
The Mechanism Study and Target Modifications on the AMP
Temporin-PEa Triggering TNF-ɑ Necroptosis Pathway in Lung
Cancer Cell Death
Jiewen Zheng
School of Pharmacy, Queen’s University Belfast, Belfast, U.K.
Keywords: Temporin-Pea, Lung Cancer, TNF, Necroptosis Pathway, Antimicrobial Peptides, Target Modifications.
Abstract: NSCLC is the most typical lung cancer with one of the highest lethal rates in the world. However, the drug
resistance and poor prognosis make the conventional therapies less effective. A previous study has reported
that a novel AMP temporin-PEa shows anticancer activity on NSCLC, which provides more potential for lung
cancer treatment. This study investigates the antiproliferation mechanism of temporin-PEa, as well as the
therapeutic effect of this AMP, and two other modified versions in both in vitro and in vivo conditions. The
experiments will utilize NSCLC cell lines A549 and NCI-H157, and HMEC-1 cell line, and Xenograft mice
models. Antiproliferation activity is measured by MTT assay. Cytotoxicity is detected by LDH assay, and
haemolytic activity is detected using horse blood. The TNF-α necroptosis will be measured by Annexin V-
FITC/PI assay and western blot. ROS over-generation will be measured by ROS assay kit with H2DCFDA
ROS probe. The results of this study will provide important information for the exploration of the anticancer
mechanism of AMPs and target modifications improving drug therapeutic values. Further studies should
mainly focus on exploiting the specific target of temporin-PEa for further anticancer drug development, and
in vivo drug delivery system design, which could help the drug overcome various biological barriers.
1 INTRODUCTION
Non-small-cell lung cancer (NSCLC) accounts for
85-90% of all lung cancers, and is one of the leading
causes of cancer death in the world (R.L. Siegel, K.D.
Miller, A. Jemal, Cancer Statistics, 2017). However,
the poor prognosis and resistance to conventional
chemotherapy and radiotherapy have greatly
motivated the development of novel efficacious
therapeutics in treating this lethal disease (Liu, Pei,
Yang, Li, Amin, Liu, Buchan, Cho 2017).
Antimicrobial peptides (AMPs), which are
commonly believed to show strong antimicrobial
activity against a broad spectrum of microorganisms,
have been reported to represent cell-line-dependent
anticancer activity (Tornesello, Borrelli, Buonaguro,
Buonaguro, Tornesello 2020). Compared to normal
cells, cancer cell membranes exhibit more anionic,
according to the changes of the tumor
microenvironment, which might lead to
dysregulation of phospholipid transporters (Ran,
Downes, Thorpe 2002). Therefore, the electrostatic
attraction between the anionic phospholipids of
cancer cell membranes and the cationic peptides
would play a vital role in the peptide-membrane
interaction (Hoskin, Ramamoorthy 2008).
A recently discovered AMP temporin-PEa,
generated through modification on a natural-derived
peptide temporin-PE, has been reported to represent
strong antiproliferation activity on lung cancer cells
and low cytotoxicity on mammalian cells (Sang, Wu,
Xi, Ma, Wang, Zhou, Burrows, Chen 2018). This
enables temporin-PEa to become a proper candidate
with high therapeutic effects for further drug
development. However, the molecular mechanisms
by which this peptide induces tumor cell death still
remain poorly understood.
Phosphatidylserine (PS), accounting for 3-9% of
the total amount of phospholipids, is negatively
charged and normally exists on the inner leaflet of the
cell membrane, and could be transferred to the outer
membrane according to apoptosis or mutations in
cancer cells (Birge et al 2016, Shklyar, Levy-Adam,
Mishnaevski, Kurant 2013). Based on the literature,
the outer membrane exposure of PS could be
mediated by Tumour Necrosis Factor (TNF), which
Zheng, J.
The Mechanism Study and Target Modifications on the AMP Temporin-PEa Triggering TNF- Necroptosis Pathway in Lung Cancer Cell Death.
