Discovery and Development of Semaglutide
Yumeng Duan
1,† a
and Ziyao Wan
2,† b
1
Jiangnan University, Wuxi, China
2
Nanjing Tech University, Nanjing, China
†
These authors contributed equally
Keywords: Diabetes, GLP-1 RAs, Semaglutide.
Abstract: Diabetes Mellitus is a universal disease around the world. There are many different types of drugs for
treatment, among which the glucagon-like peptide 1 receptor agonists (GLP-1 RAs) have attached much
attention in recent years. Semaglutide, one of the GLP-1 RAs, is a long-acting diabetes drug developed by
Novo Nordisk. Compared with traditional diabetes drugs, semaglutide possesses a longer biological half-life,
which conduces to reduce the dosing frequency. And based on the general preparation of semaglutide, Novo
Nordisk made changes to the dosage form and made it into the oral drug. In 2019, FDA launched the new
drug, oral semaglutide. Oral semaglutide has been proved that it still maintains good effectiveness, and oral
semaglutide has better safety and can reduce the occurrence of hypoglycemia through pre-clinical and clinical
trials. This review introduces the mechanism, structure, pre-clinical and clinical trials of semaglutide and
discusses the entire process from the research and development background to the market.
1 INTRODUCTION
1.1 Diabetes
Diabetes is a metabolic disease characterized by high
blood sugar, and insulin secretion defects are the main
cause of diabetes.
Long-term diabetes can damage various tissues,
such as the eyes, heart, blood vessels, and kidneys.
Diabetes can also cause symptoms such as polydipsia,
polyuria, polyphagia, and weight loss.
Table 1 shows the comparison of some indexes
between healthy people and diabetic patients. The
highest fasting blood glucose (FBG) of healthy
people is only 6.1 mmol/L, while the free blood
glucose index of diabetic patients is at least 7 mmol/L.
The glycosylated hemoglobin (HbA1c) and body
mass index (BMI) of healthy people are also lower
than those of diabetic patients. The 2-hour
postprandial blood glucose of diabetic patients is even
about twice that of healthy people.
a
https://orcid.org/0000-0001-6760-5926
b
https://orcid.org/0000-0002-9507-7208
Table 1: Comparison between healthy and diabetes patients.
(American Diabetes Association 2010, World Health
Organization, Vijan 2010).
Indexes Healthy people Patients with diabetes
mellitus
FBG 4.4 – 6.1
mmol/L
≥7.0 mmol/L
HbA1c 4 % – 6 % ≥6.5 %
(OGTT)
2hPBG
4.6 – 7.8
mmol/L
≥11.1 mmol/L
BMI 18.50 – 24.99 Overweight: 25.00-29.99
Obese Class Ⅰ: 30.00-
34.99
Obese Class Ⅱ: 35.00-
39.99
Obese Class Ⅲ: ≥40.00
C-peptide
(Kong
2016)
0.8 – 4.2 ng/ml Extremely-low (T1D)
As shown in Table 2, diabetes can be divided into
type 1 diabetes and type 2 diabetes.
Type 1 diabetes is an autoimmune disease caused
by the effects of external environmental factors based
on genetic susceptibility, leading to the damage, even
destruction of pancreatic islet cells, and finally, the
Duan, Y. and Wan, Z.
Discovery and Development of Semaglutide.
DOI: 10.5220/0011378500003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 1119-1130
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
1119
failure of their function. Type 1 diabetes is mainly
caused by insulin deficiency, and its onset is sudden.
The patient's body is mainly thin or normal, and this
type of diabetes occurs mostly in children.
Type 2 diabetes is the most common one among
diabetes, which is due to insulin resistance. Insulin
resistance is the inability of cells to respond
adequately to normal levels of insulin. The onset of
Type 2 is gradual, and patients are usually obese. This
type of diabetes occurs mostly in adults.
Table 2: Comparison between type 1 and 2 diabetes.
(Williams textbook of endocrinology).
Feature Type 1 diabetes Type 2 diabetes
Cause Insulin deficiency Insulin resistance
Onse
t
Sudden Gradual
Age at onset Mostly in
children
Mostly in adults
Body shape Thin or normal Mostly obese
Ketoacidosis Common Rare
Autoantibodies Usually present Absen
t
Endogenous
insulin
Low or absent Normal,
decreased, or
increased
Concordance in
identical twins
50% 90%
Prevalence ~10% ~90%
1.2 Glucagon-like Peptide 1
Glucagon-like peptide 1 receptor agonist drugs are an
effective option for treatment for patients with type 2
diabetes, which is used as a single treatment or
supplement to other antihyperglycemic therapies to
reduce glycosylated hemoglobin and body weight.
Glucagon-like peptide 1 (GLP-1) works by
binding to GLP-1 related receptors. GLP-1 receptors
are distributed in various tissues throughout the body,
including the pancreas, lungs, kidneys, and
cardiovascular system (Andersen 2018). GLP-1
regulates blood sugar in many ways, including
increasing blood sugar and suppressing the source of
blood sugar. The effects of GLP-1 on the pancreas
include regulating the secretion of insulin by lifetime
control on β-cells, inhibiting the secretion of
glucagon from α-cells, acting on δ-cells, and
promoting somatostatin synthesis (Smits 2016).
It is currently believed that the cAMP/Epac2/PKA
pathway is a classic activation pathway for the
regulation of insulin secretion by GLP-1 (Shigeto
2017). The protective effect of GLP-1 on β-cells is
reflected in the reduction of the damage caused by
endoplasmic reticulum stress to β-cells through
TUM1-Ex4 (Son 2018). The regulation of glucagon
may be achieved indirectly through δ cells to promote
the synthesis of somatostatin. Studies suggest that
GLP-1 can directly act on α-cells to inhibit the
secretion of glucagon (Davis 2020). The control of
GLP-1 on blood sugar is also affected by the basic
condition of patients and the type of medication. For
diabetic patients, the ones with a lower BMI can
achieve better results than those with a higher BMI
(Meier 2015).
In general, the regulation of blood sugar by GLP-
1 is a complex process, which is affected by blood
sugar levels, GLP-1 levels, receptor distribution, and
diversity (Wang 2020).
1.3 GLP-1RAs
Intravenous injection of exogenous GLP-1 to patients
with type 2 diabetes can reduce the blood glucose
concentration to the normal fasting range. However,
the short half-life and fast degradation speed limit the
further therapeutic application of GLP-1 (Gupta
2013). To overcome this problem, the development of
glucagon-like peptide 1 receptor agonists (GLP-
1RAs) was proposed.
GLP-1RAs are a class of anti-diabetic drugs with
unique characteristics. Although there are intra-class
differences in clinical efficacy due to different
biochemical structures and pharmacokinetic
characteristics, a significant hypoglycemic effect was
shown in all members of the GLP1-RA class, such as
liraglutide, abiglutide, exenatide, and semaglutide. In
addition, the safety of these drugs is generally
satisfactory (Christina 2019). Table 3 is the
comparison between liraglutide, exenatide and
semaglutide.
Table 3: Comparison of liraglutide, exenatide and
semaglutide.
