
Preparation and Adsorption Properties of Chemically Modified Isatis
Indigotica Fort Draff based Biosorbent
Xiaochun Yin
a
, Jie Li, Nadi Zhang, Hai Zhu, Ting Ke, Yonge Gu, Junliang Meng, Jianjun Wu
*
and Yongfeng Wang
*
School of Public Health, Gansu University of Chinese Medicine, Lanzhou 730000, Guansu Province, China
119261751@qq.com, 11704014@qq.com,
*
591806561@qq.com,
*
331220684@qq.com
Keywords:
Radix Isatidis Residue, Chemical Modification, Adsorbent, Heavy Metals, Removal.
Abstract:
Objective: to treat the Isatis Indigotica fort draff to enhance its adsorption capacity for heavy metals.
Methods: Isatis Indigotica fort draff was modified with NaOH, Na
2
CO
3
and citric acid to prepare Isatis
Indigotica fort draff based biosorbent; the structure was characterized by scanning electron microscopy and
Fourier transform infrared spectroscopy; then the effects of solution pH value, solution concentration,
adsorbent dosage, and adsorption time of copper ion solution on the adsorption performance of Isatis
Indigotica fort draff based adsorbent were studied by static adsorption experiments, so as to clarify the
adsorption mechanism; it provides a guide for the resource utilization of Isatis Indigotica fort draff and an
experimental basis for the preparation of new adsorbents. Results: The adsorption properties of RIR-NaOH,
RIR-Na
2
CO
3
, and RIR-CA were all better than RIR; the order of the maximum adsorption capacity of the
modified Isatis Indigotica fort draff based adsorbent for copper ions is: RIR-Na
2
CO
3
> RIR-NaOH > RIR-
CA > RIR. Conclusion: The Isatis Indigotica fort draff based adsorbent has the potential to remove heavy
metals from water, and is a new type of adsorbent.
1 INTRODUCTION
a
With the acceleration of urbanization and the
increase of industrialization, heavy metal pollution
in water has gradually become a serious problem
that plagues many countries around the world (Bai
2015, Broaga 2014). Heavy metals exist stably and
persistently in the environment and accumulate in
the human body through the food chain, causing
serious harm to human health and ecosystem (Cao
2016, Chen 2010). Therefore, the removal of heavy
metals from water is of great significance to both
human beings and ecosystems. At present, the main
methods to remove heavy metals from wastewater
are ion exchange, chemical precipitation, and
membrane filtration. However, the wide application
of these methods in commercial applications is
limited by high cost and low reusability. On the
contrary, biosorption has attracted more and more
attention in the removal of heavy metals because of
its operability and low cost. Biosorbent resources
are extensive and easy to obtain, such as algae
a
https://orcid.org/0000-0002-9749-2608
residue (Dai 2019), herb residue (Deng 2018), and
so on. As a Chinese herbal medicine with multiple
functions, Radix Isatidis can not only be used for the
treatment of some diseases such as influenza (Du
2019), but also for the prevention of diseases (Duan
2021, Huang 2015, Huang 2020, Jia 2016). Radix
Isatidis contains a variety of active substances (Jia
2010), which are currently being extensively
studied, developed and utilized. Through
processing, a variety of Radix Isatidis products can
be synthesized (Jiang 2020), such as Radix Isatidis
granules, Radix Isatidis injection, and so on.
However, if the Isatis Indigotica fort draff after
extraction is not treated in time, it will pollute the
environment to some extent. Therefore, how to treat
the waste Isatis Indigotica fort draff and reduce its
pollution to the environment has become an
important problem to be solved.
After the active substances are extracted from
Radix Isatidis, the drug residue still contains many
effective components such as lignocellulose (Kim
2003, Kommula 2013), so the preparation of Isatis
Indigotica fort draff into a cellulose based adsorbent
is a common treatment method (Pan 2018, Reddy
Yin, X., Li, J., Zhang, N., Zhu, H., Ke, T., Gu, Y., Meng, J., Wu, J. and Wang, Y.
Preparation and Adsorption Properties of Chemically Modified Isatis Indigotica Fort Draff based Biosorbent.
DOI: 10.5220/0011375600003443
In Proceedings of the 4th International Conference on Biomedical Engineer ing and Bioinformatics (ICBEB 2022), pages 1013-1021
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
1013

2019). Lignocellulose is composed of carbohydrates
(cellulose and hemicellulose), lignin, and other
components (protein, lipid and inorganics) (Tan
2019). It is a rich and renewable resource on earth
and a good source of heavy metal adsorbents (Tan
2020, Tian 2020, Ulfa 2019, Wang 2019, Wang
2019). However, due to the close connection among
the components of lignocellulose, the adsorption of
heavy metals by cellulose is hindered. Therefore, the
direct use of Isatis Indigotica fort draff for heavy
metal adsorption has the problem of low adsorption
capacity and adsorption rate. Reasonable
modification of Isatis Indigotica fort draff to remove
hemicellulose and lignin is very important to
increase its adsorption capacity.
