and
pituitary adenylate cyclase-activating polypeptide.J. J
Immunol.165:3051-7.
Freeman GJ L.A., Iwai Y, Bourque K, Chernova T,
Nishimura H, Fitz LJ, Malenkovich N, Okazaki T,
Byrne MC, Horton HF, Fouser L, Carter L, Ling V,
Bowman MR, Carreno BM, Collins M, Wood CR,
Honjo T.. (2000). Engagement of the PD-1
Immunoinhibitory Receptor by a Novel B7 Family
Member Leads to Negative Regulation of Lymphocyte
Activation.J. Exp Med.192:1027–34.
Garofalo M., Bertinato L., Staniszewska M., Wieczorek
M., Salmaso S., Schrom S., et al. (2021). Combination
Therapy of Novel Oncolytic Adenovirus with Anti-
PD1 Resulted in Enhanced Anti-Cancer Effect in
Syngeneic Immunocompetent Melanoma Mouse
Model.J. Pharmaceutics.13.
Hanahan D., Weinberg R.A. (2011). Hallmarks of cancer:
the next generation.J. Cell.144:646-74.
Huang R.Y., Francois, A., McGray, A. R., Miliotto, A., and
Odunsi, K. (2016). Compensatory upregulation of PD-
1, LAG-3, and CTLA-4 limits the efficacy of single-
agent checkpoint blockade in metastatic ovarian
cancer.J. Oncoimmunology.6:e1249561.
Imbert C., Montfort A., Fraisse M., Marcheteau E.,
Gilhodes J., Martin E., et al. (2020). Resistance of
melanoma to immune checkpoint inhibitors is
overcome by targeting the sphingosine kinase-1. J. Nat
Commun.11:437.
Kosmides A.K., Sidhom J.W., Fraser A., Bessell C.A.,
Schneck J.P. (2017). Dual Targeting Nanoparticle
Stimulates the Immune System To Inhibit Tumor
Growth.J. ACS Nano.11:5417-29.
Kottschade L.A. (2019). The Future of Immunotherapy in
the Treatment of Cancer.J. Semin Oncol
Nurs.35:150934.
LaRocca C.J., Warner S.G. (2018). Oncolytic viruses and
checkpoint inhibitors: combination therapy in clinical
trials.J. Clin Transl Med.7:35.
Leone R.D., Emens L.A. (2018). Targeting adenosine for
cancer immunotherapy.J. J Immunother Cancer.6:57.
Newton J.M., Hanoteau A., Liu H.C., Gaspero A., Parikh
F., Gartrell-Corrado R.D., et al. (2019). Immune
microenvironment modulation unmasks therapeutic
benefit of radiotherapy and checkpoint inhibition.J. J
Immunother Cancer.7:216.
Renner K., Bruss C., Schnell A., Koehl G., Becker H.M.,
Fante M., et al. (2019). Restricting Glycolysis
Preserves T Cell Effector Functions and Augments
Checkpoint Therapy.J. Cell Rep.29:135-50 e9.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
908

Robert C. (2020). A decade of immune-checkpoint
inhibitors in cancer therapy.J. Nat Commun.11:3801.
S. J. Rodig D.G., D. G. Jackson, E. Gjini, A. Giobbie-
Hurder, C. Jin, H. Chang, S. B. Lovitch, C. Horak, J. S.
Weber, J. L. Weirather, J. D. Wolchok, M. A. Postow,
A. C. Pavlick, J. Chesney, F. S. Hodi. (2018). MHC
proteins confer differential sensitivity to CTLA-4 and
PD-1 blockade in untreated metastatic melanoma.J. Sci
Transl Med.10: eaar3342.
Seliger B. (2000). Characterization of the major
histocompatibility complex class I deficiencies in B16
melanoma cells.J. CANCER RESEARCH.61:1095–9.
Tej G., Neogi K., Nayak P.K. (2019). Caffeine-enhanced
anti-tumor activity of anti-PD1 monoclonal antibody.J.
Int Immunopharmacol.77:106002.
The Application and Prospect of Immune Checkpoints based on PD1 and CTLA4
909
