
Optimized Operational Parameters for the Biodegradation of the
β-lactam Antibiotics and Intermediates in Treating High-strength
Organic Wastewater Treatment
Qian-yi Wang
a
, Zhao-Bo Chen
b
, Dong-Xue Hu
*c
, Yu-Bo Cui
d
, Li-qiang Yu
e
,
Li-Xue Liu
f
, Hui Ge
g
, Xue-Jun Zou
h
and Ze-Hao Li
i
College of Environment and Resources, Dalian Minzu University, 18 Liaohe West Road, Dalian 116600, China
616476984@qq.com, gehui@dlnu.edu.cn, zouxuejun@dlnu.edu.cn, 1293420933@qq.com
Keywords: Biodegradation, High-Strength Organic Wastewater, β-lactam Antibiotics, Target Contaminants.
Abstract: Many attentions have been devoted to the fate of antibiotics such as β-antibiotics in the soil and water,
especially in the developing countries. The problems of their fate in the environment have to be clearly
identified to prevent any environmental pollution. Therefore, it’s necessary to research the biodegradation
law of β-lactam antibiotics and intermediates if the antibiotics entered the sewage treatment plants (SATS),
and to estimate the removals of SATs. Ceftriaxone sodium, cefoperazone sodium, ampicillin sodium,
amoxicillin, 6-APA and 7-ACA, typical β-antibiotics, were chosen as the analytes. Typical biologic
treatment active sludges, which has been widely used in the developing countries, was transformed in
experimental at laboratory-sized in this study to measure the β-lactam antibiotics and intermediates’
removal efficiency during the biologic treatment on different carbon sources, temperature, dissolved oxygen
and pH value conditions. When using glucose as plus carbon source, the effect of β-lactam antibiotics and
intermediate was best, and the optimum mass ratio of glucose and analytes was 1:8. Considering the
treatment effect and the processing cost factors, then gained a relatively appropriate condition: controlling
temperature at the range from 25 to 35 ℃, pH value in neutral and dissolved oxygen at 2.5 mg/L.
1 INTRODUCTION
1
In recent years, there are many reports confirmed
that different concentration level of drugs was found
in surface water, groundwater, drinking water,
sludge, soil, biological body and the others kinds of
environmental medium (Magda 2011, Halling-
Sùren, 1998). And its influences to environment and
human health has caused the international social
attention. Antibiotics was harmless to human cells,
however, while the antibiotics abusing in the
developing countries is common, which will produce
terrible consequences, that build the pathogenic
bacteria resistant ability to antibiotics. At the same
a
https://orcid.org/0000-0001-7719-1878
c
https://orcid.org/0000-0003-1878-6139
d
https://orcid.org/0000-0003-4247-0302
e
https://orcid.org/0000-0003-3563-1956
f
https://orcid.org/0000-0003-4281-4259
i
https://orcid.org/0000-0003-0046-7631
time, the antibiotic chemical structure is so
complicated with strong bacteriostatic and
sterilization effect, belongs to the difficult
biodegradable material. Beta-lactam
antibioticcontain typical beta lactam ring structure,
occupy an important position in antibiotic use. Beta-
lactam antibiotic’s mechanism of action is to inhibit
synthesis of cell wall, it has bactericidal broad-
spectrum and very good antibacterial, a good
curative effect and hypoallergenic with low adverse
(Buynak 2016, Calamari 2003, Bel 2009).
So, antibiotics once polluted the environment,
will leave a long-term serious influence on the
environmental micro-ecological. Antibiotic
substances enter municipal sewage and sewage
treatment plants (STPs). If they are not eliminated
during sewage treatment, they are emitted into
surface water and may reach drinking water (Edward
Turos, 2007). As a consequence, the occurrence and
fate of pharmaceutically active compounds in the
natural environment has been recognized as one of
Wang, Q., Chen, Z., Hu, D., Cui, Y., Yu, L., Liu, L., Ge, H., Zou, X. and Li, Z.
Optimized Operational Parameters for the Biodegradation of the -lactam Antibiotics and Intermediates in Treating High-strength Organic Wastewater Treatment.
DOI: 10.5220/0011388700003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 1213-1220
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
1213
the emerging issues in environmental chemistry.
Knowledge of pharmaceuticals in the environment is
little.
