
Bioremediation of Antimony and Arsenic Co-contamination from
Antimony Mining Area with Sulfate-reducing Bacteria
Juan Zhong
1,2,3,4 a
, Xiaokui Che
1,2,3 b
, Xinglan Cui
1,2,* c
, Hongxia Li
1,2 d
, Qidong Zhang
1,2 e
,
Lei Wang
1,2 f
, Qi Zheng
1,2 g
and Xuewu Hu
1,2,5 h
1
GRINM Resources and Environment Tech. Co., Ltd., Beijing, 101407, China
2
National Engineering Laboratory of Biohydrometallurgy, GRINM Group Co., Ltd., Beijing, 100088, China
3
GRINM Group Corporation Limited, Beijing 100088, China
4
GRIMAT Engineering Institute Co., Ltd., Beijing, 101407, China
5
School of Metallurgical and Ecological Engineering, University of Science and Technology Beijing,
Beijing, 100083, China
Keywords: Antimony Mining Area, Bioremediation, Sulfate-Reducing Bacteria, Arsenic.
Abstract: The contamination of antimony (Sb) and arsenic (As) in the soil of antimony mining area is the most
common pollutant. Metal sulfide precipitation by sulfate-reducing bacteria (SRB) is considered to be a
promising method. In this work, antimony and arsenic contaminated soil from an antimony mining area was
treated by the application of a mixed culture of SRB. The soil samples initially contained the concentration
of 17550 mg/kg Sb and 3231 mg/kg As, and the leaching concentration exceeds the groundwater IV
standard. Changes of Eh, pH, aqueous Sb and as were monitored over 50 days in this experiment. The
results indicated that SRB was able to increase the pH value and decrease the redox potential of the solution.
When the SRB was growing well in the system, the high concentration of Sb in the solution was reduced
from 7.8 mg/L to less than 0.5 mg/L. However, As showed a completely opposite trend to antimony. The
presence of SRB can promote the release of as from contaminated soil, and the concentration of As will
gradually decrease when the activity of SRB decreased. This work demonstrates that SRB can trigger the
release of Sb, while as caused the opposite effect.
1 INTRODUCTION
1
Antimony (Sb) and Arsenic (As) are toxic and
carcinogenic metalloids of global concern and listed
as priority control pollutants by the European Union
and the United States Environmental Protection
Agency (Fu, et al., 2016). As the largest antimony
producer in the world, China risks more severe of
antimony pollution far than other countries. In
addition to high concentration of antimony pollution,
high arsenic pollution usually accompanies in
a
https://orcid.org/0000-0001-6436-9518
b
https://orcid.org/0000-0003-4913-9700
c
https://orcid.org/0000-0002-9972-9015
d
https://orcid.org/0000-0003-0654-0115
e
https://orcid.org/0000-0003-1843-6809
f
https://orcid.org/0000-0002-4476-0496
g
https://orcid.org/0000-0002-9708-660X
h
https://orcid.org/0000-0001-6643-749X
antimony mining area and surrounding environment
due to the mineral oxidation and arsenic alkali slag
leaching. Antimony and arsenic are non-essential
toxic elements for human body, which could be
absorbed and accumulated by plants when they
existed in dissolved state, and potentially entered the
human body and caused various diseases such as
cardiovascular diseases, skin lesion, reproductive
disorders, diabetes, and even cancer in the skin,
bladder, kidney, and lung (Alam, McPhedran, 2019;
Singh, et al., 2015).
In the past 20 years, significant progress has
been made in the treatment of antimony/arsenic
pollution. These technologies fall into two main
categories, directly remove antimony and arsenic
from contaminated media and reduce its biotoxicity
in the environment, both of which can reduce the
health risks of antimony and arsenic to human body
(Feng, et al., 2017). At present, remediation
technologies are mainly including biological
1174
Zhong, J., Che, X., Cui, X., Li, H., Zhang, Q., Wang, L., Zheng, Q. and Hu, X.
Bioremediation of Antimony and Arsenic Co-contamination from Antimony Mining Area with Sulfate-reducing Bacteria.