Liraglutide Exenatide Semaglutide
Chemical
Structure
H-His-
Ala-Glu-
Gly-Thr-
Phe-Thr-
Ser-Asp-
Val-Ser-
Ser-Tyr-
Leu-Glu-
Gly-Gln-
Ala-Ala-
Lys (Pal-ν-
Glu)-Glu-
Phe-Ile-
Ala-Trp-
Leu-Val-
Arg-Gly-
H-His-
Gly-Glu-
Gly-Thr-
Phe-Thr-
Ser-Asp-
Leu-Ser-
Lys-Gln-
Met-Glu-
Glu-Glu-
Ala-Val-
Arg-Leu-
Phe-Ile-
Glu-Trp-
Leu-Lys-
Asn-Gly-
Gly-Pro-
H-His-Aib-Glu-
Gly-Thr-Phe-Thr-
Ser-Asp-Val-Ser-
Ser-Tyr-Leu-Glu-
Gly-Gln-Ala-Ala-
Lys (AEEAc-
AEEAc-γ-Glu-17-
carboxyheptadeca
noyl)-Glu-Phe-
Ile-Ala-Trp-Leu-
Val-Arg-Gly-Arg-
Gly-OH
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1120
Liraglutide Exenatide Semaglutide
Arg-Gly-
OH
Ser-Ser-
Gly-Ala-
Pro-Pro-
Pro-Ser-
NH2
acetate salt
Pancreatic
β-cell
Improve
pancreatic
β-cell
function
Protect
pancreatic
β-cells
Protect pancreatic
β-cells
DPP-IV Antagoniz
e the
degration
of DPP-IV
Antagoniz
e the
degration
of DPP-IV
Cover the
hydrolysis site of
DPP-IV encyme
Half-life Long half-
life
Long half-
life
Long half-life
Cardiovasc
ular system
Improve
cardiovasc
ular risk
factors
Protect the
cardiovasc
ular
system
Protect the
cardiovascular
system
1.3.1 Liraglutide
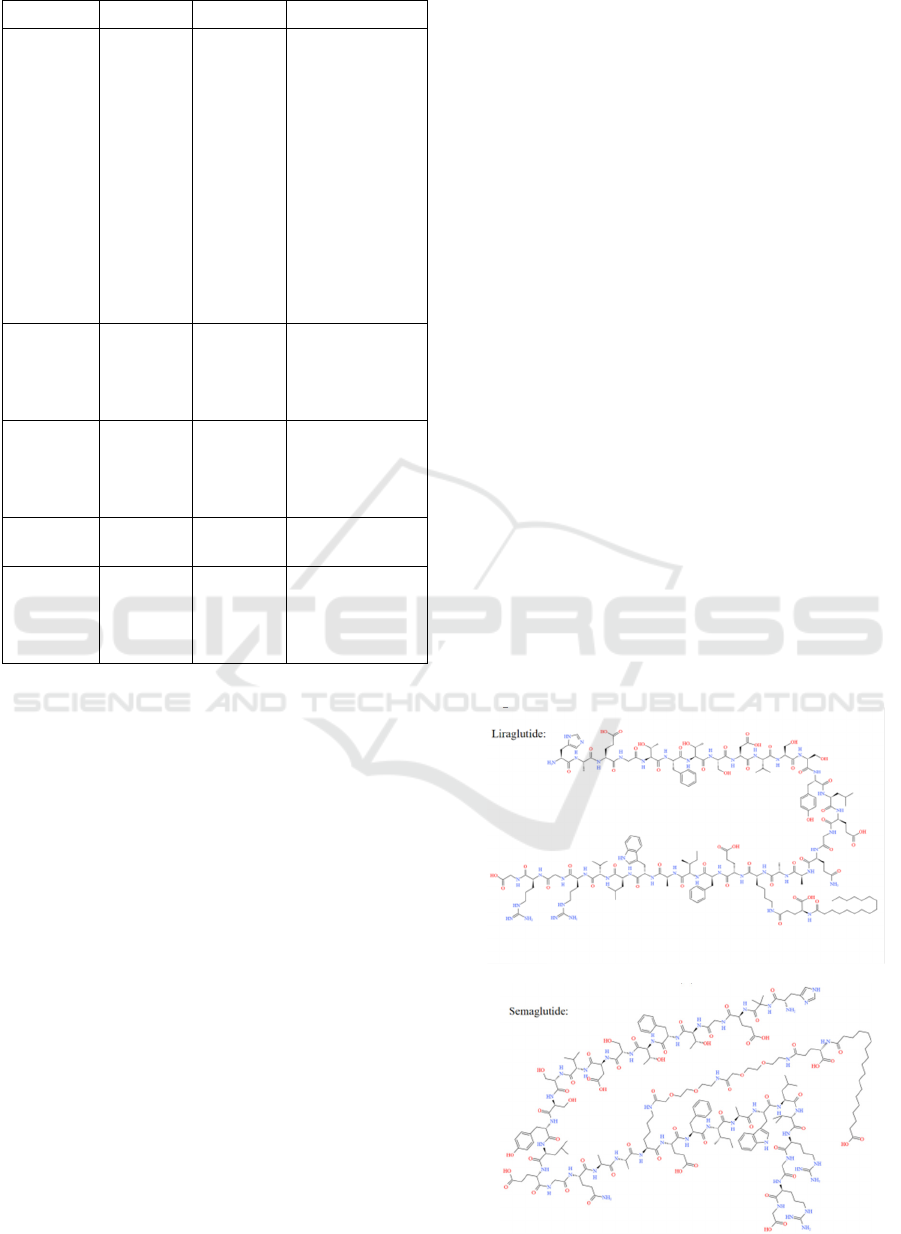
As shown in Figure 1, liraglutide is a GLP-1 analogue
formed by adding a 16-carbon acyl chain to the 26th
lysine residue of GLP-1 and substituting arginine for
the 34th lysine of GLP-1. The plasma forms a non-
covalent binding with albumin and releases slowly,
antagonizing the degradation of DPP-IV (Bock 2003).
The hypoglycemic effect of liraglutide is glucose
concentration-dependent, so the incidence of
hypoglycemia with long-term use of liraglutide is
very low. Liraglutide can also reduce patient weight,
improve cardiovascular risk factors, and at the same
time improve pancreatic β-cell function (Astrup
2012).
1.3.2 Exenatide
As shown in Figure 1, Exenatide is a GLP-1 analog
isolated from the salivary glands of the blunt-tailed
lizard distributed in the southwestern United States
and northern Mexico. It consists of 39 amino acid
residues and has a molecular mass of 4186.6, which
has 53% homology to GLP-1., And GLP-1 acts on G-
protein coupled receptors with higher affinity. The
second-to-last amino acid at the N-terminal of
exenatide is glycine (His-Gly-Glu), which is different
from the N-terminal sequence of GLP-1 (His-Ala-
Glu) and is not decomposed by DPP-IV, so it has a
longer half-life, can be more effective (HANSEN
1999, Zhou 2010). Dramatical reduction of blood
sugar and body weight was found by dosing twice a
day and protecting pancreatic β-cells and the
cardiovascular system (Bunck 2009). The main
adverse reaction of Exenatide is mild to moderate
nausea, which mostly occurs in the early stage of
medication, and its severity decreases with the
passage of time (Yoo 2006).