In this work, chemical reagent was used to
modify Isatis Indigotica fort draff. The structure of
resulting absorbent was characterized by scanning
electron microscope (SEM) and Fourier transform
infrared spectroscopy (FTIR). The effects of
solution pH value, solution concentration, adsorbent
dosage, and adsorption time of copper ion solution
on the adsorption performance of Isatis Indigotica
fort draff based adsorbent were studied through
static adsorption experiment to clarify the
adsorption mechanism. It provides guidance for the
resource utilization of Isatis Indigotica fort draff and
experimental basis for the preparation of new
adsorbents.
2 EXPERIMENTAL METHODS
2.1 Materials
The Radix Isatidis used in the experiment came
from Huirentang Pharmacy in Lanzhou, and the
experimental water was deionized water.
2.2 Methods
2.2.1 Preparation of the Absorbent
(1) Decolorization: The purchased Radix Isatidis
was washed with deionized water for 1-2 times,
boiled for 3 times (30 min each time), dried at
65 °C, crushed and passed through a 40-mesh sieve.
The prepared drug residue and methanol were mixed
in a ratio of 1:5 and stirred to remove bioactive
components and pigments. The methanol is
constantly replaced during stirring until the
methanol is colorless after stirring. After washing
with distilled water and drying at 65 °C, the
prepared drug residue was named as decolorized
Isatis Indigotica fort draff and recorded as RIR.
(2) Modification:
1) NaOH modification: The RIR was added into
an aqueous solution of NaOH (1 mol/L) at a solid-
to-liquid ratio of 10:1 and stirred magnetically for 4
h (120 r/min). The resulting residue was washed
with deionized water to be neutral, filtered, and
dried, which was named as NaOH modified Isatis
Indigotica fort draff and denoted as RIR-NaOH.
2) Na2CO3 modification: The RIR was added
into an aqueous solution of Na
2
CO
3
(1 mol/L) at a
solid-to-liquid ratio of 10:1 and stirred magnetically
for 4 h (120 r/min). The resulting residue was
washed with deionized water to be neutral, filtered
with sand filter funnel, and dried, which was named
as Na
2
CO
3
modified Isatis Indigotica fort draff and
denoted as RIR-Na
2
CO
3
.
3) Citric acid modification: The RIR was firstly
treated with 0.1 mol/L NaOH for 30 min. The
resulting residue was then mixed with an aqueous
solution of citric acid (0.6 mol/L) at a solid-to-liquid
ratio of 10:1 and stirred magnetically for 4 h (120
r/min). The resulting residue was washed with
deionized water to be neutral, filtered, and dried,
which was named as citric acid modified Isatis
Indigotica fort draff and denoted as RIR-CA. All the
Isatis Indigotica fort draff based biosorbents were
named RIR-Ts.
2.2.2 Structural Characterization of RIR-Ts
(1) FTIR: Fourier transform infrared spectroscopy
(FTIR, Nicolet Nexus, USA) was used to study the
molecular structure and chemical bonds of RIR-Ts.
The samples for analysis were dried before use,
ground in a mortar, mixed with potassium bromide
powder and pressed into transparent sheets. The
experiment was carried out in the spectral range of
4000-400 cm-1.
(2) SEM: the morphology of lignocellulose
compounds was studied by SEM (JSM-6701F,
JEOL, Japan). The samples for analysis were stored
in an oven at 50 ℃ overnight.
(3) analyzer (Perkin-Elmer Cetus Instruments,
Norwalk, CT) was used to study the thermal
stability and composition of the adsorbent. About 10
mg of the sample was placed in the sample holder
and heated from room temperature to 800℃
(heating rate = 10 ℃/min). The purging gas was
nitrogen with a flow rate of 20 mL/min.
(4) XRD: the crystalline properties of cellulose
were studied by using X-ray diffractometer (JDX-
3530,2kw, Tokyo, Japan). Before analysis, the
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1014

sample was ground into fine and uniform powder
and stored in an oven at 50 °C overnight. Using Cu
pulsed radiation with a wavelength of 0.154 nm, the
crystallization of the compound was determined by
monitoring the position, shape, and intensity of the
reflection from the distribution structure substrate.
2.2.3 Adsorption Properties and Reusability
(1) Establishment of Standard Curve A series of
concentration gradient solutions were obtained by
step dilution method, and the absorbance of copper
ions with different concentrations were measured by
flame atomic absorption photometer. This step was
repeated for three times to obtain the standard curve.
(2) Influencing Factors of Adsorption
Performance A series of Cu(NO
3
)
2
solutions (250
mL) with different concentrations were prepared.
The pH value of the solution was adjusted by 0.1
mg/L NaOH and 0.1 mg/L HCl. Different amount
(0.01 g, 0.02 g, 0.04 g, 0.06 g, 0.08 g, 0.10 g) of
Isatis Indigotica fort draff was added into the
Cu(NO
3
)
2
solutions with different pH values
(1,2,3,4,5,6) and different concentrations of copper
ions (20 mg/L, 40 mg/L, 60 mg/L, 80 mg/L, 100
mg/L, 150 mg/L). The resulting mixtures were
oscillated on the oscillator at room temperature for a
certain period of time (10 min, 20 min, 30 min, 40
min, 60 min, 75 min, 120 min). 1 mL of supernatant
was taken and diluted with pure water in a 50 ml
volumetric flask, and then the absorbance of the
solution was measured on the flame atomic
absorption analyzer after fully oscillating. The
concentration value was obtained by automatic
conversion of the instrument, and then the original
solution concentration value is obtained by
multiplying the concentration value by 50. After
calculation, the experimental adsorption capacity,
adsorption kinetic model parameters, and adsorption
isotherm parameters can be obtained.