Emad researched that UV/ZnO photocatalysis
can be used for amoxicillin, ampicillin and
cloxacillin degradation in aqueous solution (James
2010). Besides, they also examined degradation of
the antibiotics amoxicillin, ampicillin and cloxacillin
in aqueous solution by the photo-Fenton process.
Using the application of sonolysis for the
degradation of fluoroquinolone antibiotic
ciprofloxacin (Kümmerer 2013), the pH effect on
the degradation rate is studied and changes in
biodegradability.
Besides chemical and photochemical degradation
(Kummerer 2003), the biodegradability of antibiotic
released into the environment is an important aspect
of their partial or complete decomposition by
microorganisms (Chaudhuri 2009), which is the
major progress in the sewage treatment plants. Thus,
it is important to study on the biodegradation of
antibiotic substances in the active sludges. As
antibiotics are considered to have an adverse effect
on microorganisms (Brosillon 2013).
Using the Closed Bottle test (CBT) (Emad 2010),
the biodegradation of some clinically important
antibiotic drugs was investigated as the first step of
an environmental risk assessment. The CBT was
performed according to test guidelines in the dark
room temperature (20±1℃) was described elsewhere
in detail. The standard test period of the CBT is 28
days. The CBT conditions are low bacterial density
and low concentration of organic carbon i.e. test
compound. As a result, the antibiotics tested were
not biodegraded in the CBT. The results indicate that
the various antibiotics were active against different
groups of bacteria present in wastewater.
Hybrid processes (Reddy 2006) involving a
physical-chemical and pre-treatment like
photocatalysis coupled to a biological treatment
have been considered for antibiotics’ removal. Using
photocatalysis as pre-treatment as prior to a
biological treatment, an irradiation time of 2h ensure
a significant residual organic content for the
biological treatment. A decrease of the residual
amount of antibiotics contained in the irradiated
solution s was recorded, which can be related to an
inherent biodegradation since these residual
concentrations were below their inhibitory
thresholds, 18 and 9 mg/L for TC and TYL.
So, the biodegradation of the beta-lactam
antibiotics in the environment, especially in the
SATs, is important to the antibitics problems. This
study, therefore, investigated the biodegradability of
some typical beta-lactam antibiotics in the actives on
lab-scales: ceftriaxone sodium, cefoperazone
sodium, ampicillin sodium, amoxicillin, 6-APA and
7-ACA to observe the existing conventional biologic
treatments ability to remove the antibiotics.
2 EXPERIMENTAL
2.1 Materials
This context selected four beta lactam antibiotics
(ceftriaxone sodium, cefoperazone sodium,
ampicillin sodium, amoxicillin) and two
intermediates (6 - APA ,7 - ACA) as the research
object, with a purity of > 99 % determined by HPLC
according to the Chinese Pharmacopoeia 2005 (ChP
2005). But methanol (MeoH), formic acid, disodium
hydrogen phosphate (Na
2
HPO
4
), potassium
dihydrogen phosphate (KH
2
PO
4
) (purchased from
Tianjin Chemical Reagent Co., China) were of
analytical-reagent grade. The water used was
domestic sewage. They were filtered by 0.45 µm
filter membrane before experiment. Pencil core
aerator was used to control the DO of the system,
and electric-heated thermostatic water bath was used
to control the temperature.
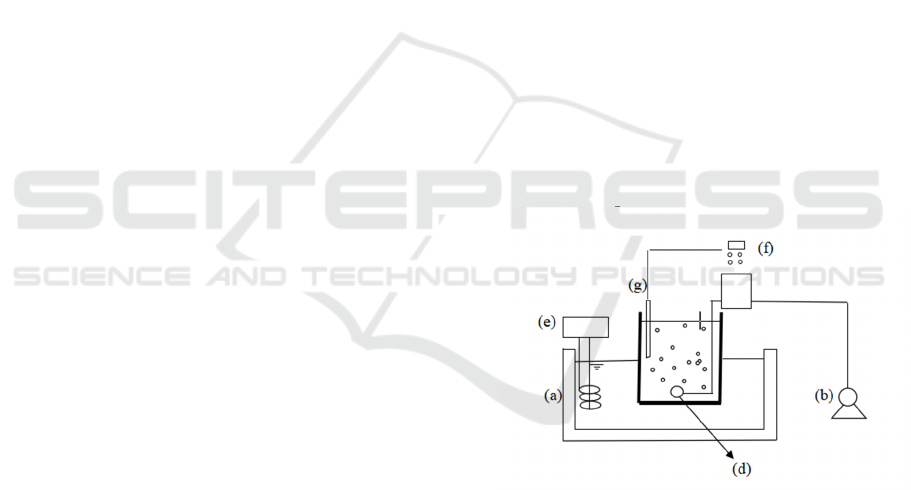
Figure 1: Schematic view of the active sludges. (a)
thermostatic bath; (b) air pump; (c) tank; (d) pencil core
aerator; (e) thermostatic devices; (f) pH, DO and detector;
(g) electrode.