1.3.3 Semaglutide
As shown in Figure 1, Semaglutide is a long-acting
dosage developed from the structure of liraglutide
(Lau 2015). A1a at position 8 on the GLP-1 (7-37)
chain is replaced with Aib, and Lys at position 34 is
replaced with Arg. The Lys at position 26 is
connected to the fatty acid chain of octadecanoic acid.
Compared with Liraglutide, semaglutide has a longer
aliphatic chain with higher hydrophobicity. However,
semaglutide has been modified with a short chain of
PEG to greatly increase its hydrophilicity. The
modified semaglutide can bind tightly to albumin,
cover up the DPP-4 enzymatic hydrolysis site, and
reduce renal excretion, prolong the biological half-
life, and achieve the effect of long circulation
(Kapitza 2012).
(a)
(b)
Discovery and Development of Semaglutide
1121
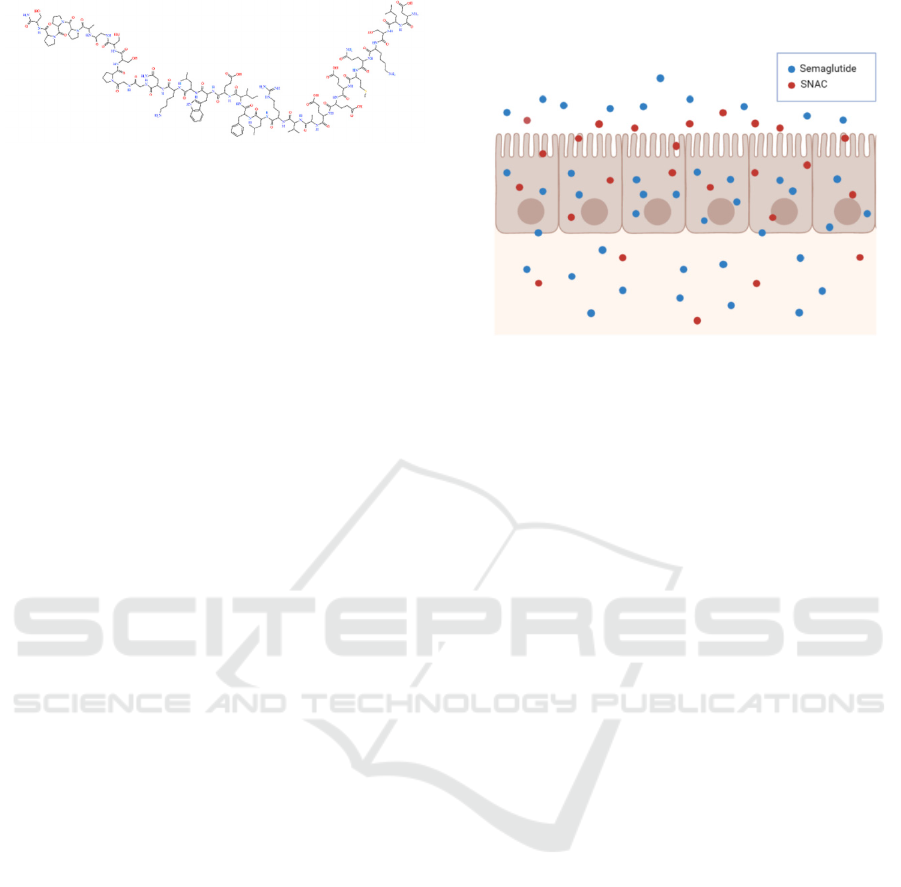
(c)
Figure 1: Structure of liraglutide (a), exenatide (b) and
semaglutide (c).
2 DEVELOPMENT OF ORAL
SEMAGLUTIDE
Their main disadvantage is that the subcutaneous
route of administration can cause malabsorption.
Therefore, the development of oral GLP1-RA
preparations will further consolidate its beneficial
effects in clinical practice. Oral semaglutide is a
modified form of semaglutide with the addition of a
carrier sodium N-(8-[2-hydroxybenzoyl] amino)
caprylate.
2.1 The Mode of Action of Oral
Semaglutide
Figure 2 shows the mode of action of oral semaglutide.
Oral semaglutide is limited to extensive degradation
by proteolytic enzymes in the gastrointestinal tract
and poor absorption across the gastrointestinal
epithelium (Mahato 2003). To achieve adequate
bioavailability of semaglutide after oral
administration, oral semaglutide has been co-
formulated with a 300 mg concentration of the
absorption enhancer called SNAC (Rasmussen 2020).
One disadvantage of using SNAC is the fast
absorption rate. Because of its low potency as a type
of permeation enhancer, it is difficult to maintain a
threshold concentration in the intestinal wall for a
long enough time. Therefore, semaglutide tablets
must use a 300 mg concentration of SNAC. SNAC
protects semaglutide against enzymatic degradation
via a local pH buffering effect (Rasmussen 2020). As
the oral semaglutide tablet rapidly erodes, SNAC
causes a local increase in pH, leading to the higher
solubility of semaglutide and protection from
proteolytic degradation (Rasmussen 2020). SNAC
also promotes the absorption of semaglutide across
the gastric epithelium in a concentration-dependent
manner by effects on transcellular pathways, which
are transient and fully reversible (Rasmussen 2020).
This absorption of semaglutide is highly localized and
depends on the spatial proximity of semaglutide and
SNAC (Buckley 2018).
Figure 2: Mode of action of oral semaglutide. SNAC,
sodium N-(8-hydroxybenzoyl] amino) caprylate.
2.2 Dosing and Medical Condition
Now researchers face another question, which is the
appropriate dosing condition.
Clinically, they found out food intake can have a
negative impact on the absorption of oral semaglutide,
so when oral semaglutide is taken in a fasting sate,
sufficient exposure can be reached. After the dosage
condition was figured out, the clinical pharmacology
of oral semaglutide becomes the focal point of the
research. Through several studies, researchers get
deep insight into how the exposure of semaglutide
following oral administration is influenced by
comorbidities or medication and how oral
semaglutide might impact the exposure of
concomitant medications (Rasmussen 2020).
Besides, some special medical conditions, such as
hepatic or renal impairment, might have a strong
impact on the pharmacokinetics of oral semaglutide.
To assess this, researchers clinically assessed how
oral semaglutide changes in its efficacy or absorption
under those medical conditions. From the results,
there is no apparent effect observed on the
pharmacokinetics or tolerability of oral semaglutide,
which indicates the dose redesign is not necessary at
this point.
Last but not the least, they investigated the effect
of oral semaglutide on exposure to various
medications normally taken by T2D patients. Based
on their results, when co-administered, oral
semaglutide had no clinically relevant effect on the
exposure of lisinopril, warfarin, and digoxin in
healthy subjects (Rasmussen 2020). When co-
administrated with metforminm furosemide, and
rosuvastatin, it only resulted in small change of oral
semaglutide. Similarly, they observed that there was
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1122
an increase in thyroxine exposure when co-
administered with levothyroxine. Until now, they still
keep doing studies about drug-drug interaction for
oral semaglutide. When enough information is
gathered, it will be more lucid and straightforward for
researchers to give more useful suggestions when oral
semaglutide is co-administered with other
medications by T2D patients.