(3) Reusability After the adsorption process is
completed, the adsorbent was separated from the
copper ion solution. After being washed, the
adsorbent is desorbed with eluent. After desorption,
the adsorbent was separated, and the next
adsorption-desorption process is cycled for a total of
four adsorptions-desorption experiment. Two sets of
parallel experiments were carried out to calculate
the average value of desorption efficiency.
3 RESULTS AND DISCUSSION
3.1 Characterization of Chemically
Modified Isatis Indigotica Fort
Draff based Biosorbent
3.1.1 SEM Analysis
The SEM images of RIR, RIR-NaOH, RIR-Na
2
CO
3
,
and RIR-CA are shown in Figure1-(a). The results
show that the structure of RIR is relatively dense,
and the surface of RIR-NaOH, RIR-Na
2
CO
3
, and
RIR-CA show signs of fiber surface fracture and
fiber disintegration, and the surface pores increase.
The increase in pores is beneficial to increase the
contact area between heavy metal ions and the
surface area of cellulose, and to adsorb more heavy
metal ions.
3.1.2 FTIR Analysis
The IR spectra of RIR, RIR-NaOH, RIR-Na
2
CO
3
,
and RIR-CA are shown in Figure1-(b). In general,
before and after the modification, the peak shape of
the IR spectrum is roughly the same, and there is no
big shift. Before modification, the peak is broad and
strong near 3417 cm-1, indicating that there were
many stretching vibration absorption peaks of O-H
and N-H on the surface of the Isatis Indigotica fort
draff -based biosorbent; the absorption peak of 2927
cm-1 comes from the stretching vibration of
saturated C-H bonds, the absorption peak of 1647
cm-1 comes from the stretching vibration of C=O of
aliphatic aldehyde, the absorption peak of 1415cm-1
is from the deformation vibration of CH
3
- and -CH
2
-
, the absorption peak of 1153 cm-1 is from the
stretching vibration of ester bond, and the
absorption peak of 1029cm-1 is from the bending
vibration of -OH. After modification, the peak
intensity of Isatis Indigotica decreased, the
amplitude decreased, and the wave peaks became
wider, indicating that the content of various groups
on the surface of the modified Isatis Indigotica were
reduced (Wei 2003).
3.1.3 TG Analysis
The thermograms of RIR, RIR-NaOH, RIR-
Na2CO3, and RIR-CA are shown in Figure1-(c).
According to the thermal decomposition of each
component of lignocellulosic biomass, Reddy et al.
(Xu 2021), Chen et al. (Yang 2016), and Braga et al.
(Yang 2020) divided the TG curves of the
Lignocellulosic biomass into three stages, namely,
Preparation and Adsorption Properties of Chemically Modified Isatis Indigotica Fort Draff based Biosorbent
1015
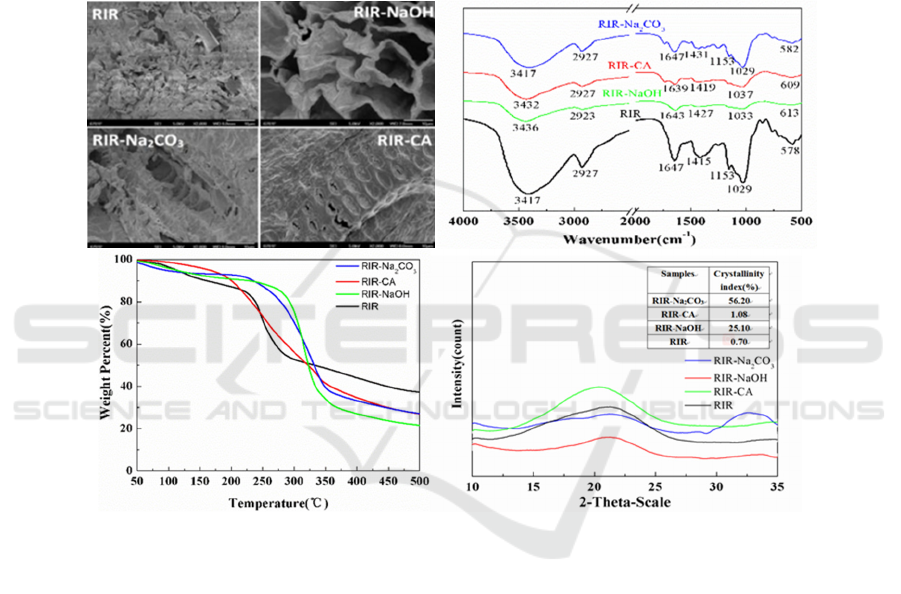
the dehumidification stage, the decomposition of the
cellulose molecular skeleton and the loss of
hemicellulose, and the decomposition of solid
residues. In this experiment, according to the
thermogravimetric curve, when the temperature is
lower than 120-220 ℃, the weight loss of the
sample is between 5% and 8%, mainly due to the
evaporation of water in the sample. When the
temperature is between 220 and 350 ℃, the curve
drops faster, mainly due to the decomposition of
cellulose and hemicellulose. The order of the weight
loss rate of the four adsorbents is as follows: RIR-
NaOH > RIR-Na
2
CO
3
> RIR-CA > RIR, which
shows that the loss rate of cellulose and
hemicellulose of the modified adsorbent is higher.