DOI: 10.5220/0011390000003443
In Proceedings of the 4th International Conference on Biomedical Engineering and Bioinformatics (ICBEB 2022), pages 1221-1229
ISBN: 978-989-758-595-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
1221
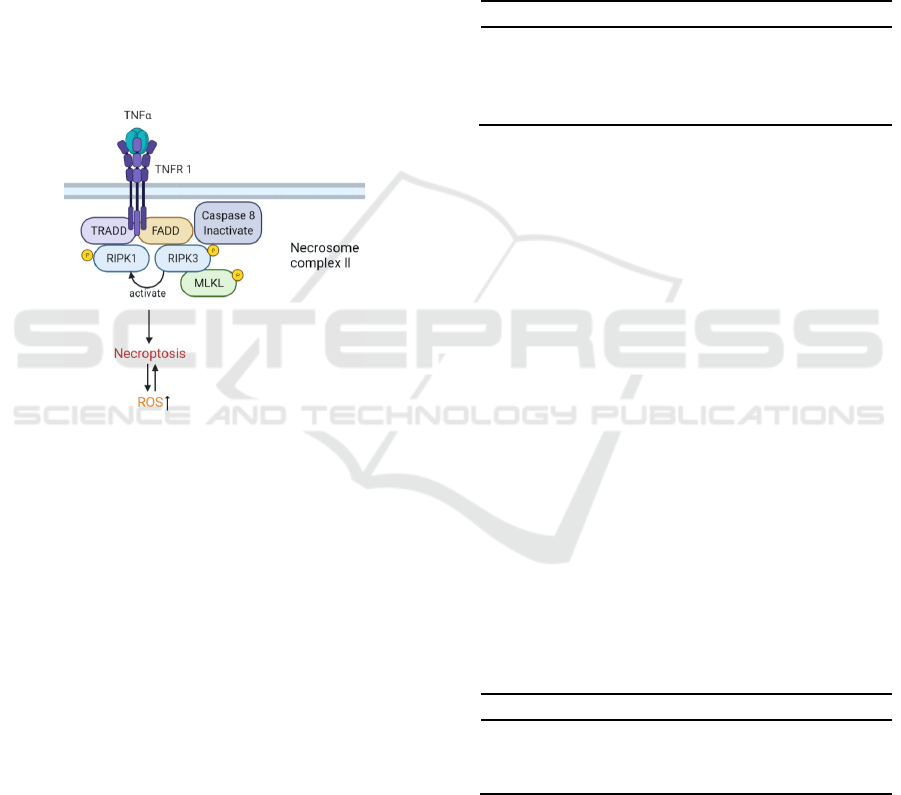
is significantly important for cellular homeostasis,
and is a master regulator in cell necroptosis (Parvy,
Yu, Dostalova, Kondo, Kurjan, Bulet, Lemaitre,
Vidal, Cordero 2019, Kalliolias, Ivashkiv 2016).
Necroptosis is a regulated cell death program without
caspase activation, and it is mainly mediated through
Receptor-Interacting Protein Kinase 1/3 (RIPK1/3)
and Mixed Lineage Kinase Domain-Like
(MLKL)(Gong et al 2019). Necroptosis plays a
pivotal role in oncogenesis, cancer metastasis, and
cancer immunity (Seehawer 2018). In the necroptosis
signaling pathway, the interaction of TNF-α and
TNFR would induce a downstream signaling cascade,
followed by energy depletion due to reactive oxygen
species (ROS) over-generation, and ultimately leads
to cell death (Figure 1) (Christofferson, Yuan 2010,
Vanden Berghe 2014).
Figure 1: Schematic of TNF-α-induced necroptosis
pathway and ROS accumulation.
TRADD: TNF receptor-associated death domain;
FADD: Fas-associated cell death domain.
Therefore, I hypothesize that temporin-PEa
exhibits certain antiproliferation activity on NSCLC
via the TNF-α-induced necroptosis pathway and ROS
over-generation. In this study, the molecular
mechanisms of temporin-PEa inducing NSCLC cell
death are investigated. Also, the bioactivities of
temporin-PEa and two other modified peptides are
studied, in both in vitro and in vivo occasions.
2 MATERIALS AND METHODS
2.1 Peptides Design and Synthesis
In order to improve the cell-penetrating activity and
better investigate the intracellular mechanism of
temporin-PEa, two analogues are designed. The RGD
peptide (Arg-Gly-Asp) and the TAT
(GRKKRRQRRR) peptide have been reported to
effectively deliver drugs as the cell-penetrating
peptide (CPP) in treating lung cancers, as described
previously (Duan et al 2017, Diao et al 2012).
Therefore, RGD is added to the N-terminus of the
template peptide, generating the novel peptide RGD-
temporin-PEa (RGD-PEa). Meanwhile, the other
analogue is designed by linking the TAT sequence to
the C-terminus of the original peptide, namely TAT-
PEa. The specific sequences are listed in Table 1.
Table 1: Anticancer peptide sequences.
Peptide Sequence
Temporin-PEa FLYIVAKLLSGLL-NH
2
RGD-PEa RGD- FLYIVAKLLSGLL-NH
2
PEa-TAT FLYIVAKLLSGLL-
GRKKRRQRRR-NH
2
*-NH
2
: C-terminal amidation
Temporin-PEa and the other modified peptides
will be synthesized using a Tribute 2-channel
automated peptide synthesizer through solid-phase
peptide synthesis. Briefly, the process will employ
resin with rink amide and standard Fmoc protection
chemistry, and will be performed in the PS4 peptide
synthesizer. Then the crude peptides will be purified
by the RP HPLC and then characterized by MALDI-
TOF MS.
2.2 Cell Lines, Cell Culture, and
Chemicals
Human NSCLC cell lines A549, NCI-H157 will be
obtained and cultured in the medium of DMEM/F12
with 10% FBS and 1% penicillin-streptomycin.
Human microvascular epithelial cell line HMEC-1
will be cultured in a full-growth MCDB-131 medium
with 10 mM L-Glutamine as well as 10 ng/mL of
epidermal growth factor. The specific cell lines are
shown in Table 2.
Table 2: Cell lines applied in this study.