DOI: 10.5220/0011381600003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 1174-1178
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

oxidation/reduction, electrokinetics,
phytoremediation, coagulation–flocculation, ion
exchange, and solidification/stabilization (Wan, et
al., 2019). Biological methods have been widely
used in the treatment of heavy metal pollution due to
their advantages of low remediation cost,
environmental friendliness and simple operation. In
particular, sulfide precipitation generated by sulfate
reducing bacteria (SRB) is considered to be an
effective method for the removal of heavy metal
pollution. Under anaerobic conditions, SRB can
reduce sulfate to sulfide, which is able to precipitate
metals and metalloids, decreasing its solubility and
pervasiveness (Zacarias-Estrada, et al., 2020).
Microbial redox reactions can significantly affect
the conversion of valence states between Sb (III) and
Sb (V). Although there are relatively few studies on
bioreduction of Sb (V), it has been shown that
microorganisms have the ability to reduce Sb(V). Sb
is a strong chalcophile element, thus it can be easily
reacted with the dissolved sulfide produced by SRB
to form antimony sulfide. Sb (V) usually exists as Sb
(OH)
6-
and can be reduced to HSb
2
S
4−
, Sb
2
S
4
2−
,
Sb
2
S
3
, and Sb (III)-sulfide complexes by the
dissolved sulfide under anoxic conditions (Xi et al.,
2020). Sb and As belong to the same main group and
their properties are similar. As(V) usually exists in
the form of H
2
AsO
4−
and HAsO
4
2−
and the sulfide
produced by SRB can potentially precipitate As(V)
as arsenic sulfides (realgar (AsS) and orpiment
(As
2
S
3
)), or form soluble thio-As species when As
concentration reaches equilibrium, thus promoting
or reducing As migration and immobilization (Fan,
et al., 2018).
There are many reports of As remediation by
SRB, and the Sb biotransformation has also been
studied in recent years. However, few studies have
investigated the bioremediation of Sb and As co-
contamination by SRB. Therefore, this study
investigated the potential of As/Sb removal and
immobilization ability mediated by SRB in the soil
of antimony mining area. The objectives of this
study are (1) investigate the bioremoval efficiency of
As/Sb during the growth of a mixed culture of SRB,
and (2) evaluate the long-term stability of the
remediation process.
2 MATERIALS AND METHODS
2.1 Site Location and Soil Sampling
Soil samples were derived from an antimony mining
area located in Qinglong County, Guizhou Province,
Southwest China. The soil samples were collected
from the upper surface (5-15 cm) in April, 2021. The
collected samples were transferred with ice to the
laboratory within two days, and stored at 4℃ before
the incubation experiments. To determine the main
elemental compositions in the soil, samples were
freeze-dried, sieved through 100 mesh and then
measured using X-ray fluorescence (XRF,
PANalytical BV).
2.2 Bacterial Culture
The SRB consortium used in this study was
originated from National Engineering Laboratory of
Biohydrometallurgy. Prior to the experiments, the
bacteria were cultured in liquid medium containing
per liter: 0.01 g CaCl
2
·2H
2
O, 0.5 g MgSO
4
·7H
2
O, 1
mL sodium lactate, 1.0 g Yeast extract, 0.5 g KCl,
0.5 g K
2
HPO
4
. The SRB were incubated for 3 days
to a late exponential phase at 30°C in an anaerobic
reactor.
2.3 Bioreduction Experiment
The microcosm experimental system was performed
simultaneously in 500 mL serum bottles, containing
200 g contaminated soil, 375 mL of liquid medium,
with 10% v/v of SRB enriched anaerobic consortium
as inocula and nitrogen as gas headspace to impose
anaerobic condition. In the early stage of the
reaction, medium was not supplemented during the
culture process. After 35 days, the same volume of
medium solution was added after each sampling.
Control treatments without addition of SRB were
also set up for comparison. All the bottles were
anaerobic incubation at 25-30°C in static conditions.
Each experiment was carried out in triplicate. At
regular intervals, liquid supernatant samples (5.0
mL) were filtered through a sterile syringe to
analysis of aqueous Sb and As. The redox potential
(Eh) and pH were also monitored at each sample
internal.
2.4 Analytical Methods
Soil samples were heated at 105℃ for 2 h to
determine water content. The pH of soil samples was
measured at solid/water ratio of 1:2.5 with a pH
meter (Thermo Scientific Orion 3-Star, Germany).
To determine the leaching toxicity of heavy metals
in soil samples, sulfuric acid and nitric acid method
were applied.