3 PRE-CLINICAL TRAILS OF
SEMAGLUTIDE
3.1 AME Test
Semaglutide is an analogue of human glucagon-like
peptide 1 and is in clinical development to treat type
2 diabetes. In Lene Jensen’s study, the radioactivity
in blood, plasma, urine, and feces was measured in
rats and monkeys; the radioactivity in exhaled air was
measured in rats. The researchers quantified
metabolites in plasma, urine, and feces after analysis
and radiological testing. The blood-to-plasma ratio
and pharmacokinetics of both radiolabelled
semaglutide-related materials were assessed (Lene
2017).
This trial studied 0.5 mg of Semaglutide,
providing sufficient exposure to estimate the relevant
PK endpoint. Semaglutide was radiolabelled in the
octadecanedioic acid moiety in the side chain of
lysine 26 to characterise the metabolism of the most
modified part of the molecule (Lene 2017).
3.1.1 Blood-to-Plasma Ratio
The researchers collected blood and plasma samples
for 24 hours in rats to assess the ratio of blood to
plasma. The average blood-to-plasma ratio of the rats
was found to be 0.44-0.46 (Lene 2017). For monkeys,
in the absence of a small amount of tritium water, the
average blood-to-plasma ratio of samples collected
48 hours after dosing is in the range of 0.54-0.60
(Lene 2017).
3.1.2 Excretion of
[
3
H]-semaglutide-related Material
Table 4 shows the excretion of radioactivity in rats
and monkeys. In rats, the recovery of total excretion
of [
3
H]-semaglutide related substances after 168
hours showed that urine and feces are important
excretion pathways (Table 4). Radioactivity was still
detectable in urine 168h after dose administration,
and 22.4% was recovered in the carcasses. Less than
0.5% of the radioactivity was detected in expired air
(Lene 2017).
For monkeys, the total recovery of [
3
H]-
semaglutide-related material after 336 h showed that
similar to rats, urine and faeces were important routes
of excretion. When the collection is stopped, the
recovery of urine and feces is not complete. This can
be seen from the slightly positive slope of the
cumulative excretion versus the time curve. Unlike
mice, the radioactivity in the carcass has not been
determined. The total recovery rates of intact and dry
urine and stool samples were 12.6% and 7.0%,
respectively (Lene 2017).
Table 4: Excretion of radioactivity. (Lene 2017).
Species Rat
(% of dose [%
coefficient of
variation])
n=3
Monkey
(% of dose [%
coefficient of
variation])
n=3
Dose 0.3 mg/kg
10 MBq/kg
0.03 mg/kg
14 MBq/kg
Time 0-1week 0-2weeks
Gender Male Male
Urine 35.6 (27.7) 30.3 (25.3)
Faeces 32.6 (9.7) 20.7 (3.0)
Expired air <0.2 Not applicable
Carcass 22.4 (27.0) Not applicable
Cage wash and
debris
3.7 (22.2) 7.2 (28.4)
Total excretion 72.1 (8.8) 58.3 (10.2)
Total recovery 94.5 (0.5) 58.2
3.1.3 Metabolite Profiling
(1) Plasma
As shown in Figure 3, twelve components were
detected in the plasma of rats. At all time points of the
analysis, [
3
H]-semaglutide is the main component in
plasma, and the retention time of [
3
H]-semaglutide
reference substance is similar to the main peak in
plasma chromatogram. [
3
H]-semaglutide accounts for
69% of the total semaglutide related substances. As
shown in Table 5, another 10 metabolites were
detected in the plasma, each accounting for <1-7% of
the total AUC
0-last
(Lene 2017).
Figure 3 shows that 6 components were detected
in plasma of monkeys. Semaglutide was the primary
component in plasma at all time-point analysed. As
shown in Table 5, in the other peak areas, 4
metabolites are eluting close to semaglutide, each
accounting for <1-9% of the total AUC
0-last
(Lene
2017).
Discovery and Development of Semaglutide
1123
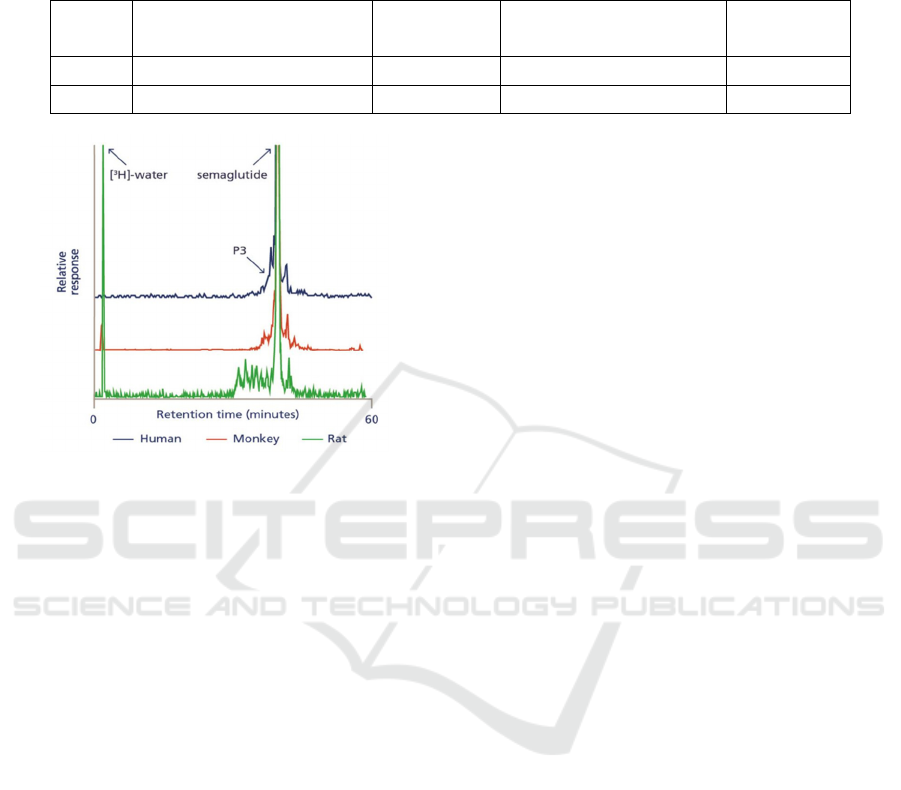
The retention time for the first eluting peak was
characteristic of tritiated water for both rats and
monkeys, and this was confirmed by data from
freeze-dried samples (Lene 2017).
Table 5: Exposure of semaglutide and metabolites in plasma across species. (Lene 2017).
AUC
total
Timepoint of samples profiled % semaglutide Total number of metabolites % metabolites
Rat 2-72 hours 69 10 <1-7
Monkey 0.5-168 hours 71 5 <1-9
Figure 3: HPLC analysis of metabolite profile in plasma
from rat, monkey, and human (Lene 2017).