When the temperature is higher than 350 ℃, the
weight of the sample changes slowly, which is
mainly related to the decomposition of lignin (Yi
2019). In addition, it can also be observed that the
thermal stability of the adsorbent after modification
is higher than that before modification.
(a) (b)
(c) (d)
Figure 1: Characterization of RIR, RIR-NaOH, RIR-Na2CO3, and RIR-CA.(a)SEM of RIR, RIR-NaOH, RIR-Na2CO3, and
RIR-CA. (b)FTIR spectra of RIR, RIR-NaOH, RIR-Na2CO3 and RIR-CA. (c) TG of RIR, RIR-NaOH, RIR-Na2CO3 and
RIR-CA. AR-Na2CO3 and AR-CA. (d)XRD profiles of RIR, RIR-NaOH, RIR-Na2CO3 and RIR-CA.
3.1.4 XRD Analysis
The XRD patterns of RIR, RIR-NaOH, RIR-
Na
2
CO
3
, and RIR-CA are shown in Figure1-(d). The
results show that the crystallinity indexes of RIR,
RIR-NaOH, RIR-Na
2
CO
3
, and RIR-CA are 0.7%,
25.10%, 56.20%, and 1.08% respectively. It was
found that the crystallinity of the modified adsorbent
was higher than that before modification, indicating
that the structure of lignin and hemicellulose was
destroyed after modification, making the overall
structure loose. Secondly, it is found that the
crystallinity index of RIR and RIR-CA is very low,
which may be due to the changes of Radix Isatidis
in the process of chemical treatment since its
composition is different from those of the other two
Chinese herbal medicines. We need to conduct in-
depth research in future.
3.2 Study on the Absorption Behavior
of Isatis Indigotica Fort Draff
based Biosorbent on Copper ions
3.2.1 The Influence of Solution pH on
Adsorption
The effect of solution pH on the adsorption capacity
of copper ions is shown in Figure 2-(a). When the
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1016
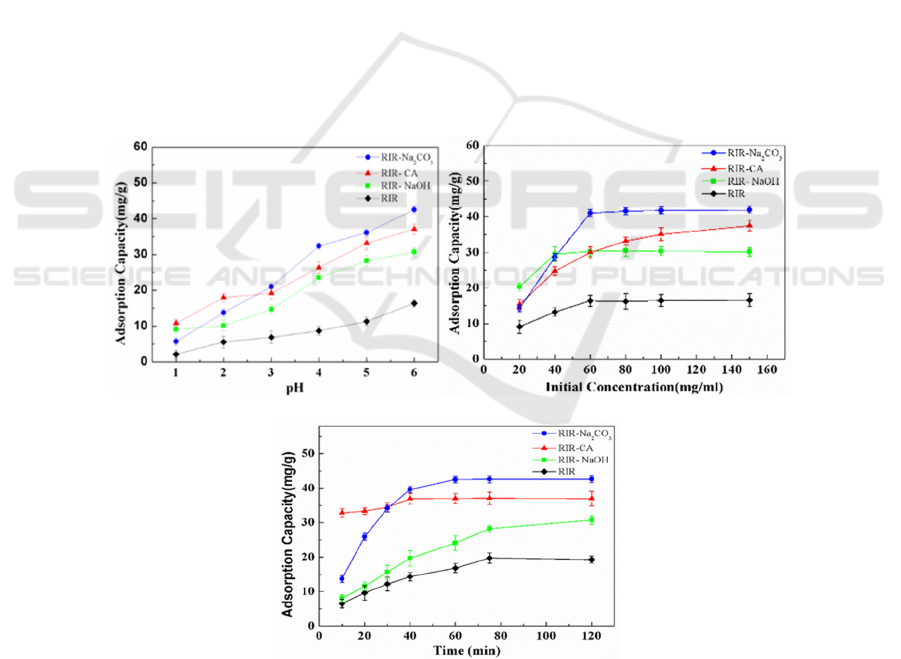
pH is too high, copper ions will exist in the form of
precipitation, which is not conducive to adsorption.
Therefore, the maximum pH of the solution studied
in this experiment is about 6.0. It can be seen from
the figure that the adsorption capacity of RIR, RIR-
NaOH, RIR-Na
2
CO
3
, and RIR-CA for copper ions
all increases with the increase of pH value. This is
mainly due to the high concentration of H+ in the
solution at the beginning, which competes with
copper ions for the active sites of the adsorbent.
Therefore, the adsorption capacity is low. As the pH
value increases, the concentration of H+ decreases,
which weakens the competition gradually.
Therefore, the adsorption capacity is increased. In
addition, in the process of pH change, the adsorption
capacity of RIR-NaOH and RIR-CA is always
higher than that of RIR, and the adsorption capacity
of RIR-CA is always higher than RIR-NaOH.