The pH and Eh of water samples were
immediately measured after sample collection with
Bioremediation of Antimony and Arsenic Co-contamination from Antimony Mining Area with Sulfate-reducing Bacteria
1175

pH meter and redox potential meter (pH/ORP
controller PC-350, China), respectively. Total
dissolved Sb and As content were quantified using
Inductively Coupled Plasma Optical Emission
Spectroscopy (ICP-OES, Agilent 725, USA).
3 RESULTS AND DISCUSSION
3.1 Characterization of The Soil
The antimony mining area soil sampled at Qinglong
County were reddish brown in appearance. The
contaminated soil was almost neutral, and the
average pH values are 7-7.8. The soil moisture
content was 6.8%. The main element of the sample
as conducted by XRF analysis were summarized in
Table 1. Totally, Sb contents were several times
greater than that of As, and the concentration of Sb
and As was 17550 and 3231 mg/kg, respectively.
Besides, the soil also contained high concentrations
of Fe (18.74%), Ca (6.85%), S (6570 mg/kg), Ti
(4720 mg/kg). According to the Chinese national
standards for groundwater quality, the leaching
concentration of Sb and As exceeds the groundwater
Ⅳ standard, in which As exceeds 1.256 times and
Sb exceeds 728 times.
Table 1: The XRF results of the antimony mining area
soil.
Elements Con.
(mg/kg)
Elements Con.
(mg/kg)
Elements Con.
(mg/kg)
Fe 187400 Ti 4720 Sr 330
Ca 68500 As 3231 Ba 273
Sb 17550 Cl 1070 Cr 215
K 10610 Zn 861 Cu 115
S 6570 Mn 498 V 114
3.2 Variation of Physicochemical
Properties in the Soluble Medium
After 3 days of reaction, the solution in the
remediation group began to turn black and hydrogen
sulfide was produced (Figure 1), while there was no
significant change in the control group, indicating
that SRB in the remediation group could quickly
adapted to the high concentration of antimony and
arsenic pollution environment and played a
biological reduction function in this system.
Figure 1: Solution color changes during remedation.
ORP and pH are fundamental properties which
can reveal the activity of microorganism and the
process of mineral transformation (Gao, et al., 2021.
Changes of Eh and pH during the 50d reaction
period was shown in Figure 2. The pH in biotic
systems increased from the initial value of 7.2 to 8.3
within 28 d, which may be attributed to the
alkalinity (HCO
3
−
) generation and biogenic H
2
S
accumulation due to the sulfate reduction by SRB
(Fan, et al., 2018). Subsequently, pH decrease was
observed in the treatment may be relative to the
death of SRB in the system. Thus, when liquid
medium was supplemented to the system, the pH of
solution began to increase again. With the initiation
of the remediation treatment by SRB, the Eh was
decreased in the first 4 days, then increased
gradually and reached a peak to approximately 50
mV (Figure 3), reflecting the decrease of SRB
activity. After the addition of medium in 35d, the Eh
decreased markedly from 40 mV to -220 mV,
indicated that SRB was growing well and resulted in
a strong reducing condition in the culture. In the
abiotic control treatments, the pH presented an
upward trend first and then falling, and the Eh nearly
remain unchanged at ~100 mV throughout the entire