2.2 Characterization
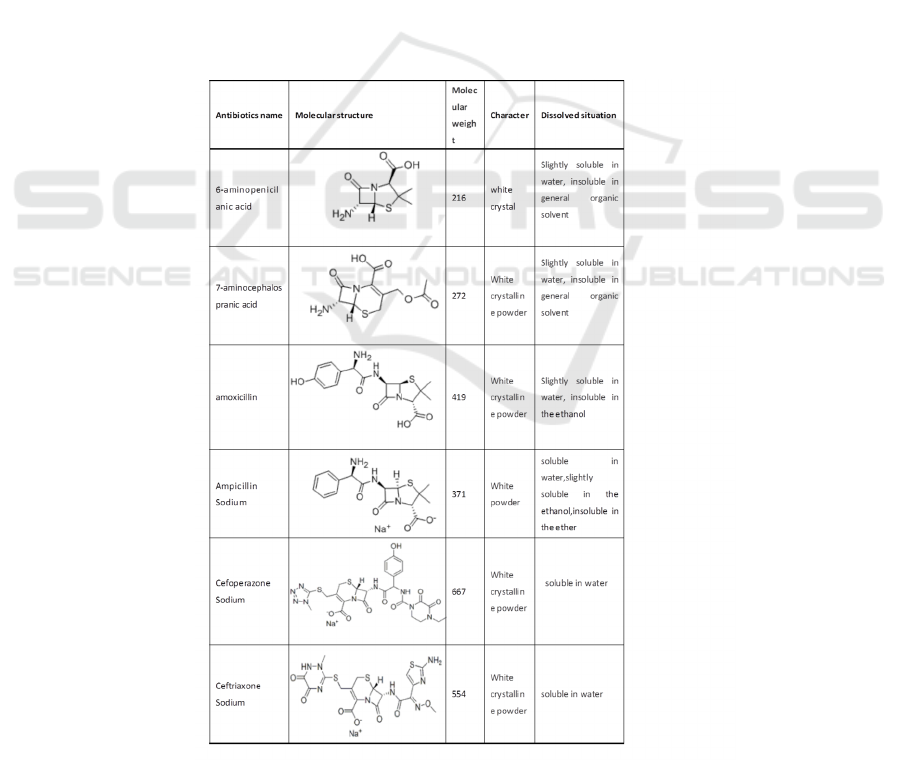
This context selected four beta lactam antibiotics
(ceftriaxone sodium, cefoperazone sodium,
ampicillin sodium, amoxicillin) and two
intermediates (6 - APA 7 - ACA) as the research
object. The experimentally characterization for the
research object are summarized in Table 1. An
Agilent Technologies 1200 HPLC system with an
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1214
UV detector and an Eclipse XDB-C18 column (150
mm × 4.6 mm×5 µm) were employed to study the
degradation process (Yahiat 2011).
2.3 Degradation Experiments
Activated sludge was collected from municipal
wastewater treatment. The basic culture medium
contains the following mineral supplementation:
K
2
HPO
4
(34g/L), CaCl
2
(18g/L), MgSO
4
(25g/L).
Firstly, biodegradation in different carbon source
(regarded as co-metabolism substance) including
glucose, sugar, starch and was investigated at 298 K
and almost neutral pH with the initial respective
concentration of 80 mg/L, and the check test was not
added in the culture medium. Selected glucose as the
carbon source used in co-metabolism action and was
investigated at 298K and almost neutral pH and
maintianed DO at 2.5 mg/L, tested biodegradation in
series of glucose concentration/respective target
contaminants concentration (1:1, 1:3, 1:5, 1:8, 1:10
and 1:15) system.