Cell line Cell Type
NCI-H157 Human Non-small cell lung cancer cell
A549 Human Non-small cell lung cancer cell
HMEC-1 Human microvascular epithelial cell
2.3 MTT Cell Proliferation Assay
3-(4, 5-Dimethylthiazol-2-yl)-2, 5-
diphenyltetrazolium bromide (MTT) cell viability
assay is utilized to evaluate the antiproliferation
activity of temporin-PEa, and its analogues.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1222

Firstly, the cells with the amount of 5× 103 cells
per well will be plated in the 96-well plate and
cultured under 5% CO2 at 37°C for 24 h. The cells
will be then starved by the medium without FBS for
6 h, followed by treatment with three peptides
gradient dilutions (10-9-10-4 M) for 24 h.
Meanwhile, PBS solution will be used in the vehicle
control group, and 1% Triton X-100 will be utilized
as the positive control. After incubation for another
24 h, a total amount of 10 μL MTT with the
concentration of 5 mg/mL will be added to each well
and co-incubated for 4 h at the conventional
incubator. Finally, after removing the medium, a
certain amount of DMSO will be added to each well,
and the optical density (OD) value of each well will
be subsequently measured by a Synergy HT plate
reader at the wavelength of 490 nm. After the
experiment, the half-maximal inhibitory
concentration (IC50) values of three peptides will be
determined by GraphPad Prism Software. Also, the
Geometric Mean (GM) of each peptide inhibiting
cancer cell growth is calculated.
2.4 Lactate Dehydrogenase Release
(LDH) Assay
LDH will release from the damaged cells, and the cell
membrane integrity will be measured to estimate the
cytotoxicity of the three peptides on normal cells. The
degree of LDH release from destroyed cells after
peptide treatment will be determined by the Pierce
LDH Cytotoxicity Assay Kit. In brief, cells with a
density of 5 × 103 each well will be seeded into 96-
well plates and incubated for 24 h. The cells will be
treated with a range of doses (10-9-10-4 M) of the
three peptides or PBS solution for 24 h incubation as
sample groups and negative control group,
respectively. Also, in the positive control group, cells
will be mixed with 1% Triton X-100 and then
incubated at 37°C under 5% CO2 for 30 min to
acquire a relatively maximum LDH release. After the
incubation, the cell supernatant of each group will be
transferred to another 96-well plate, and the reaction
buffer will be added with incubation for about half an
hour at room temperature. At last, the stop solution
will be added, and the OD value will be determined
at the wavelength of 490 nm. Also, the therapeutic
index (TI) of each peptide will be calculated as the
ratio of the IC50 value for the HMEC-1 cell line to
the GM value for two NSCLC cell lines.
2.5 Haemolysis Assay
The haemolytic effect of three peptides will be
determined using defibrinated horse red blood cells.
A total volume of 100 μL peptide serial dilutions,
ranging from 1 to 128 μM, will be incubated with an
equal volume of 4% erythrocytes for 120 min at
37°C. The positive control and negative control
groups will contain the same volume of 2% Triton X-
100 and PBS solution, respectively. Then, the OD
value of each well will be measured at 550 nm.
2.6 Annexin V-PI Assay
Cancer cells (1×106) will be treated with peptide
solution at half IC50, IC50, and 2×IC50
concentrations. Z-VAD-FMK, the pan-caspase
inhibitor, followed by TNF-α, and PBS solution will
be utilized in the positive control the negative control
group, respectively. The Annexin V-FITC/PI assay
will be conducted based on the protocol, as reported
previously (Yu et al 2019). Briefly, after incubation
for 24 h, cells will be rinsed with PBS solution,
trypsinized, and resuspended in the binding buffer.
Then, the cell suspension will be incubated with
Annexin V-FITC and propidium iodide (PI) for 5 min
at room temperature in the dark. The cells will then
get analyzed for morphological changes and DNA
content by flow cytometry with a ZE5 Cell Analyzer.
2.7 Western Blot Analysis
Harvested cells will be lysed with 400 μl of lysis
buffer. Protein concentration will be determined
according to the Bradford method. A certain amount
of protein extraction will be loaded into SDS gels to
separate the protein components. After the membrane
transfer, the blotted membrane will be blocked with
skim milk for 60 min at room temperature and
incubated with the appropriate primary antibody for
10 h at 4˚C. At last, HRP will be coupled with
appropriate secondary antibodies. After 60 min,
signals from the combined antibodies will be detected
by using the chemiluminescence kit.
2.8 ROS Accumulation Detection
The ROS assay kit will be used in this section. A total
amount of cells with a density of 5×105 per dish (10
cm) will be incubated for 24 h, and then will be
treated with the peptide solution at half IC50, IC50,
and 2×IC50 for a specific time length. Ten microliters
of the ROS probe H2DCFDA will be added to the
cells suspension after PBS-rinsing, and incubated for
The Mechanism Study and Target Modifications on the AMP Temporin-PEa Triggering TNF- Necroptosis Pathway in Lung Cancer Cell
Death
1223

at 37˚C, 5% CO2 for half an hour in the dark. Then,
cells will be treated with trypsin and instantly
analyzed for DCF fluorescence intensity by flow
cytometry. Hydrogen peroxide (H2O2) dilution and
PBS will be used as the positive control and negative
control respectively.