(2) Urine and feces
For rats, the radioactive content in urine
accounted for about 89% of the total excreted
radioactivity (0-168 hours) from 0-120 hours after
administration, and a total of six components were
detected. The total amount of radioactivity in the
feces accounted for 89% of the total excreted
radioactivity (0-168 hours) from 0-120 hours after the
dose, and 14 ingredients were detected (Lene 2017).
In monkeys, the total amount of radioactivity in
urine from 0-216 hours after administration
accounted for 63% of the total excreted radioactivity
(0-336 hours). A total of 9 components were detected;
none of them had a retention time similar to [
3
H]-
semaglutide. The total amount of radioactivity in the
feces from 0-216 hours after the dose accounts for
74% of the total excreted radioactivity (0-336 hours),
and 15 ingredients were detected in monkeys (Lene
2017).
For both rats and monkeys, the retention time for
the first eluting peak was characteristic of tritiated
water (Lene 2017).
3.2 Nonclinical Toxicology
Carcinogenesis, mutagenesis, impairment of fertility.
In a 2-year carcinogenicity study in CD-1 mice,
subcutaneous doses of 0.3, 1, and 3 mg/kg/day of
semaglutide were administered to the males, and 0.1,
0.3, and 1 mg/kg/day were administered to the
females. A statistically significant increase in thyroid
C-cell adenomas and a numerical increase in C-cell
carcinomas were observed in males and females at all
dose levels (
http://www.novonordisk-
us.com/products/product-patents.html.)
.
In a 2-year carcinogenicity study in Sprague
Dawley rats, subcutaneous doses of 0.0025, 0.01,
0.025, and 0.1 mg/kg/day of semaglutide were
administered. A statistically significant increase in
thyroid C-cell adenomas was observed in males and
females at all dose levels, and a statistically
significant increase in thyroid C-cell carcinomas was
observed in males at ≥0.01 mg/kg/day, at clinically
relevant exposures (http://www.novonordisk-
us.com/products/product-patents.html.).
In a combined fertility and embryo-fetal
development study in rats, subcutaneous doses of 0.01,
0.03, and 0.09 mg/kg/day of semaglutide were
administered to male and female rats. Males were
dosed for 4 weeks before mating, and females were
dosed for 2 weeks prior to mating and throughout
organogenesis until Gestation Day 17. No effects were
observed on male fertility. In females, an increase in
estrus cycle length was observed at all dose levels,
together with a small reduction in numbers of corpora
lutea at ≥0.03 mg/kg/day. These effects were likely an
adaptive response secondary to the pharmacological
effect of semaglutide on food consumption and body
weight (http://www.novonordisk-us.com/products/
product-patents.html.).
3.2.1 Animal Toxicology and Pharmacology
An increase in lactate levels and decreased glucose
levels in the plasma and cerebrospinal fluid (CSF)
were observed in mechanistic studies with SNAC in
rats. Small but statistically significant increases in
lactate levels were observed in a few animals at
approximately the clinical exposure. These findings
were associated with moderate to marked adverse
clinical signs (lethargy, abnormal respiration, ataxia,
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1124

reduced activity, body tone, and reflexes) and marked
decreases in plasma and CSF glucose levels at higher
exposures. These findings are consistent with
inhibition of cellular respiration and lead to mortality
at SNAC concentrations >100-times the clinical
Cmax (http://www.novonordisk-us.com/products/
product-patents.html.).
4 CLINICAL TRAILS
4.1 Overview of Clinical Development
of Semaglutide
The clinical trial is a carefully designed study that
tests the benefits and risks of a specific medical
treatment or intervention in humans. It is to determine
the efficacy and safety of the trial drug. It consists of
four phases, phase I, phase Ⅱ, phase Ⅲ, and phase IV
(after the drug marketed).
As shown in Figure 4, a comprehensive global
clinical development program was conducted for
semaglutide. At the time of cut-off for the NDA, 25
trials with semaglutide s.c. once-weekly had been
completed: 16 phases 1 clinical pharmacology trials,
one phase 2 dose-finding trial, and 8 phase 3a trials
(including a 2-year cardiovascular outcomes trial
[CVOT]). A total of 9,384 individuals were included
in the clinical development program, of whom 5,710
were exposed to semaglutide and 3,674 to
comparators, including placebo. Approximately 1/3
of the total population was recruited from sites in the
US. (https://www.fda.gov/media/108291/download).
Figure 4: Semaglutide development program: Overview of completed clinical trials.
4.2 Phase 1
4.2.1 Trail Design
The purpose of phase 1 clinical trial is to investigate
the first safety, pharmacokinetic and
pharmacodynamic data for oral semaglutide in
healthy subjects and subjects with T2D.
In the first-in-human experiment, there are two
kinds of trials, single-dose trial, and multiple-dose
trial. The single-dose trial is tested in healthy subjects,
and the multiple-dose trial is tested between the
healthy subjects and the subjects with T2D. Both
trials were randomized, placebo-controlled, double-
blind trials, each conducted at single sites (Parexel
International, Harrow, UK, and Parexel International
GmbH, Berlin, Germany, respectively). (Granhall
2019)
4.2.2 Selection Condition of Subjects
Table 6 shows the selection conditions of subjects. In
the single-dose trial, eligible subjects are healthy men
aged 18-50, weighing 65.0-95.0 kg, and having a
Completed Trials
Phase 1
Clinical pharmacology trials
Phase 2 trial
1821-Dose finding
Phase 3a trials
SUSTAIN 1(3623) sema vs placebo
(Mono)
SUSTAIN 2(3626) sema vs sita
(OADs)
SUSTAIN 3(3624) sema vs exe ER
(OADs)
SUSTAIN 4(3625) sema vs IGlar
(OADs)
SUSTAIN 5(3627) sema vs placebo
(Insulin)
SUSTAIN JP Mono (4092) sema vs
sita (Mono), JP
SUSTAIN JP OADs (4091) sema vs
OAD (OAD), JP
Healthy subjects
1820-First human dose
3697-Equivalence-product strength
3687-Equivalence/bioavailability
4010-Bioequivalence-
manufacturing process
3633-Multiple dose-Caucasian/JP
3634-Pk/Pd-Caucasian/JP
3789-Metabolism
3652-QTc
Special populations
3616-Renal impairment
3651-Hepatic impairment
Drug-drug interaction
3817-DDI-metformin and warfarin
3818-DDI atorvastatin and digoxin
3819-DDI-oral contraceptives
Phase 3b trial
Long-term outcomes trial
SUSTAIN 6 COVT (3744) sema vs
placebo (SoC)
Pharmacodynamics
3635-Beta-cell function
3684-Hypoglycaemia
3685-Ener
gy
intake
Discovery and Development of Semaglutide
1125
body mass index (BMI) of 18.5-27.5 kg/m
2
. In the
multi-dose trial, eligible subjects are healthy men
aged 18-64 with a BMI of 20.0-29.9kg/m
2
and men
who have been diagnosed with T2D after diet,
exercise, and/or metformin treatment within the last
10 years, aged 18-64 years old, with a BMI of 20.0-
37.0 kg/m
2
, glycosylated hemoglobin (HbA1c) of
6.5-9.0%.