In the glucose concentration/respective target
concentration contaminants (1:8) system, the
influence of the pH on the degradation of target
contaminants was determined at 5, 7, 9 and with the
initial concentration of 400 mg/L, with glucose (50
mg/L) and primary nitrogen source ammonium (32.5
mg/L NH
4
Cl) at 298 K and DO at 2.5 mg/L, each
condition was aerated for 20h, deposited for 1h per
day and run for 20 days. The temperature effect on
the degradation of target contaminants was
determined at 288, 298 and 308K with the initial
concentration of 400 mg/L under pH around 7 and
DO around 2.5 mg/L, and also each condition was
aerated for 20h, deposited for 1h per day and run for
20 days. Finally the DO effect on the degradation
was determined at 1, 2.5 and 4 mg/L with initial
concentration of 400 mg/L.
Table 1: Overview of study β-lactam antibiotics.
Optimized Operational Parameters for the Biodegradation of the -lactam Antibiotics and Intermediates in Treating High-strength Organic
Wastewater Treatment
1215
3 RESULT AND DISCUSSION
3.1 Degradation of Target
Contaminants in Different Carbon
Sources System
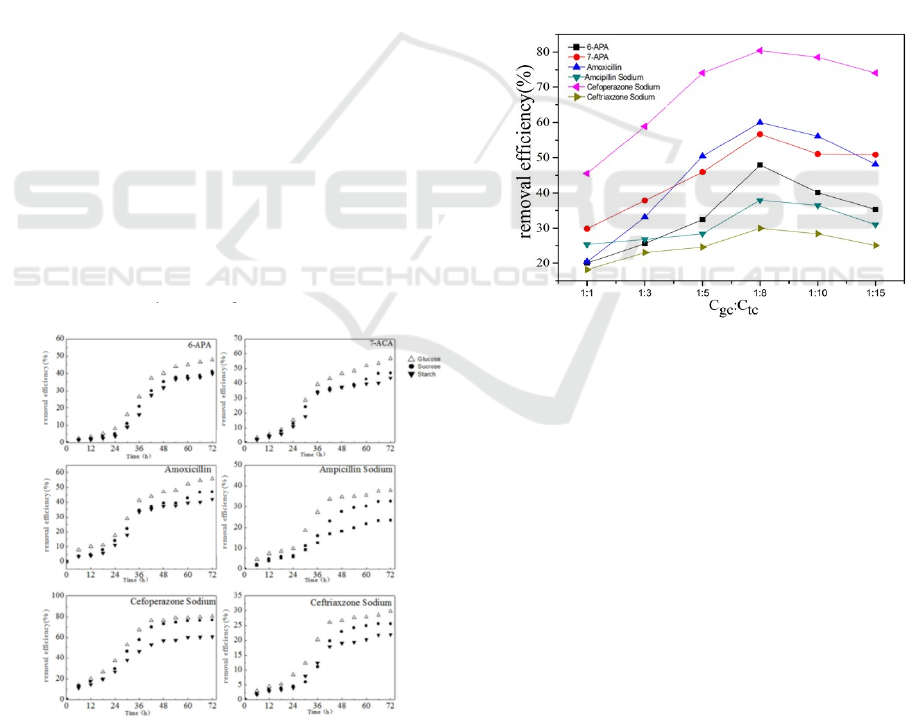
Fig.2 demonstrates the temporal evolution of the
concentration during the temporal evolution of in
different carbon sources solvent systems. It shows
that, on the basis of the degradation in the out-added
carbon sources, the introduction of different carbon
sources increased the degradation rate of the target
contaminants, the possible reason is that the out-
added carbon sources were oxidative decomposed
by microorganisms, which used the release of large
amounts of energy in decomposition, for the
synthesis of their own cellular material, so that the
microorganism bloomed, and also letted key enzyme
of the β-lactam antibiotics and intermediates’s
decomposition keep active, and it was conducive to
the further degradation (Heberer 2002). And this
process is called Co-Metabolism (Smital 2004).
Among the three out-added carbon sources, glucose
has the monosaccharide structure, and sucrose and
starch has disaccharides and polysaccharides
structure, so their molecular structure is more
complex compared with glucose, the reactions
required more kinds of enzymes involved, not as
directly and quickly as glucose. Obviously all the
target contaminants had the best removal efficiency
in carbon sources system of glucose.