2.9 In Vivo Antiproliferation Assay
The cell line that peptides exhibit a stronger
anticancer level will be chosen to conduct the in vivo
assay. Also, the peptide with the highest TI value and
no significant haemolytic activity will be selected for
the in vivo assay.
In vivo antiproliferation will be detected by
subcutaneous injection of a total amount of 2×106
cancer cells in the right flank of the seven-week-old
BALB/c-nu/nu nude mice, based on the Guide for the
Care and Use of Laboratory Animals published by the
US NIH. When tumor volumes reach 200 mm3, mice
will be divided into six groups, including a negative
control group treated with PBS solution, a positive
control group treated with Cisplatin (DDP), and three
doses of sample groups treated with a high, medium,
and low concentration of peptide solutions,
respectively. Moreover, mice with no tumor will be
treated as the blank group.
Mice of peptide groups will receive intratumoral
injections on a daily basis, with a volume of 20 μL of
peptide solutions with different concentrations for
two weeks. Additionally, mice in the negative control
group will be daily treated with 20 μL PBS solution,
while the mice in the positive control group will
receive the seven-day intraperitoneal injections with
200 μL DDP and then will be continuously fed until
day 14. Tumour growth inhibition will be then
detected by tumor size measurement twice a week
using a digital caliper.
2.10 Statistical Analysis
Each experiment will be repeated five times. And the
statistical significance of data acquired will be
analyzed with the student’s T-Test or one-way
ANOVA on GraphPad Prism software at (p <0.05).
3 RESULTS
3.1 Possible Results on the Mechanisms
of the Peptide Temporin-Pea
Inducing Lung Cancer Cell Death
(The Overview of Four Possible
Results is Demonstrated in Table
3).
Possible Result 1: Temporin-PEa induces lung
cancer cell death via the TNF-α necroptosis
pathway and ROS over-generation.
Based on western blot analysis, there is a
significant up-regulation of the expression level of
TNF-α, phosphorylated MLKL (pMLKL), pRIPK1,
and pRIPK3, and no significant changes in the level
of the procaspase-8 as well as the cleaved caspase-8
expression. Also, ROS accumulation is detected in
the peptide-treated cells.
Possible Result 2: Temporin-PEa induces lung
cancer cell death via the TNF-α necroptosis
pathway, but not ROS over-generation.
Based on western blot analysis, there is a
significant up-regulation of the expression level of
TNF-α, pMLKL, pRIPK1, and pRIPK3, and no
significant changes in the level of the procaspase-8 as
well as the cleaved caspase-8 expression. However,
ROS accumulation is not detected.
Possible Result 3: Temporin-PEa induces lung
cancer cell death through ROS over-generation,
but not the TNF-α necroptosis pathway.
After the treatment of the peptide, the expression
levels of TNF-α, pMLKL, pRIPK1, and pRIPK3 have
not been increased significantly, and/or there are
significant changes in the expression level of
procaspase-8 and cleaved caspase-8. However, ROS
accumulation is detected.
Table 3: Possible results on the mechanisms of the peptide temporin-PEa inducing lung cancer cell death.
Result 1 Result 2 Result 3 Result 4
TNF-α + + - -
pRIPK 1/3 + + - -
pMLKL + + - -
Procaspase 8 - - + +
Cleaved caspase 8 - - + +
ROS over-generation + - + -
Note. “+” represents a significant increase from the negative control. “-” represents there is no significant difference from the
negative control.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1224

Possible Result 4: Temporin-PEa induces lung
cancer cell death via other signalling pathways,
instead of the TNF-α necroptosis pathway and
ROS over-generation.
After the treatment of the peptide, the expression
level of TNF-α, phosphorylated RIPK1,
phosphorylated RIPK3, and phosphorylated MLKL
has not been increased significantly, and/or there are
significant changes in procaspase-8 and cleaved
caspase-8 expression. Also, ROS accumulation is not
detected.
3.2 Possible Results on the Therapeutic
Index (TI) of the Peptides (The
Overview of Four Possible Results
is Demonstrated in Table 4).
Possible Result 5: RGD-PEa shows the highest TI
value, while PEa-TAT and temporin-PEa show
the second and the third TI value respectively.
According to the peptide antiproliferation activity
on lung cancer cells and cytotoxicity to normal cells,
the peptide RGD-PEa exhibits the highest TI value
among the three peptides, while peptide PEa-TAT
shows the second TI value, and temporin-PEa shows
the lowest.
Possible Result 6: RGD-PEa shows the highest
TI value, while temporin-PEa and PEa-TAT show
the second and the third antiproliferation activity
respectively.
Based on the antiproliferation activity and
cytotoxicity of the peptide, RGD-PEa exhibits the
highest TI value among the three peptides, while
temporin-PEa shows the medium TI value, and PEa-
TAT shows the lowest.