If the subject has a clinically significant
concomitant disease or disorder, clinically significant
outliers in laboratory screening tests, any history of
gastrointestinal surgery (except for simple surgical
procedures such as appendectomy and hernia
surgery), or if they smoked more than five cigarettes
or the equivalent per day. (Granhall 2019).
Table 6: The selection conditions of subjects.
Single-dose
trial
Multiple-dose trial
Healthy males Healthy males Males with T2D
(Within the last
10 years, treated
with diet and
exercise and/or
metformin
)
18~50
years old
65.0~95.0
kg
BMI
18.5~27.5
kg/m
2
18~64
years old
BMI
20.0~29.9
kg/m
2
18~64
years old
BMI
20.0~37.0
kg/m
2
HbA1c
6.5~9.0%
4.2.3 Trail Methods
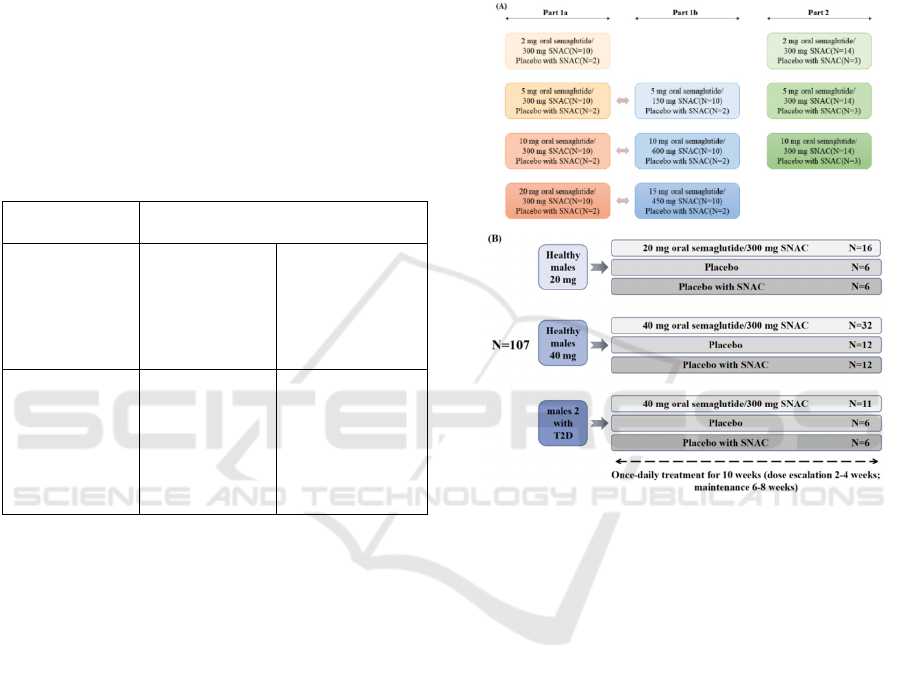
Figure 5 shows the details of the trail design. The
single-dose trial is to investigate the best dose of oral
semaglutide. It is divided into 3 parts, part 1a, part 1b,
and part 2.
In part 1a, four ascending dose groups are tested
in a sequential design. When the first dose level is
proved safe, test the next dose level until it reached
the specified maximum dose. In part 1b, there are
three additional dose groups in a parallel design. This
process is to confirm which dose level of the
semaglutide with SNAC has a better curative effect.
In part 2, three of the doses from part 1 were selected
to be repeated in a parallel design in another three
groups. At the same time, intravenous and
subcutaneous semaglutide are also tested to
investigate absolute and relative biotechnology.
The multiple dose trial is to reveal the therapeutic
potential in the treatment of T2D. As the subjects are
divided into healthy males and males with T2D, their
tests are also different. This trial is to identify if the
semaglutide gets the therapeutic potential in the
treatment of T2D. Healthy males receive oral
semaglutide maintenance doses of 20 and 40 mg, and
also compared them with receiving placebo and
placebo with SNAC. But the subjects with T2D only
receive an oral semaglutide dose of 40 mg. In the
multiple-dose trial, subjects are randomized to once-
daily treatment for 10 weeks with different tablets.
(Granhall 2019)
Figure 5: Trail design.
4.2.4 Results
Overview two trials, it confirmed that there are no
safety concerns identified and the pharmacokinetic
properties of oral semaglutide are comparable in
healthy subjects and subjects with T2D.
About the single-dose trial, it concluded that
semaglutide with 300mg SNAC gets the best
pharmacokinetics. Through comparing different dose
levels, 300mg compared with 150 or 600mg is the
optimal amount of SNAC to enhance absorption of
oral semaglutide. Next, at a fixed amount of 300 mg
SNAC, both the proportion of subjects with
measurable semaglutide in plasma and the
semaglutide exposure appeared to increase with
increasing dose of oral semaglutide from 2 to 10 mg,
as shown in Figure 6. And in healthy subjects of the
multiple-dose trial, semaglutide plasma exposure was
about twofold higher with oral semaglutide 40 mg
compared with 20 mg, as shown in Figure 7.
Furthermore, semaglutide plasma exposure did not
differ between healthy subjects receiving 40 mg and
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1126
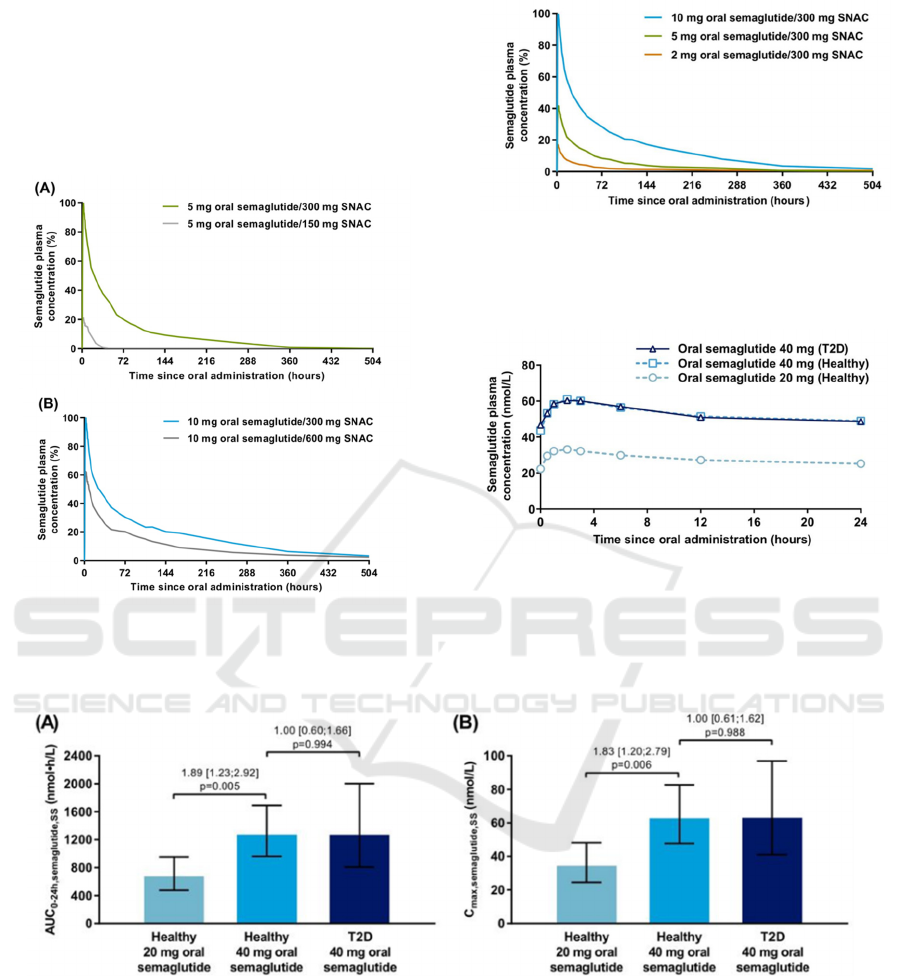
subjects with T2D receiving 40 mg, as shown in
Figure 8 and 9.