3.2.2 The Effect of Initial Solution
Concentration on Adsorption
As shown in Figure 2-(b), the adsorption capacity of
RIR, RIR-NaOH, RIR-Na
2
CO
3
, and RIR-CA for
copper ions varies with the adsorption
concentration. It can be seen from the figure that
with the increase of the initial concentration of the
solution, the change trend of adsorption capacity
increases first and then remains basically
unchanged. The possible reason is that because the
dosage of the adsorbent is fixed, the number of
adsorption sites is fixed, and the maximum amount
of copper ions that can be adsorbed is also fixed.
When the concentration of copper ions in the
solution is low, the adsorption sites are unsaturated.
When the concentration of copper ions in the
solution increases to a certain extent, the adsorption
sites reach saturation, at which time the adsorption
capacity is the largest. Even the concentration of
copper ions further increases, the adsorption
capacity will no longer change. When RIR, RIR-
NaOH, RIR-Na
2
CO
3
, and RIR-CA reach adsorption
equilibrium, their optimal copper ion concentrations
in the solution that can be adsorbed are 60, 100, 60,
and 60 mg/L, respectively.
(a) (b)
(c)
Figure 2: Study on the adsorption effect of Isatis In digotica Fort Draff residue-based bio-adsorbent on Cu2+. (a) Effect of
solution pH on the adsorption capacity of Isatis Indigotica Fort Draff based biosorbent.. (b) Effect of initial concentration of
solution on the adsorption capacity of Isatis Indig otica Fort Draff based biosorbent. (c) Effect of adsorption time on the
adsorption capacity of Isatis Indigotica Fort Draff based biosorbent.
Preparation and Adsorption Properties of Chemically Modified Isatis Indigotica Fort Draff based Biosorbent
1017

3.2.3 The Effect of Adsorption Time on
Adsorption
Figure 2-(c) shows the change of adsorption
capacity of copper ions by RIR, RIR-NaOH, RIR-
Na2CO3, and RIR-CA with adsorption time. It can
be seen from the figure that with the increase of
adsorption time, the adsorption capacity increases
first and then remains basically unchanged. The
possible reason is that at the beginning of the
adsorption, there are many sites on the surface of the
adsorbent that can be used to adsorb copper ions and
there are more free copper ions in the solution.
However, as the reaction proceeds, these adsorption
sites gradually reach saturation, the copper ion in the
solution decreases, the adsorption tends to be
balanced, and the adsorption capacity reaches the
maximum. In the whole process of adsorption, the
adsorption capacity of RIR-NaOH, RIR-Na
2
CO
3
,
and RIR-CA are all higher than that of RIR, and the
adsorption capacity follows an order of RIR-
Na
2
CO
3
> RIR-NaOH > RIR-CA. Therefore, the
three modification methods can all increase the
adsorption capacity of RIR, and the modification
effect of Na
2
CO
3
is better than the other two. In
addition, in the whole adsorption process, the
adsorption time when RIR-CA, RIR-Na2CO3, RIR,
and RIR-NaOH reach equilibrium is 40, 60, 75, and
75 min respectively. Therefore, there is no
significant change in the adsorption time when RIR-
NaOH reaches adsorption equilibrium compared
with RIR. The adsorption time of RIR-CA and RIR-
Na
2
CO
3
is reduced compared with RIR when
reaching the adsorption equilibrium, that is, the
Na
2
CO
3
modification can shorten the adsorption
time of the adsorbent.
3.2.4 Adsorption Isotherm
The Langmiur isotherm model and the Freundlich
isotherm model were used to fit the adsorption data
of the Isatis Indigotica fort draff-based adsorbent,
and the formula is as follows:
Langmiur model:
(1)
Freundlich model:
(2)
It can be seen from Table 1 that the fitting
constant obtained by the Langmiur isotherm model
is closer to 1, so the Langmiur isotherm model can
better describe the adsorption data. It shows that the
adsorption of heavy metal ions by the adsorbent is
single-layer adsorption, and the adsorption process
is chemical adsorption. According to the Langmuir
adsorption isotherm, the saturated adsorption
capacities of RIR, RIR-NaOH, RIR-Na
2
CO
3
, and
RIR-CA for copper ions are 18.72, 31.91, 54.23, and
47.73 mg/g respectively. It can be seen that the
performance of Na
2
CO
3
modified Isatis Indigotica
fort draff based adsorbent is the best.
Table 1: Adsorption isotherm model and parameters of Isatis Indigotica Fort Draff based biosorbent.
Adsorbent
Langmuir Model
Freundlich Model
qmax(mg/g) K(L/mg) RL2 KF n RF2
RIR 18.72 6.50×10-2 0.987 4.10 3.30 0.769
RIR-NaOH 31.91 1.67×10-1 0.993 4.88 5.47 0.556
RIR-Na
2
CO
3
54.23 3.25×10-2 0.919 4.24 1.99 0.742
RIR-CA 47.73 2.63×10-2 0.995 1.91 5.13 0.920
3.2.5 Adsorption Kinetics
The first-order and second-order equations of
adsorption kinetics are respectively used to fit the
adsorption data of the adsorbent, and the formula is
as follows:
The first-order equation of adsorption kinetics:
(3)
The second-order equation of adsorption
kinetics:
(4)
The results are shown in Table 2. The linear
correlation coefficient R
1
2
obtained by quasi first-
Lmm
e
e
e
KQQ
C
q
C 1
+=
eFe C
n
Kq log
1
loglog ×+=
t
k
qqq ete )
303.2
()log()log(
1
−=−
e
e
t q
t
qkq
t
+=
2
2
1
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1018
order kinetic fitting of the three adsorbents is
relatively small, indicating that the reaction does not
accord with the relevant characteristics of the quasi
first-order reaction. The linear correlation
coefficient R
2
2
obtained by the quasi second-order
kinetics fitting is greater than that of the quasi first-
order kinetics, and the degree of fit is high,
indicating that the quasi second-order kinetics
model can describe the adsorption data of these
three adsorbents well. This also shows that the
adsorption process of heavy copper ions on the
surface of the Isatis Indigotica fort draff based
biosorbent is chemical adsorption.