Figure 2. Different target contaminants’ removal
efficiency in different carbon system.
3.2 Degradation of Target
Contaminants under Different Cgc:
Ctc (Gc: Glucose Concentration,
Tc: Respective Target
Contaminants Concentration)
Fig.3 presents removal of target contaminants under
different Cgc: Ctc. Under the constant target
contaminants concentration condition, between 1:15
and 1:8 the co-substance increased with the increase
of removal efficiency of all the target contaminants,
and it had a maximum value of all with Cgc at 1/8 of
Ctc. When Cgc: Ctc was above 1:8, the removal
efficiency decreased with the increase of the co-
substance glucose concentration, excessive growth
of substance would appear. Therefore, refractory
organic matters were disadvantageous for contesting
enzymes, resulting in the decreased removal
efficiency.
Figure 3: Removal of target contaminants under different
Cgc:Ctc.
3.3 pH-Dependent Degradation in the
Glucose System
The concentration of target contaminants was
monitored during the degradation under different pH
condition ranging from 5 to 9, as shown in Fig.3. It
shows the process of the active sludges cultivated
under different pH with time series. And the average
removal efficiency and effluent concentration of all
target contaminants was shown in Fig.4. From Fig.4
and Fig.5, it can see that under acidic conditions,
degradation is relatively low. And with pH
increased, ceftriaxone sodium and ampicillin
sodium’s degradation efficiency increased with a
little margin compared with pH=7, the removal
efficiency of 6-APA almost constant, but removal
efficiency of cefoperazone sodium, amoxicillin and
7-APA decreased. Considered the appropriate pH of
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1216
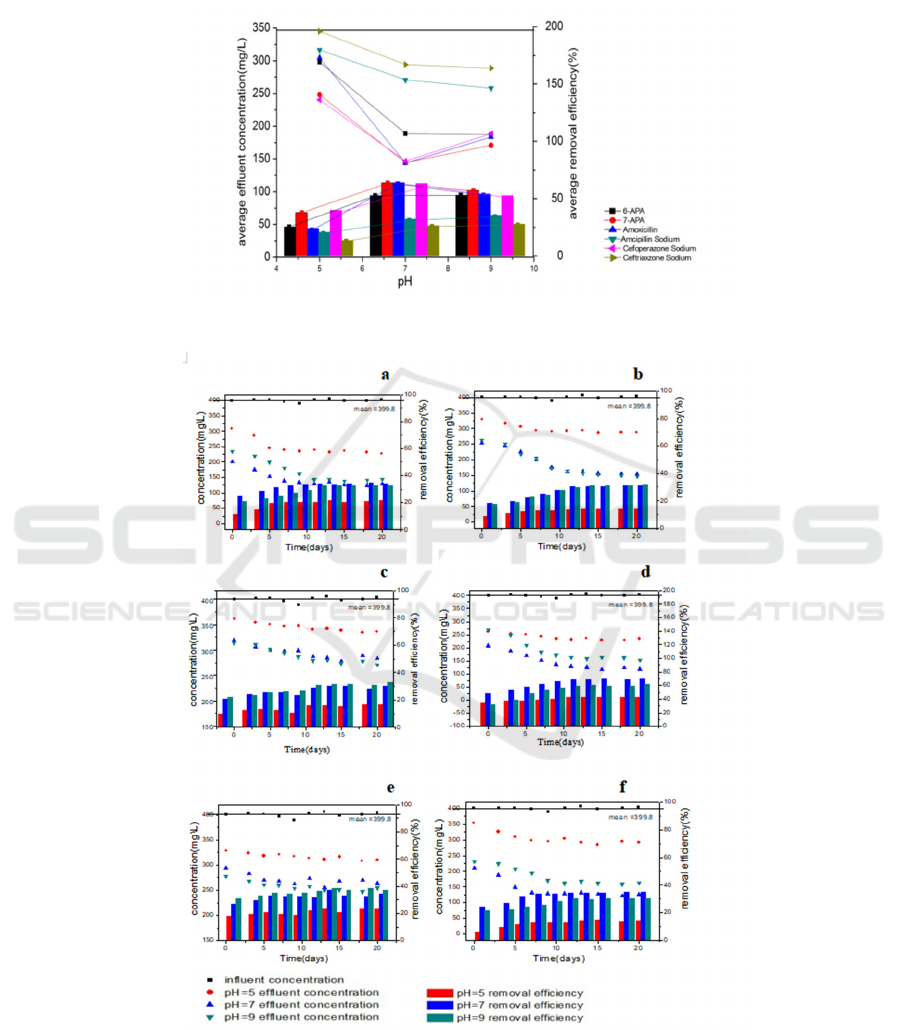
water treatment plants’ flows is ranging from 6.5 to
8.5. Fig.3 or Fig.4 showed that when under pH was
around 7 (neuter), removal efficiency of all the
target contaminants was better considered the
relative conditions. Adjusting the pH around 7 for
degradation will be helpful to improve the removal
efficiency.