Possible Result 7: PEa-TAT shows the highest
TI value, and RGD-PEa and temporin-PEa show
the second and the third TI value respectively.
Based on the antiproliferation activity and
cytotoxicity of the peptide, PEa-TAT exhibits the
highest TI value among the three peptides, while
peptide RGD-PEa shows the second TI value, and
temporin-PEa shows the lowest.
Possible Result 8: PEa-TAT shows the highest
TI value, and temporin-PEa, as well as RGD-PEa
shows the second and the third TI value
respectively.
According to the peptide antiproliferation activity
on lung cancer cells and cytotoxicity to normal cells,
the peptide PEa-TAT exhibits the highest TI value
among the three peptides, while temporin-PEa shows
the medium level of TI value, and RGD-PEa shows
the lowest.
Table 4: Possible results on the TI values of the three peptides.
Result 5 Result 6 Result 7 Result 8
RGD-PEa +++ +++ ++ +
PEa-TAT ++ + +++ +++
Temporin-PEa + ++ + ++
Note. “+” represents the peptide with the lowest TI value, “++” represents the peptide with the medium TI value, and “+++”
represents the peptide that shows the highest TI value
3.3 Possible Results on The Haemolytic
Activity of The Peptides (The
Overview of Four Possible Results
is Demonstrated in Table 5).
Possible Result 9: None of the peptides shows
significant haemolytic activity.
Compared with the negative control, all of the
three peptides display no significant haemolytic
activity on mammalian red blood cells.
Possible Result 10: RGD-PEa and temporin-PEa
both show no significant haemolytic activity, while
PEa-TAT shows significant haemolytic activity.
Compared with the negative control, the peptide
RGD-PEa and temporin-PEa both display no
significant haemolytic activity on red blood cells.
However, PEa-TAT exhibits significant haemolytic
activity.
Possible Result 11: PEa-TAT and temporin-
PEa both show no significant haemolytic activity,
while RGD-PEa shows significant haemolytic
activity.
Compared with the negative control, the peptide
RGD-PEa and temporin-PEa both display no
significant haemolytic activity. However, PEa-TAT
exhibits significant haemolytic activity.
Possible Result 12: Both PEa-TAT and RGD-
PEa show significant haemolytic activity, and only
temporin-PEa shows no significance with the
negative control.
Both of the modified peptides exhibit significant
haemolytic activity, and only the original peptide
temporin-PEa shows no significance with the
negative control group.
The Mechanism Study and Target Modifications on the AMP Temporin-PEa Triggering TNF- Necroptosis Pathway in Lung Cancer Cell
Death
1225

Table 5: Possible results on the haemolytic activity of three peptides.
Result 9 Result 10 Result 11 Result 12
RGD-PEa - - + +
PEa-TAT - + - +
Temporin-PEa - ---
Note. “+” represents a significant difference in haemolytic activity from the negative control. “-” represents there is no
significant difference from the negative control.
3.4 Possible Results on the In Vivo
Antiproliferation Activity of the
Peptides (The Overview of Four
Possible Results is Shown in Table
6).
Possible Result 13: The chosen peptide represents
stronger antiproliferation activity on the NCI-
H157 cancer cell line, and induces significant lung
cancer cell death in vivo.
In the in vitro detection, the chosen peptide shows
stronger antiproliferation activity on the NCI-H157
cancer cell line, which is used to establish in vivo
models. Also, the peptide induces significant lung
cancer cell death in the animal model.
Possible Result 14: The chosen peptide
represents stronger antiproliferation activity on
the NCI-H157 cancer cell line, but does not show
significant in vivo antiproliferation activity on
lung cancer.
In the in vitro detection, the chosen peptide shows
stronger antiproliferation activity on the NCI-H157
cancer cell line, which is used to establish in vivo
models. However, the peptide does not induce
significant lung cancer cell death in the animal model.
Possible Result 15: The chosen peptide
represents stronger antiproliferation activity on
the A549 cancer cell line, and induces significant
lung cancer cell death in vivo.
In the in vitro detection, the chosen peptide shows
stronger antiproliferation activity on the A549 cancer
cell line, which is used to establish in vivo models.
Also, the peptide induces significant lung cancer cell
death in the animal model.
Possible Result 16: The chosen peptide
represents stronger antiproliferation activity on
the A549 cancer cell line, but does not show
significant in vivo antiproliferation activity on
lung cancer.
In the in vitro detection, the chosen peptide shows
stronger antiproliferation activity on the A549 cancer
cell line, which is used to establish in vivo models.
However, the peptide does not induce significant lung
cancer cell death in the animal model.
3.5 Additional Possible Results on the
Temporin-Pea and Modification
Peptides Different from Previous
Researches
Possible Results 17: Compared with temporin-
PEa, modified peptides RGD-PEa and PEa-TAT
both show lower TI values.
Temporin-PEa shows the highest TI value, while
the other two modification strategies decrease the PI
value of the original peptide.
Possible Results 18: The chosen peptide shows
no significant difference in antiproliferation
activity between the two cancer cell lines.