(Granhall 2019)
Comparison of a AUC
0–24 h, semaglutide, SS(steady state)
and b C
max, semaglutide, SS
between 20 and 40 mg doses of
oral semaglutide in healthy males and between
healthy males and males with T2D receiving 40 mg
oral semaglutide (multiple-dose trial)
Figure 6: Arithmetic mean semaglutide plasma
concentration-time pro-files after a single dose of oral
semaglutide with varying amounts of SNAC in healthy
male subjects in single-dose trial.
(Granhall 2019)
Figure 7: Arithmetic means semaglutide plasma
concentration-time profiles after ascending single doses of
oral semaglutide with 300 mg SNAC in healthy male
subjects in single-dose trial. (Granhall 2019)
Figure 8: Geometric mean semaglutide plasma
concentration-time pro-files at steady state in multiple-dose
trial. Profiles represent geometric means of the last 3 days
of once-daily oral semaglutide treatment for 10 weeks. T2D
type 2 diabetes.
(Granhall 2019).
Figure 9. Comparison of a AUC0–24 h, semaglutide, SS and b Cmax, semaglutide, SS between 20 and 40 mg doses of oral
semaglutide in healthy males and between healthy males and males with T2D receiving 40 mg oral semaglutide in multiple-
dose trial. (Granhall 2019).
4.3 Phase 2
4.3.1 Trail Design
Phase 2 compares the effects of oral semaglutide with
placebo (primary) and open-label subcutaneous
semaglutide (secondary) on glycemic control in
patients with type 2 diabetes.
From December 2013 to December 2014, a 2-
week randomized, parallel grouping, the dose-
determined 26-week trial was conducted in 100
locations (hospital clinics, general practice, and
clinical research centers) in 14 countries/regions. 5-
week follow-up. Among the 1106 participants
evaluated, 632 patients with type 2 diabetes and
insufficient blood glucose control only through diet
Discovery and Development of Semaglutide
1127

and exercise or a stable dose of metformin were
randomly assigned. Randomization was stratified by
metformin use. (Davies 2017)
4.3.2 Trail Methods
Subjects took orally semaglutide 2.5 mg (n=70), 5 mg
(n=70), 10 mg (n=70), 20 mg (n=70), 40 mg orally
once a day in 4 weekly dose escalations (standard
Escalation; n=71), 40 mg 8-week dose escalation
(slow escalation; n=70), 40 mg 2-week dose
escalation (rapid escalation, n=70), oral placebo
(n=71; double-blind) or every Semaglutide 1.0 mg
(n=70) was injected subcutaneously once a week for
26 weeks. (Davies 2017)
4.3.3 Results
The baseline characteristics of each treatment group
are comparable. Among 632 randomized patients, 583
(92%) completed the test. From baseline to week 26,
the average change in HbA1c levels decreased with
oral semaglutide (dose-dependent range, -0.7% to -
1.9%) and subcutaneous semaglutide (-1.9%) and
placebo (-0.3%); Compared with placebo, the
reduction in oral semaglutide was significant
compared to the dose (the dose-dependent) estimated
treatment difference between oral semaglutide and
placebo [ETD] ranged from –0.4% to –1.6%; for 2.5
mg, P = 0.01, for all other doses, <0.01). Oral
simaglutide (dose-dependent range, -2.1 kg to -6.9 kg)
and subcutaneous simaglutide (-6.4 kg) have greater
weight loss than placebo (-1.2 kg), and oral
simaglutide doses Significantly compared to placebo
at 10 mg or higher (dose-dependent ETD range –0.9
to –5.7 kg; P<0.01). The reported incidence of adverse
events was 63% to 86% in the oral semaglutide group
(371 of 490 patients), 81% of the subcutaneous
semaglutide group (56 of 69 patients), and placebo
group 68% (48 of 71 patients); mild to moderate
gastrointestinal events are the most common. Within
26 weeks, oral semaglutide can control blood sugar
better than a placebo and is effective for patients with
type 2 diabetes. (Davies 2017)
4.4 Phase 3
4.4.1 Trail Design
Phase 3 is a series of PIONEER programs. The
PIONEER program includes 10 trials, including a
pre-approval cardiovascular outcome trial, which
aims to evaluate the efficacy and safety of oral
semagluide in a wide range of patients with type 2
diabetes. The program includes eight global trials,
including Japanese patients, and two trials conducted
only in Japan. All trials started in 2016, and the main
treatment period ended in 2018. (Rasmussen 2020)
4.4.2 Results
The PIONEER clinical trial plan includes several
studies that recruit Japanese patients.
Throughout the plan, oral semaglutide 14 mg
reduced HbA1c significantly more than placebo,
empagliflozin, and sitagliptin, and was not inferior to
liraglutide. In the PIONEER trial in Japan, the
reduction in HbA1c of 14 mg of oral semaglutide was
significantly higher than that of liraglutide 0.9 mg or
dulaglutide 0.75 mg, and the reduction of HbA1c at a
7 mg dose was similar to that of dulaglutide 0.75 mg.
Compared with oral placebo, sitagliptin, and
liraglutide, oral sitagutide 14 mg can also
significantly reduce body weight. And the weight loss
was similar to ipaglifozin. Oral semaglutide also has
more beneficial effects in achieving blood sugar
control and weight loss than sitagliptin, even when
flexible dosage adjustments are made, which reflect
the actual dosage setting.
Whether it is based on the estimated value of the
treatment strategy (regardless of whether the trial
product is discontinued or the use of emergency drugs)
or the estimated value of the trial product (the patient
continues to use the experimental drug and does not
use emergency drugs), the results are usually
consistent.
In all PIONEER trials, oral semaglutide was well
tolerated, and its adverse events were consistent with
other GLP-1RA administered subcutaneously. There
were no unexpected safety risks in individual trials.
For patients with moderate renal insufficiency, the
safety of oral semaglutide seems to be acceptable. In
Japanese patients, oral semaglutide is also well
tolerated. The incidence of adverse events of oral
semaglutide is similar to that of dulaglutide, and its
safety is consistent with that of injectable GLP-1RA.