incubation period.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1176
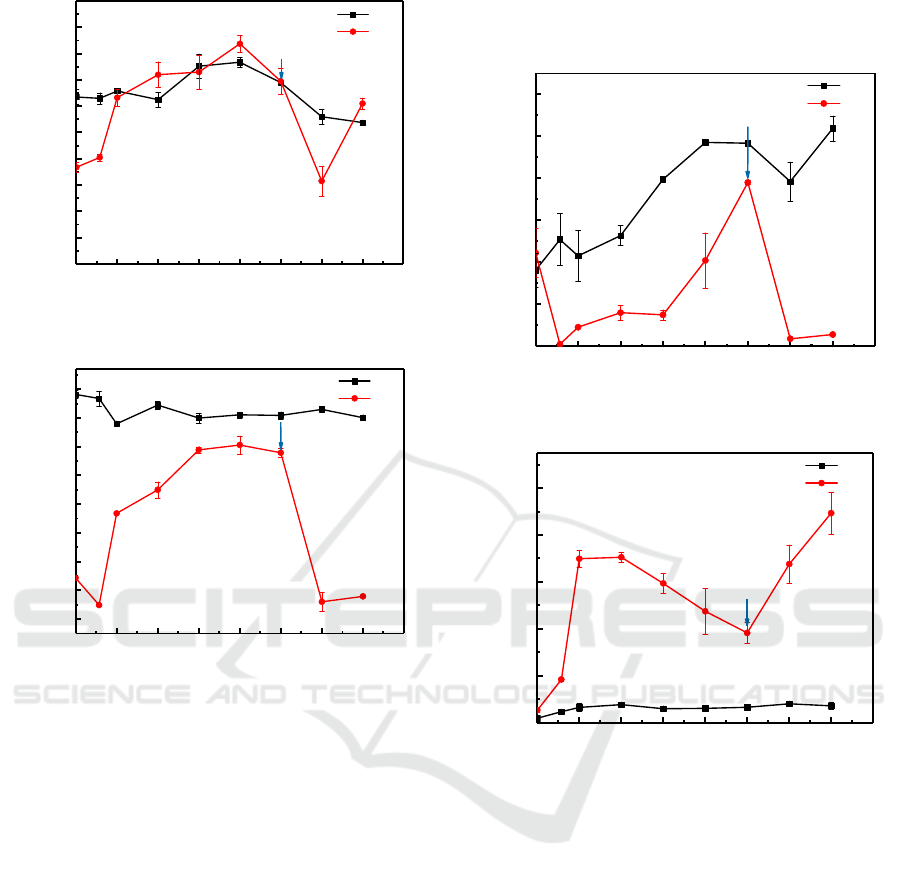
Figure 2: pH changes during remedation.
Figure 3: ORP changes during remedation.
3.2 Concentration Changes of Sb and
As in Solution
The Sb concentration in the CK group increased
from 3.62 mg/L to 10.37 mg/L (Figure 4). While in
the SRB bioremediation group, the Sb concentration
decreased rapidly from 4.45 mg/L to 0.91 mg/L
within 7 days, after that, there was a slightly
increase, and then began to increase sharply,
reaching 7.80 mg/L on the 35 days. After
supplemented with the medium, Sb concentration
quickly decreased to 0.36 mg/L, and maintained a
low concentration during the whole experiment. The
change of As concentration was shown in Figure 5.
The As concentration in the CK group increased
from 0.37 mg/L to 1.30 mg/L from 0-7d, and then
remained relatively stable. The As concentration in
the SRB bioremediation group decreased from 1.05
mg/L to 13.97 mg/L from 0-7d, and it began to
decrease significantly on the 14 days, and the
concentration decreased to 7.64 mg/L on the 35
days. After supplemented with medium, As
concentration started to increase rapidly at the end of
this experments.
Figure 4: Sb concentration changes during remedation.
Figure 5: As concentration changes during remedation.
SRB mediated sulfate reduction to produce H
2
S,
and H
2
S reacted with Sb to produce insoluble
precipitate Sb
2
S
3
, which may be the reason for the
decrease in Sb concentration in the initial stage.
With the consumption of the substrate, we observed
that the ORP gradually increased, the activity of
SRB decreased and the production of H
2
S decreased,
and inability to precipitate the dissolved Sb
completely, resulting in an increase in the
concentration of Sb in the solution. In addition, the
medium was supplemented in the later stage, and
ORP and the concentration of Sb decreased rapidly,
confirming that the highly active SRB can
precipitate Sb by mediating sulfate reduction to
produce H
2
S. However, the change of As
concentration in solution was opposite to Sb, which
indicated that SRB may lead to the release of
arsenic. This may be because As (V) was reduced to
0 7 14 21 28 35 42 49 56
6.6
6.8
7.0
7.2
7.4
7.6
7.8
8.0
8.2
8.4
8.6
pH
Time (d)
CK
SRB
Addition of medium
0 7 14 21 28 35 42 49 56
-250
-200
-150
-100
-50
0
50
100
150
Eh (eV)
Time (d)
CK
SRB
Addition of medium
0 7 14 21 28 35 42 49 56
0
2
4
6
8
10
12
Sb (mg/L)
Time (d)
CK
SRB
Addition of medium
0 7 14 21 28 35 42 49 56
0
4
8
12
16
20
As (mg/L)
Time (d)
CK
SRB
Addition of medium
Bioremediation of Antimony and Arsenic Co-contamination from Antimony Mining Area with Sulfate-reducing Bacteria
1177

As (III) in the presence of SRB, while As (III) is
more unstable and easily released into the
environment. Although H
2
S can react with As to
form insoluble As
2
S
3
, this precipitate is not stable
(Matos et al., 2018). In the presence of high
concentrations of sulfide and at near-neutral pH, the
transformation of insoluble As
2
S
3
into soluble As
(OH)S
2
2-
could be observed in the system (Sun et al.,
2019). Therefore, it is difficult to remeditation of As
by SRB alone, which requires strict reaction
conditions.