In the in vitro detection, the chosen peptide shows
no significant difference in the anticancer activity
between NCI-H157 and A549 cancer cell lines. In
this case, cell line NCI-H157 is used for mechanism
study and in vivo detection.
Table 6: Possible results on the in vivo antiproliferation activity of the chosen peptide.
Result 13 Result 14 Result 15 Result 16
Cell line for xenograft model NCI-H157 NCI-H157 A549 A549
In vivo antiproliferation + - + -
Note. “+” represents a significant difference in antiproliferation activity from the negative control. “-” represents there is no
significant difference from the negative control.
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1226

4 DISCUSSION
Previous studies report that AMPs show certain
anticancer activity in a cell-line-dependent manner
(Tornesello, Borrelli, Buonaguro, Buonaguro,
Tornesello 2020). An AMP temporin-PEa has been
reported to present strong antiproliferation activity on
human lung cancer cells (Sang, Wu, Xi, Ma, Wang,
Zhou, Burrows, Chen 2018). To unravel the
molecular mechanisms of this peptide-inducing lung
cancer cell death, this study further investigates
whether temporin-PEa would trigger the TNF-α
necroptosis signaling pathway and ROS
accumulation. Also, in order to assess potential target
modification strategies to improve the therapeutic
value of the peptide, the in vitro bioactivity assays are
designed, and the peptide with no significant
haemolytic activity and relatively higher TI value is
used for xenograft animal model detection.
Possible Result 1 shows that temporin-PEa would
induce lung cancer cell death through the TNF-α
necroptosis pathway and ROS accumulation, which
fully supports the hypothesis of this study. According
to the literature, there are few studies on the
exploration of temporin-peptide inducing cancer cell
death, and the mechanisms include membrane
destruction, intracellular Ca
2+
leakage, and inducing
apoptosis pathway (Wang et al 2013, Shaheen et al
2018). Here, temporin-PEa would trigger the TNF-α
necroptosis pathway and ROS accumulation, which
could provide a new research direction for the
mechanism study on the anticancer activity of AMPs
from the temporin family. This might also provide a
new strategy for novel anticancer drug development
based on the structure of temporin-PEa. Moreover,
further investigation should be conducted on the
specific intracellular target of the peptide for target
drug delivery system design.
ROS accumulation, which always occurs
downstream of the necroptosis pathway, can lead to
energy depletion and initiate oxidative stress-induced
cancer cell death (Moloney 2018). In Possible Result
2, ROS accumulation is not detected, which might
indicate that there are other mechanisms as the
downstream of TNF-α necroptosis pathway,
including ER (endoplasmic reticulum) stress,
mitochondria membrane disruption, and Ca
2+
leakage
(Kim, Kim 2018, Wang, Zhou, Li, Li, Tian, Wang
2013).
The negative results of temporin-PEa not
triggering the TNF-α necroptosis pathway are
described in Possible Results 3 and 4. Possible Result
3 might indicate that lung cancer cell death is induced
by other signaling pathways, such as the apoptosis
pathway or FasL-mediated necroptosis pathway,
which might trigger ROS over-generation as well
(Otani 2018, Sauler, Bazan, Lee 2019). This could be
further verified by flow cytometry and western blot
detection. In Possible Result 4, neither TNF-α
necroptosis nor ROS over-generation is detected,
which contradicts the hypothesis and probably means
that temporin-PEa could directly break the cancer cell
membrane instead of triggering the intracellular
pathway. Based on the two results, further
investigation should be done on the molecular
mechanisms of temporin-PEa inducing lung cancer
cell death.
In order to improve the therapeutic value of the
original peptide, two modifications are conducted on
temporin-PEa. Possible Results 5 and 7 show that
both of the modified peptides have higher TI value
than temporin-PEa, which is consistent with former
studies and support the hypothesis of this study (Diao
et al 2012, Hu, Chen, Huang, Chen 2018). This would
indicate that both RGD and TAT motifs could
increase the cell penetration activity and tumor
selectivity of the peptide, and thus increase the
anticancer activity while reducing the cytotoxicity to
normal cells. RGD motif shows better efficacy in
Possible Result 5, while TAT peptide behaves better
in Possible Result 7. While in Possible Results 6 and
8, only one of the two modification strategies could
improve the therapeutic efficacy.
Possible Result 9 demonstrates that all of the three
peptides show no haemolytic activity, which enables
them to be proper candidates for further clinical
therapeutic development. Except for temporin-PEa,
PEa-TAT and RGD-PEa show significant
cytotoxicity or haemolytic activity in Possible
Results 10 and 11 respectively. The modified peptide,
which is harmful to the normal cells, cannot be
utilized for in vivo experiments or further clinical
research. Both of the modified peptides display
significant haemolytic activity in Possible Result 12,
which suggests that the novel peptide might generate
new structures that change the properties of the
original peptide and result in haemolysis. These two
modification strategies are not suitable for temporin-
PEa so that further modification approaches should
be studied. Based on the Possible Results (5-12), the
peptide with no haemolytic activity and relatively
higher TI value is chosen for in vivo detection.