In the PIONEER 6 trial, oral glucosamine showed
good cardiovascular safety compared with
conventional care and compared with placebo,
cardiovascular death and all-cause mortality were
significantly reduced. (Rasmussen 2020)
5 CONCLUSIONS
Oral semaglutide is a novel tablet containing the
human glucagon-like peptide-1 (GLP-1) analogue
semaglutide, co-formulated with the absorption
enhancer SNAC. It has three major effects of
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1128

lowering blood glucose, weight loss, and reducing
cardiovascular risk. For non-clinical species, intact
semaglutide is a major component circulating in
plasma and is metabolized prior to excretion.
Additionally, it has been found to affect the thyroid's
fertility and C cell adenomas in preclinical studies. In
the single-dose experiment of clinical trials, it was
found that semaglutide with 300mg SNAC will exert
the maximum effect. Multi-dose experiments have
proved that semaglutide has the therapeutic properties
of T2D. In phase 2 trials, semaglutide has better
potency and effects than other therapeutic drugs
already on the market. Phase 3 trial further proved
that semaglutide has the function of reducing HbA1c
and body weight. In conclusion, the successful design
of the oral formulation of Semaglutide paves the way
for the subsequent development of oral forms of
GLP-1RAs.
REFERENCES
American Diabetes Association, Standards of Medical Care
in Diabetes-2010, J. Position Statement, 2010, pp. S11-
S61.
Andersen A, Lund A, Knop FK, et al. Glucagon-like
peptide 1 in health and disease, J. Nat Rev Endocrinol,
2018, pp.390-403.
Astrup A1, Carraro R, Finer N. Safety, tolerability and
sustained weight loss over 2 years with the once-daily
human GLP-1 analog, liraglutide, J. Int J Obes (Lind),
2012, pp.843-854.
Bock T, Pakkenberg B, Buschard K. The endocrine
pancreas in non-diabetic rats after short-term and long-
term treatment with the long-acting GLP-1 derivative
NN2211, J. AP-MIS, 2003, p.1117.
Buckley ST, Bækdal TA, Vegge A, et al. Transcellular
stomach absorption of a derivatized glucagon-like
peptide-1 receptor agonist, J. Sci Transl Med, 2018,
pp.7047.
Bunck MC1, Diamant M, Cornér A. One-year treatment
with eventide improves beta-cell function, compared
with insulin glaring, in metformin-treated type 2
diabetic patients: a randomized, controlled trial, J.
Diabetes Care, 2009, pp.762-768.
Christina A, Krishnarajah N, Ioannis D, et al. The
development of an oral GLP-1 receptor agonist for the
management of type 2 diabetes: evidence to date, J.
Drug Design, Development and Therapy, 2019,
pp.2985–2996.
C. Granhall et al. Safety and Pharmacokinetics of Oral
Semaglutide. Clinical Pharmacokinetics (2019)
58:781–791.
Davies M, Pieber TR, Hartoft-Nielsen ML, Hansen OKH,
Jabbour S, Rosenstock J. Efect of oral semaglutide
compared with placebo and subcutaneous semaglutide
on glycemic control in patients with type 2 diabetes: a
randomized clinical trial. JAMA. 2017; 318: 1460–70.
Davis EM, Sandoval DA, Glucagon-like peptide-1: actions
and influence on pancreatic hormone function, J.
Compr Physiol, 2020, pp.577-595.
Gupta V. Glucagon-like peptide-1 analogues: an overview.
Indian, J. Endocrinol Metab, 2013, pp.413– 421.
HANSEN L, DEACON CF, ORSKOV C, et al Glucagon-
like peptide-1(7-36) amide is transformed to glucagon-
like peptide-1 (9-36) amide by dipeptidyl peptidese IV
in the capillaries supplying the L cells of the porcine
intestine, J. Endocrinology, 1999, pp.5356-5363.
Information on
https://www.fda.gov/media/108291/download
Information on http://www.novonordisk-
us.com/products/product-patents.html.
Kapitza C,Lynge J,Düring M, et al. Safety, tolerabiliy
pharmacokinetics (PK)/ pharmacodynarnjcs (PD) of
single escalating doses of semaglutide, a unique once
weekly GLP-l analogue, in healthy male subjects, J.
Diabetologia, 2012, pp. S341.
Kong L E. Analysis of the role of serum C-peptide and
glycosylated hemoglobin in the diagnosis of diabetes, J.
Tribune of Primary Medicine, 2016, pp. 1078-1079.
Lau J, Bloch P, Schäffer L, et al. Discovery of the once-
weekly glucagon-like peptide-1 (GLP-1) analogue
Semaglutide, J. Journal of medicinal chemistry, 2015,
pp.7370-7380.
Lene J, Hans H, Everton R, et al. Absorption, metabolism
and excretion of the GLP-1 analogue semaglutide in
humans and nonclinical species, J. European Journal of
Pharmaceutical Sciences, 2017, pp.31-41.
Mahato RI, Narang AS, Thoma L, Miller DD, Emerging
trends in oral delivery of peptide and protein drugs, J.
Crit Rev Ther Drug Carrier Syst, 2003, pp.153–214.
Meier JJ, Rosenstock J. Hincelin-Mery A, et al. Contrasting
effects of lixisenatide and liraglutide on postprandial
glycemic control , gastric emptying , and safety
parameters in patients with type 2 diabetes on op-
timized insulin glargine with or without metformin: a
randomized, open-label trial, J. Diabetes Care, 2015,
pp.1263-1273.
Rasmussen M. F, The development of oral semaglutide, an
oral GLP-1 analog, for the treatment of type 2 diabetes,
J. Diabetology international, 2020, pp.76–86.
Reference to a chapter in an edited book: Williams textbook
of endocrinology.
Reference to a chapter in an edited book: World Health
Organization.
Shigeto M, Cha CY, Rorsman P, et al. A role of PLC /PKC-
dependent pathway in GLP-1-stimulated insulin
secretion, J. J Mol Med ( Berl) , 2017, pp.361-368.
Smits MM, Tonneijck L, Muskiet MH, et al.
Gastrointestinal actions of glucagon-like peptide-1-
based therapies: glycaemic control beyond the pancreas,
J. Diabetes Obes Metab, 2016, pp.224-235.
Son S, Park EJ, Kim Y, et al. Chemical chaperone-
conjugated exendin-4 as a cytoprotective agent for
pancreatic β-cells, J. Int J Biochem Cell Biol, 2018,
pp.13-19.
Vijan, Sandeep, Type 2 Diabetes, J. Annals of Internal
Discovery and Development of Semaglutide
1129

Medicine. 152 (2010).
Wang X, Jia D, Research progress in clinical application
and mechanism of GLP-1, J. Sichuan Medical Journal,
2020, pp.1089-1093.
Yoo BK, Triller DM, Yoo DJ. Eventide: A new option for
the treatment of type 2 diabetes, J. Ann Pharmaeother,
2006, p.1777.
Zhou Lingyun, Zhang Bikui, Zuo Xiaocong. Inhibitors of
dipeptidyl peptidase IV for the treatment of diabetes
mellitus, J. Chin J New Drugs and Clinics, 2010,
pp.401-405. (in Chinese)
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1130