4 CONCLUSIONS
During the reductive process of contaminated soil
from antimony mining area by SRB, the changing
trend of Sb and As were different. The change of
aqueous Sb concentration showed two stages, firstly,
when the activity of SRB decreased, the Sb
immobilization by SRB would be gradually released
in the early stage of the reaction. next, the released
Sb quickly removed from the solution when the
OPR value decreased to negative. However, SRB
caused a negative effect on As in this study. This
process verified a cost-effective biological process
to remove Sb from Sb-As contaminated soil, while
the remediation of As through SRB needs further
study.
ACKNOWLEDGEMENTS
The project was funded by the Youth Fund Project
of GRINM (No. 12120), the National Key Research
and Development Project (No. 2020YFC1807700),
the Open Foundation of State Key Laboratory of
Vanadium and Titanium Resources Comprehensive
Utilization (No. 2021P4FZG13A), and the National
Key Research and Development Project (No.
2019YFC1805900).
REFERENCES
Alam, R. & McPhedran, K. (2019). Applications of
biological sulfate reduction for remediation of arsenic
– A review. Chemosphere, 222, 932-944.
Fan, L., Zhao, F., Liu, J., & Frost, R. L. (2018). The As
behavior of natural arsenical-containing colloidal
ferric oxyhydroxide reacted with sulfate reducing
bacteria. Chemical Engineering Journal, 332, 183-191.
Feng, C., Aldrich, C., Eksteen, J. J., & Arrigan, D. W. M.
(2017). Removal of arsenic from alkaline process
waters of gold cyanidation by use of
Fe3O4@SiO2@TiO2 nanosorbents. Minerals
Engineering, 110, 40-46.
Fu, Z., Wu, F., Mo, C., Deng, Q., Meng, W., & Giesy, J.
P. (2016). Comparison of arsenic and antimony
biogeochemical behavior in water, soil and tailings
from Xikuangshan, China. Sci Total Environ, 539, 97-
104.
Matos, L. P. d., Costa, P. F., Moreira, M., Gomes, P. C. S.,
Silva, S. d. Q., Gurgel, L. V. A., & Teixeira, M. C.
(2018). Simultaneous removal of sulfate and arsenic
using immobilized nontraditional SRB mixed culture
and alternative low-cost carbon sources. Chemical
Engineering Journal, 334, 1630-1641.
Singh, R., Singh, S., Parihar, P., Singh, V. P., & Prasad, S.
M. (2015). Arsenic contamination, consequences and
remediation techniques: a review. Ecotoxicol Environ
Saf, 112, 247-270.
Sun, J., Hong, Y., Guo, J., Yang, J., Huang, D., Lin, Z.,
Jiang, F. (2019) Arsenite removal without thioarsenite
formation in a sulfidogenic system driven by sulfur
reducing bacteria under acidic conditions. Water Res,
151, 362-370.
Wan, X., Lei, M., & Chen, T. (2019). Review on
remediation technologies for arsenic-contaminated
soil. Frontiers of Environmental Science &
Engineering, 14(2).
Xi, Y., Lan, S., Li, X., Wu, Y., Yuan, X., Zhang, C., Yun,
G. L., Huang, Y. Quan, B., Wu, S. (2020).
Bioremediation of antimony from wastewater by
sulfate-reducing bacteria: Effect of the coexisting
ferrous ion. International Biodeterioration &
Biodegradation, 148.
Zacarias-Estrada, O. L., Ballinas-Casarrubias, L.,
Montero-Cabrera, M. E., Loredo-Portales, R.,
Orrantia-Borunda, E., & Luna-Velasco, A. (2020).
Arsenic removal and activity of a sulfate reducing
bacteria-enriched anaerobic sludge using zero valent
iron as electron donor. J Hazard Mater, 384, 121392.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1178