Possible Results 13-16 demonstrate the in vivo
detection of the chosen peptide. The results that the
peptide shows higher anticancer activity on the NCI-
H157 cell line are listed in Possible Results 13 and
14. This is consistent with the former study, as
temporin-PEa exhibits strong anticancer activity on
The Mechanism Study and Target Modifications on the AMP Temporin-PEa Triggering TNF- Necroptosis Pathway in Lung Cancer Cell
Death
1227

the NCI-H157 cell line. Possible Results 15 and 16
show that the chosen peptide has better anticancer
activity on the A549 cancer cell line, which indicates
that the peptide might behave differently in various
lung cancer cell types. Also, Possible Results 14 and
16 demonstrate that the peptide would probably show
no antiproliferation activity in the xenograft animal
models, which might due to the biological barriers
and poor drug delivery efficiency.
The Possible Result 17 contradicts the hypothesis
and the current understanding of RGD and TAT
peptides improving drug therapeutic value. This
might indicate systemic errors of the experiment
design, and these two modification strategies cannot
promote the peptide cell-penetration ability or tumor
selectivity. Thus, further studies on peptide structure-
function relationship as well as peptide modifications
should be carried out. In Possible Result 18, the
peptide shows no significant difference between
NCI-H157 and A549 cell lines, which would indicate
that the choice of the two cancer cell lines might not
be appropriate, and the antiproliferation activity of
the peptide exhibiting on other lung cancer cell lines
should be further exploited.
5 CONCLUSION
In summary, this study investigates the molecular
mechanisms of the AMP temporin-PEa inducing lung
cancer cell death, and target modifications on this
peptide. The results of this study will test the
hypothesis that whether the peptide would induce
lung cancer cell death via the TNF-α necroptosis
pathway and ROS over-generation, and whether the
RGD and TAT motifs will enhance the therapeutic
value of the peptide.
The possible results on the anticancer mechanism
of temporin-PEa indicate that the peptide would
trigger TNF-α necroptosis or other pathways
followed by energy depletion processes. Also, the
results might suggest that there would be a signaling
network of AMPs triggering immunogenic cell death
(ICD), which involves both death-receptor signaling
pathways and the engagement of other organelles.
Additionally, the possible results on CPP modifying
temporin-PEa would provide potential peptide
modification strategies for further anticancer drug
development. However, novel drug delivery systems
should get further studied to help the peptide
overcome the biological barriers, and to reduce
cytotoxicity to normal cells.
Researchers have started to explore the anticancer
activity of AMPs in recent years, and the detailed
understanding of the intracellular mechanisms of
AMPs still remains largely unclear. Therefore, more
studies on the AMPs anticancer mechanisms should
be conducted to provide more therapeutic potentials
for peptide biologics development.
REFERENCES
A.L. Tornesello, A. Borrelli, L. Buonaguro, F.M.
Buonaguro, M.L. Tornesello, Antimicrobial Peptides
as Anticancer Agents: Functional Properties and
Biological Activities, Molecules 25(12) (2020).
B. Shklyar, F. Levy-Adam, K. Mishnaevski, E. Kurant,
Caspase activity is required for engulfment of apoptotic
cells, Mol Cell Biol 33(16) (2013) 3191-201.
C. Hu, X. Chen, Y. Huang, Y. Chen, Synergistic effect of
the pro-apoptosis peptide kla-TAT and the cationic
anticancer peptide HPRP-A1, Apoptosis 23(2) (2018)
132-142.
C. Kim, B. Kim, Anti-Cancer Natural Products and Their
Bioactive Compounds Inducing ER Stress-Mediated
Apoptosis: A Review, Nutrients 10(8) (2018).
C. Wang, L.L. Tian, S. Li, H.B. Li, Y. Zhou, H. Wang, Q.Z.
Yang, L.J. Ma, D.J. Shang, Rapid cytotoxicity of
antimicrobial peptide tempoprin-1CEa in breast cancer
cells through membrane destruction and intracellular
calcium mechanism, PLoS One 8(4) (2013) e60462.
C. Wang, Y. Zhou, S. Li, H. Li, L. Tian, H. Wang, D.
Shang, Anticancer mechanisms of temporin-1CEa, an
amphipathic alpha-helical antimicrobial peptide, in
Bcap-37 human breast cancer cells, Life Sci 92(20-21)
(2013) 1004-14.
D.E. Christofferson, J. Yuan, Necroptosis as an alternative
form of programmed cell death, Curr Opin Cell Biol
22(2) (2010) 263-8.
D.W. Hoskin, A. Ramamoorthy, Studies on anticancer
activities of antimicrobial peptides, Biochim Biophys
Acta 1778(2) (2008) 357-75.
F. Shaheen, M. Nadeem-Ul-Haque, A. Ahmed, S.U.
Simjee, A. Ganesan, A. Jabeen, Z.A. Shah, M.I.
Choudhary, Synthesis of breast cancer targeting
conjugate of temporin-SHa analog and its effect on pro-
and anti-apoptotic protein expression in MCF-7 cells,
Peptides 106 (2018) 68-82.