Figure 4: Different contaminants’ average removal efficiency and average effluent concentration under different pH.
Figure 5: The effluent concentrations and removal efficiency of the different β-lactam antibiotics and intermediates during
the 20 days. (a) 7-APA; (b) 6-APA; (c) ceftriaxone sodium; (d) cefoperazone sodium; (e) ampicillin sodium; (f)
amoxicillin.
Optimized Operational Parameters for the Biodegradation of the -lactam Antibiotics and Intermediates in Treating High-strength Organic
Wastewater Treatment
1217
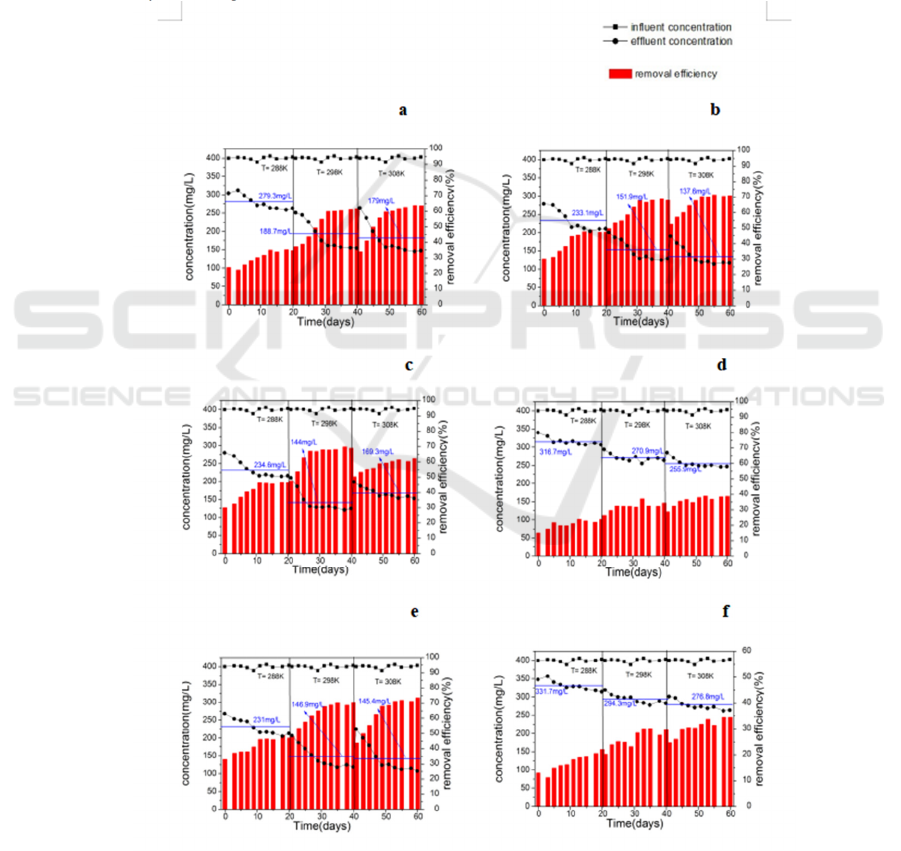
3.4 Temperature Effect on the
Degradation of β-lactam Antibiotics
and Intermediates
Temperature has a remarkable effect on reaction
according to the biodegradation theory. To
determine the effect of temperature on target
contaminants degradation, the average effluent
concentrations and removal efficiency was
monitored at 288, 298 and 308K, as shown in Fig.6.