Table 2: Adsorption kinetic model and parameters of Isatis Indigotica Fort Draff based biosorbent.
Adsorbent
qe,exp
(mg/g)
Quasi first order dynamics model Quasi second order dynamics model
qe,cal(mg/g) k1(min−1) R
1
2
qe,cal(mg/g) k
2
(g/mg·min-1) R
2
2
RIR 16.54 14.85 3.71×10-2 0.566 20.56 1.71×10-3 0.982
RIR-NaOH 31.00 49.18 4.05×10-2 0.527 44.68 4.36×10-4 0.992
RIR-Na
2
CO
3
42.65 42.67 7.03×10-2 0.862 51.15 1.09×10-3 0.971
RIR-CA 37.55 3.85 2.14×10-2 0.564 37.69 1.47×10-2 0.991
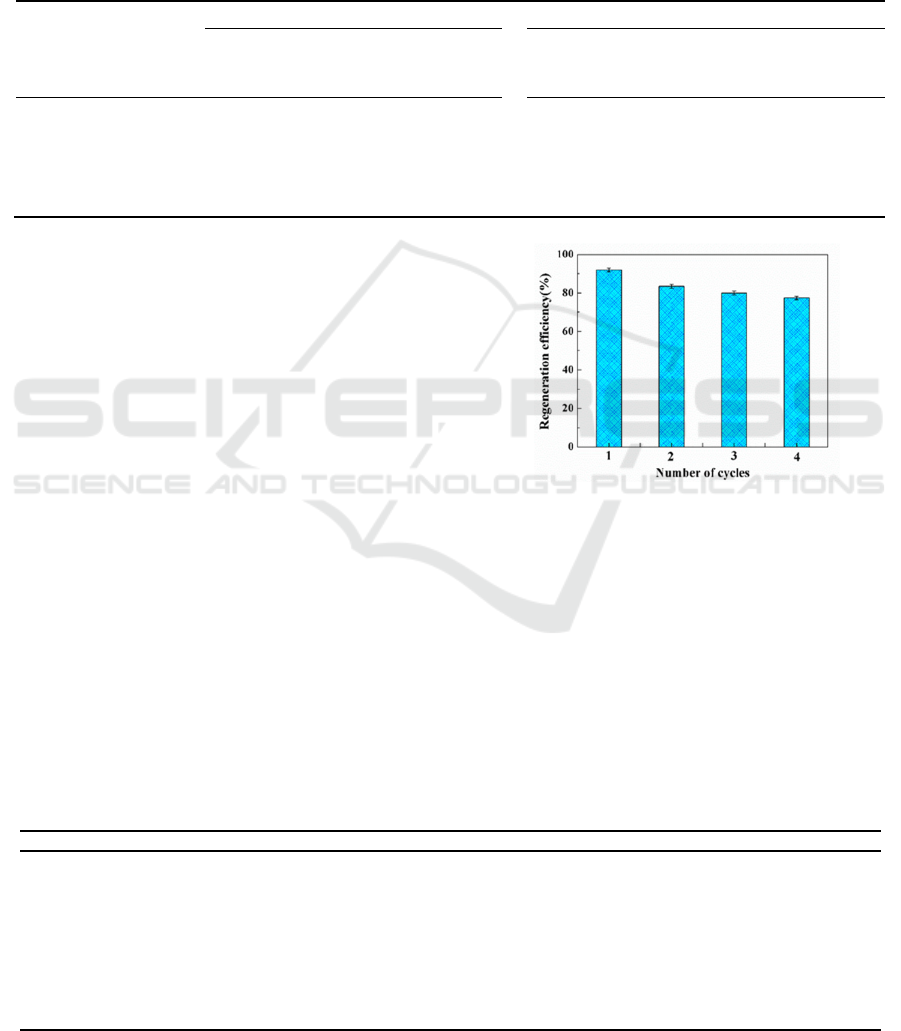
3.2.6 Reusability
LR-Na
2
CO
3
, the best bio-adsorbent for Cu
2+
in our
experiment, was selected to study the adsorption and
desorption conditions. The regeneration and
reusability of LR-Na
2
CO
3
for heavy metals Cu
2+
were evaluated by four consecutive adsorption–
desorption cycles (Figure 3). It showed that LR-
Na
2
CO
3
has good reusability for Cu
2+
, the
adsorbability remained higher than 74% after four
consecutive adsorption–desorption cycles,
consistent with other reports, which indicated that
the LR-Na
2
CO
3
is a suitable potential adsorbent for
the removal of the heavy metal ions Cu
2+
from
water.