G. Liu, F. Pei, F. Yang, L. Li, A.D. Amin, S. Liu, J.R.
Buchan, W.C. Cho, Role of Autophagy and Apoptosis
in Non-Small-Cell Lung Cancer, Int J Mol Sci 18(2)
(2017).
G.D. Kalliolias, L.B. Ivashkiv, TNF biology, pathogenic
mechanisms and emerging therapeutic strategies, Nat
Rev Rheumatol 12(1) (2016) 49-62.
J.N. Moloney, T.G. Cotter, ROS signalling in the biology
of cancer, Semin Cell Dev Biol 80 (2018) 50-64.
J.P. Parvy, Y. Yu, A. Dostalova, S. Kondo, A. Kurjan, P.
Bulet, B. Lemaitre, M. Vidal, J.B. Cordero, The
antimicrobial peptide defensin cooperates with tumour
necrosis factor to drive tumour cell death in Drosophila,
Elife 8 (2019).
ICBEB 2022 - The International Conference on Biomedical Engineering and Bioinformatics
1228

M. Sang, Q. Wu, X. Xi, C. Ma, L. Wang, M. Zhou, J.F.
Burrows, T. Chen, Identification and target-
modifications of temporin-PE: A novel antimicrobial
peptide in the defensive skin secretions of the edible
frog, Pelophylax kl. esculentus, Biochem Biophys Res
Commun 495(4) (2018) 2539-2546.
M. Sauler, I.S. Bazan, P.J. Lee, Cell Death in the Lung: The
Apoptosis-Necroptosis Axis, Annu Rev Physiol 81
(2019) 375-402.
M. Seehawer, F. Heinzmann, L. D'Artista, J. Harbig, P.F.
Roux, L. Hoenicke, H. Dang, S. Klotz, L. Robinson, G.
Dore, N. Rozenblum, T.W. Kang, R. Chawla, T. Buch,
M. Vucur, M. Roth, J. Zuber, T. Luedde, B. Sipos, T.
Longerich, M. Heikenwalder, X.W. Wang, O. Bischof,
L. Zender, Necroptosis microenvironment directs
lineage commitment in liver cancer, Nature 562(7725)
(2018) 69-75.
R.B. Birge, S. Boeltz, S. Kumar, J. Carlson, J. Wanderley,
D. Calianese, M. Barcinski, R.A. Brekken, X. Huang,
J.T. Hutchins, B. Freimark, C. Empig, J. Mercer, A.J.
Schroit, G. Schett, M. Herrmann, Phosphatidylserine is
a global immunosuppressive signal in efferocytosis,
infectious disease, and cancer, Cell Death Differ 23(6)
(2016) 962-78.
R.L. Siegel, K.D. Miller, A. Jemal, Cancer Statistics, 2017,
CA Cancer J Clin 67(1) (2017) 7-30.
S. Ran, A. Downes, P.E. Thorpe, Increased exposure of
anionic phospholipids on the surface of tumor blood
vessels, Cancer Res 62(21) (2002) 6132-40.
T. Otani, M. Matsuda, A. Mizokami, N. Kitagawa, H.
Takeuchi, E. Jimi, T. Inai, M. Hirata, Osteocalcin
triggers Fas/FasL-mediated necroptosis in adipocytes
via activation of p300, Cell Death Dis 9(12) (2018)
1194.
T. Vanden Berghe, A. Linkermann, S. Jouan-Lanhouet, H.
Walczak, P. Vandenabeele, Regulated necrosis: the
expanding network of non-apoptotic cell death
pathways, Nat Rev Mol Cell Biol 15(2) (2014) 135-47.
W.N. Yu, Y.J. Lai, J.W. Ma, C.T. Ho, S.W. Hung, Y.H.
Chen, C.T. Chen, J.Y. Kao, T.D. Way, Citronellol
Induces Necroptosis of Human Lung Cancer Cells via
TNF-alpha Pathway and Reactive Oxygen Species
Accumulation, In Vivo 33(4) (2019) 1193-1201.
Y. Diao, W. Han, H. Zhao, S. Zhu, X. Liu, X. Feng, J. Gu,
C. Yao, S. Liu, C. Sun, F. Pan, Designed synthetic
analogs of the alpha-helical peptide temporin-La with
improved antitumor efficacies via charge modification
and incorporation of the integrin alphavbeta3 homing
domain, J Pept Sci 18(7) (2012) 476-86.
Y. Gong, Z. Fan, G. Luo, C. Yang, Q. Huang, K. Fan, H.
Cheng, K. Jin, Q. Ni, X. Yu, C. Liu, The role of
necroptosis in cancer biology and therapy, Mol Cancer
18(1) (2019) 100.
Z. Duan, C. Chen, J. Qin, Q. Liu, Q. Wang, X. Xu, J. Wang,
Cell-penetrating peptide conjugates to enhance the
antitumor effect of paclitaxel on drug-resistant lung
cancer, Drug Deliv 24(1) (2017) 752-764.
The Mechanism Study and Target Modifications on the AMP Temporin-PEa Triggering TNF- Necroptosis Pathway in Lung Cancer Cell
Death
1229