The curve lines and histograms in Fig.5 shows the
consequent changes of effluent concentrations and
removal efficiency in sludge domestication under
different temperature. Generally, temperature had a
strong effect on degradation, especially under 288K
and 298K. It is found that the increase in
temperature dramatically accelerates the degradation
efficiency, and participated in aerobic
biodegradation process microorganism are classified
to mesophile microorganism, its optimum growth
temperature ranges from 293 to 310K. It also shows
that when under 298k or under 308k, the active
sludges has similar effluent concentrations and
removal efficiency. Considered the energy-
consumed, temperature should be controlled under
298K.
Figure 6: Temperature effect on the effluent concentrations and removal efficiency of the different β-lactam antibiotics and
intermediates during the 20 days. (a) 6-APA; (b) 7-APA; (c) amoxicillin; (d) ampicillin Sodium; (e) cefoperazone sodium;
(f) ceftriaxone sodium.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1218
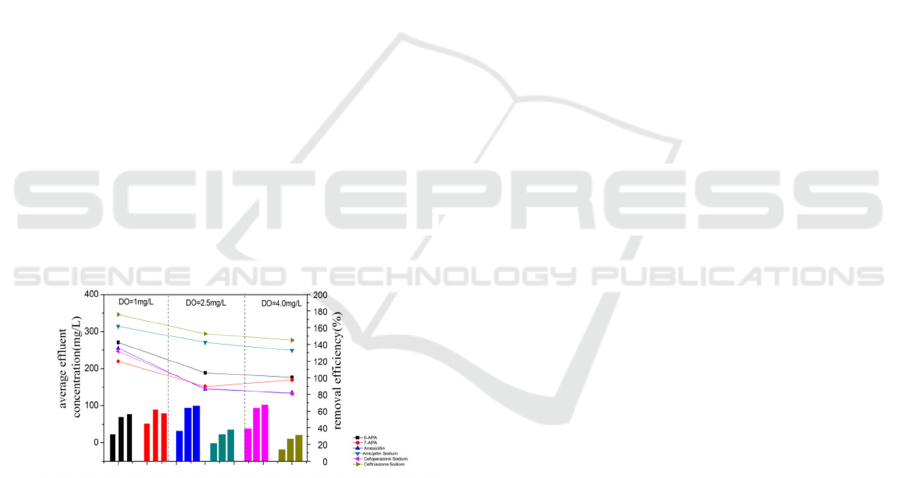
3.5 DO Effect on the Degradation of
β-lactam Antibiotics and
Intermediates
Dissolved oxygen is an important element
affecting microbial metabolism. The
microorganisms involved in wastewater treatment
are mainly based in aerobic respiration. The DO in
the reactor must be guaranteed in order to maintain
normal aerobic respiration and physiological
metabolism of microorganisms in the active sludges.
So the low DO concentration has a seriously
negative effects, even worse, it decrease the
performance of the treatment reactor. While the high
DO contents would not only increase the operation
costs and reduce the feasibility of the treatment
process, but also increase decomposition of
pollutants, causing a lack of nutrients for
microorganisms, loosely structures of active sludges
and poor performance. Average removal efficiency
and effluent concentration of the target contaminants
during the 20 days’ active sludges domestication are
show in Fig.6. It shows that with the DO increased,
the degradation efficiency increased. There have a
little improvement between DO under 2.5 mg/L and
under 4.0 mg/L, while removal efficiency of 7-APA
decreased when DO contents increased. So
considered the more operation costs under a higher
DO contents, controlling the DO contents under 2.5
mg/L is more rational.
Figure 7: Different contaminants’ average removal
efficiency and average effluent concentration under
different DO (points represented concentration, columns
represented removal efficiency).
4 CONCLUSIONS
The degradation of the target contaminants in
different systems was determined using HPLC. Out-
added carbon sources can accelerate the degradation
and out-added-glucose system has higher removal
efficiency than sucrose and starch as the carbon
sources. And when the initial glucose concentration
the initial target contaminants concentration was
under 1:8, it has the best removal efficiency.
Investigating the effects of the pH on the
degradation of target contaminants in the glucose
system revealed that the degradation of 6-APA,
amoxicillin and ceftriaxone sodium accelerated
generally with the increasing pH, but which was a
little improvement compared with under pH=7. So
when pH around 7, all the target contaminants had a
rational removal efficiency.