Figure 3: Reusability of RIR-Na
2
CO
3
.
3.3 Comparison with Other
Adsorbents
The qmax value obtained in this study was
compared with various biosorbents reported in the
literature for removing
Cu2+
, as shown in Table3. The
results show that the adsorption capacity of RIR-CA
and RIR-Na2CO
3
are higher than that of other
biosorbents (such as activated carbon fiber,
modified sawdust cellulose, and so on).
Table 3: Comparison of Isatis Indigotica Fort Draff and other adsorbents.
Absorben
t
Metal Ion qmax(mg/g) Reference
RIR-CA
Cu
2+
47.73 this study
RIR-Na
2
CO
3
54.23 this study
Autotrophic nitrifying granular
sludge
15.02 Zhang et al. (Yu 2019)
Activated carbon fiber 25.51 Yu J, Chi C, Zhu B, Qiao K, Yan
S.(Yuan 2019)
Modified sawdust cellulose 4.33 Ulfa S M, Chamidah N, Kurniawan
A.(Zhang 2020)
Papermaking sludge 28.788 Dai C, Zhang Y. (Zhang 2020)
Preparation and Adsorption Properties of Chemically Modified Isatis Indigotica Fort Draff based Biosorbent
1019

4 SUMMARY
The Isatis Indigotica fort draff was used as raw
materials and modified with NaOH, Na
2
CO
3
, and
citric acid to prepare the Isatis Indigotica fort draff
based biosorbent, which was used in the study of the
adsorption of heavy metal copper in water. The
structure of the modified biosorbent was
characterized by FTIR, SEM, TG and XRD. It was
found that the structure of the modified biosorbent
was loose and the cellulose surface active groups
increased. The adsorption performance of RIR, RIR-
NaOH, RIR-Na
2
CO
3
, and RIR-CA is better than that
of RIR. The order of the maximum adsorption
capacity of the modified Isatis Indigotica fort draff
based adsorbent for copper ions is: RIR-Na
2
CO
3
>
RIR-NaOH > RIR-CA > RIR. The adsorption
process of copper ion conforms to the quasi first-
order kinetic model and Langmuir model, that is, the
adsorption of copper ion on its surface is chemical
adsorption. The adsorption capacity of Na
2
CO
3
modified Radix Isatidis for copper ions is the best,
and the maximum adsorption capacity after four
consecutive adsorption-desorption cycles is about
77% of that at the first adsorption. In conclusion, the
modification of NaOH, Na
2
CO
3
, and citric acid can
not only improve the adsorption performance of
Isatis Indigotica fort draff, but also reduce the
environmental pollution caused by the unreasonable
use of Isatis Indigotica fort draff and improve the
resource utilization rate of Isatis Indigotica fort
draff.
ACKNOWLEDGEMENT
This work was supported by NSFC (82160900),
Innovation Fund Project of Higher Education in
Gansu Province (2021B-159), Open Foundation of
Collaborative Innovation Center for Prevention and
Control by Chinese Medicine on Disease Related
Northwestern Environment and Nutrition
(998/99860202) , Open Foundation of Traditional
Chinese Medicine Research Center of Gansu
Province (ZYZX-2020-ZX16) and Research on
prevention and control of COVID-19 by integrated
Traditional Chinese and Western Medicine(2020C-
36).
REFERENCES
Bai L, Wu C, Gu F. The Adsorption of Lead-containing
Wastewater by Modified Isatis indigotica Fort
Draff[J]. Hubei Agricultural Sciences. 2015; 54(24):
6219-6222.
Braga R M, Costa T R, Freitas J C O, Barros M F, Melo D
M A, Melo M A F. Pyrolysis Kinetics of Elephant
Grass Pretreated Biomasses. J. Therm. Anal. Calorim.
2014; 117(3): 1341-1348.
Cao K. Correlation between Chemical Composition in
Process of Isatidis Radix Preparation and Its
Pharmacological Activity [J]. Journal of North
Pharmacy. 2016; 13(11): 119-120.
Chen W, Tu Y, Sheen H K. Impact of Dilute Acid
Pretreatment on the Structure of Bagasse for
Bioethanol Production. Int. J. Energy Res. 2010;
34(3):265-274.
Duan N, Fang L, Zhang X. Non-carcinogenic Health Risk
Assessment of Soil Heavy Metals Based on Target
Organ[J]. Safety and Environmental Engineering.
2021; 28(04): 207-212.
Du Z, Chen H, An Y, Guo X. Research Progress on
Preparation Techniques of Lignocellulose-based
Heavy Metal Adsorbents[J]. Journal of Agro-
Environment Science. 2019; 38(12): 2659-2671.
Deng C, Ma H, Wang Z, Zhou J. Characterization and
Pyrolysis of Apricot Shell Cellulose[J]. Journal of
Forestry Engineering. 2018; 3(6): 87-92.
Dai C, Zhang Y. Study on the Preparation of Functional
Adsorption Material from Paper Sludge and Its
Adsorption Properties for Cu2+[J]. Environmental
Pollution and Control. 2019; 41(6): 621-625+630.
Huang W, Chen H. Research Progress on Antiviral of
Active Components of Radix Isatidis[J]. Journal of
Mathematical Medicine. 2015; 28(1): 96.
Huang Y, Dong F, Li C. Research Progress on the
Determination Methods of Main Chemical
Components in Radix Isatidis[J]. China
Pharmaceuticals. 2020; 29(7): 150-156.
Jia J, Cao R, Du Y, Zhao Z, Li L. Review of the
Researches on Polysaccharides from Isatis Indigatica
Fort. Science and Technology of Food Industry. 2016;
37(18): 378-383.
Jia W, Qin K, Liu L. Research on the Components
Determination of Isatis Indigotica Fort Draff and Its
Application[J]. Journal of Taishan Medical College.
2010; 31(7): 520-521.
Jiang S, Zhou M, Deng W, Dai H, Fang G, Wu W. High-
wet-strength Paper-based Lignocellulosic Adsorbents
and Its Heavy Metal Ion Adsorption Properties[J].
Journal of Forestry Engineering. 2020; 5(3):101-107.
Kim T H, Kim J S, Sunwoo C, Lee Y Y. Pretreatment of
Corn Stover by Aqueous Ammonia. Bioresour.
Technol. 2003; 90(1): 39-47.
Kommula V P, Reddy K O, Shukla M, Marwala T, Rajulu
A. V. Physico-chemical, Tensile, and Thermal
Characterization of Napier Grass (Native African)
Fiber Strands. Int. J. Polym. Anal. Charact. 2013;
18(4): 303-314.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1020

Pan H, Zhu P, Wei X, Zhang Y. Research Progress of
Radix Isatidis [J]. Guide of China Medicine. 2018;
16(30): 30-32.
Reddy K O, Maheswari C U, Reddy D J P, Rajulu A V.
Thermal Properties of Napier Grass Fifibers. Mater.
Lett. 2009; 63(27): 2390-2392.
Tian Y, Zhao J, Feng B, Sun X. Research Progress in the
Removal of Heavy Metal by Algae[J]. Journal of
Kunming University of Science and Technology
(Natural Science). 2020; 45(05): 97-104.
Tan S, Bai X, Wang X, An Y, Ning G, Zhang X.
Adsorption of Pb (II) on Modified
Lignocellulose/Montmorillonite[J]. Journal of Inner
Mongolia Agricultural University (Natural Science
Edition). 2019; 154(4): 76-83.
Tan S, Bai X, Wang X, An Y, Shen Y, Zhang X. Study of
Adsorption Property of Lignocellulose-based
Nanocomposite towards Mn2+[J]. Journal of Inner
Mongolia Agricultural University (Natural Science
Edition). 2020; 159(3): 74-80.
Ulfa S M, Chamidah N, Kurniawan A. Adsorption of
Cu(II) in Aqueous Solution by Modified Sawdust
Cellulose[J]. IOP Conference Series Earth and
Environmental Science. 2019; 239: 012008.
Wang J, Li W. Determination of Effective Components in
Banlangen Granules and Research Progress of
Pharmacological Effects[J]. China Medical Herald.
2019; 16(18): 49-52.
Wang J, Li W. Determination of Effective Components in
Banlangen Granules and Research Progress of
Pharmacological Effects[J]. China Medical Herald.
2019; 16(18): 49-52.
Wei P, Wei D, Mo D. Biosorption of Lead by Isatis
Indigotica Fort Draff[J]. Ion Exchange and
Adsorption. 2003; 19(4): 351-356.
Xu S, Wang Y, Bi W, Zhou J, Chen W. Preparation of a
Modified Microbial Adsorbent and Its Adsorption
Characteristics for Cd[J]. Acta Scientiae
Circumstantiae. 2021; 41(04): 1342-1350.
Yang Y, Zhong Y, Li S, Deng M, Yang X, Yang Y, Yao
Y. Research Progress on the Harm of Heavy Metals to
Human Body in Aquatic Products[J]. Agricultural
Technology and Equipment. 2020; (10): 55-56. More
references
Yuan T, Hu S. Current Situation and Treatment Methods
of Heavy Metal Pollution in Water[J]. Construction
and Budget. 2019; (06): 75-78.
Yang C. Advances in Pharmacological Research of Radix
Isatidis[J]. Chinese Journal of Modern Drug
Application. 2016; 10(9): 282-283.
Yi J, Huo Z, Abdullah M A, Khalid A A, Li J. Application
of Agroforestry Waste Biomass Adsorption Materials
in Water Pollution Treatment[J]. Progress in
Chemistry. 2019; 31(5): 760-772.
Yu J, Chi C, Zhu B, Qiao K, Yan S. High Adsorptivity
and Recycling Performance Activated Carbon Fibers
for Cu(II) Adsorption. Sci. Total Environ. 2019; 700:
134412-134435.
Zhang B. Heavy Metal Pollution in Water and Its
Remediation Methods[J]. Chemical Enterprise
Management. 2020; (17): 63-64.
Zhang B, Zeng M, Zhang L, Wang H, Zeng Y, Huang S,
Wu J, Cheng Y, Long B. Adsorption of Cu2+ by
Autotrophic Nitrifying Granular Sludge and Its
Adsorption Isotherm[J]. Chemical Industry and
Engineering Progres. 2020; 39(4): 1583-1590.
Preparation and Adsorption Properties of Chemically Modified Isatis Indigotica Fort Draff based Biosorbent
1021