The effect of temperature on the degradation of
target contaminants is strong. On the basis of the
experimental results, when temperature increased
from 288K to 298K, the degradation accelerated a
lot. While under 298k or 308K, the degradation was
similar. So main active microorganism were
mesophile.
DO also has a strong effect on the degradation.
Experiments results shows that with the DO contents
increased, the degradation increased. But there was a
small improvement between 2.5 mg/L and 4 mg/L.
Considered the operation costing, keep DO under
2.5 mg/L is more practical.
REFERENCES
Bel, E. D. Dewulf, J. De, B. (2009). Influence of pH on
the sonolysis of ciprofloxacin: Biodegradability,
ecotoxicity and antibiotic activity of its degradation
products. Chemosp. 77, 291–295.
Brosillon, S. (2013). Guideline for Testing of Chemicals
(301D). Closed Bottle Test. 54, 134-156. Buynak, D.
(2016). Understanding the longevity of the β-lactam
antibiotics and of antibiotic/β-lactamase inhibitor
combinations. Biochem Pharmacol. 71(30), 930–940.
Calamari,. D. Zuccato, E. Castiglioni,.S. Bagnati,.R.
Fanelli, R. (2003). Strategic survey of therapeutic
drugs in the rivers Po and Lambro in northern Italy.
Environ. Sci. Technol. 37, 1241–1248.
Chaudhuri, M. (2019). Degradation of the antibiotics
amoxicillin, ampicillin and cloxacillin in aqueous
solution by the photo-Fenton process. J Hazar Mater.
30, 1476–1481.
Edward Turos, G. Greenhalgh, K. (2007). Penicillin-
bound polyacrylate nanoparticle: Restoring the activity
of β-lactam antibiotics against MSRA. Bioorganic &
Medicinal Chemistry Letters. 17(12), 3468-3472.
Emad, S. (2010). Degradation of amoxicillin, ampicillin
and cloxacillin antibiotics in aqueous solution by the
UV/ZnO photocatalytic process. J Hazar Mater. 12,
445–449.
Halling-Sùren sen, B. NorsNielsen, S. Lanzky, P.F.
Ingerslev, Holten, F. Utzhùft, L. Jùrgensen, H.C.
(1998). Occurrence fate and effects of pharmaceutical
substances in the environment - a review. Chemosp.
36, 357-393.
Optimized Operational Parameters for the Biodegradation of the -lactam Antibiotics and Intermediates in Treating High-strength Organic
Wastewater Treatment
1219

Heberer, T. (2002). Occurrence, fate, and removal of
pharmaceutical residues in the aquatic environment: a
review of recent research data. Toxicol. Lett. 131, 5–
17. James, H. (2010). Fate of amoxicillin in mixed-
culture bioreactors and its effects on microbial growth
and resistance to silver ions. Sci. Technol. 44, 1827-
1832.
Kummerer, K. Henninger, A. (2003). Promoting resistance
by the emission of antibiotics from hospitals and
households into effluent, Clin. Microbiol. Infect. 9,
1203–1214.
Kümmerer, K. Ahmad, A. Sundermann, V. (2103).
Biodegradability of some antibiotics, elimination of
the genotoxicity and aection of wastewater bacteria in
a simple test. Chemosp. 11(14), 15-23.
Magda, A. Mona, A. Pharma, J. Ramadan, A. (2011).
Validation of an HPLC-UV method for the
determination of ceftriaxone sodium residues on
stainless steel surface of pharmaceutical
manufacturing equipments. Bio Analysis. 55, 247-252.
Reddy, S.K. (2006). Effects of Drugs: The International
Encyclopedia of adverse. Fifteenth Edition. 7, 478–
502.
Smital, T. Luckenbach, T. Sauerborn, R. (2004). Emerging
contaminants-pesticides, PPCPs, microbial
degradation products and natural substances as
inhibitors of multixenobiotic defense in aquatic
organisms. Mutation Res. 552 (1-2), 101-117.
Yahiat, S. Fourcade, F. Amrane, A. (2011). Removal of
antibiotics by an integrated process coupling
photocatalysis and biological treatment -Case of
tetracycline and tylosin. International Biodeterioration
& Biodegradation. 65, 997-1003.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1220
