
Anti-inflammatory Activities of Pineapple (Ananas comosus) Core
Extract in Lipopolysaccharide-induced RAW264.7 Cell Line
Hanna Sari Widya Kusuma
1,* a
, Hartini Tiono
2b
, Philips Onggowidjaja
2c
,
Selonan Susang Obeng
2d
, Wahyu Widowati
2* e
, Cintani Dewi Wahyuni
1f
,
Cahyaning Riski Wijayanti
1g
, Muhamad Aldi Maulana
1h
, Tri Handayani
1i
and Rizal Rizal
1,3 j
1
Biomolecular and Biomedical Research Center, Aretha Medika Utama, Jl. Babakan Jeruk II No. 9, Bandung,
West Java, Indonesia
2
Faculty of Medicine, Maranatha Christian University, Jl. Prof. Drg. Surya Sumantri No. 65, Bandung,
West Java, Indonesia
3
Biomedical Engineering, Department of Electrical Engineering, Faculty of Engineering, Universitas Indonesia,
Jl. Kampus UI, Depok, West Java, Indonesia
wahyu_w60@yahoo.com, cintanidewi@gmail.com, cahyaningwidodo@gmail.com, aldimaulana.srl@gmail.com,
mbaktrihandayani@gmail.com, rizal_biotek@yahoo.com
Keywords: Anti-inflammatory, IL-1β, Pineapple, RAW 264.7 Cell Lines.
Abstract: Inflammation is a biological response process by the immune system that may induce acute/chronic
inflammatory and leading tissue damage or diseases. Pineapple (Ananas comosus) cores that have been
investigated for anti-inflammatory properties and immunomodulator. This research aims to evaluate the anti-
inflammatory potency of pineapple core extract (PCE) in lipopolysaccharide-induced macrophage cells
(RAW 264.7). The viability assay of PCE was determined by MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-
carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) to ensure the safe and non-toxic concentration in
RAW 264.7 cells. The pro-inflammatory induction of cells using 200 μL of lipopolysaccharide (LPS). Levels
of PGE-2 and pro-inflammatory cytokines IL-1β and TNF-α levels were measured using enzyme-linked
immunosorbent assay. RESULTS: PCE 4 and 20 µg/mL showed high viability (>90%) with the values
95.03% and 92.94%, respectively. PCE 20 µg/mL showed the lower of PGE-2 and TNF-α levels (507.68
pg/mL; 345.90 pg/mL) compared to PCE 4 µg/mL (795.37 pg/mL; 474.19 pg/mL) and positive control (870.48
pg/mL; 581.71 pg/mL). In IL-1β level, PCE 20 µg/mL showed the lower (217.63 pg/mL) compared to PCE 4
pg/mL (350.78 pg/mL) and positive control (433.53 pg/mL). Pineapple core extract has beneficial for anti-
inflammatory by downregulating inflammatory mediators including PGE-2, TNF-α, and IL-1β in LPS-
induced RAW 264.7 cell lines.
1
INTRODUCTION
Inflammation is an immune system reaction that can
a
https://orcid.org/0000-0002-7422-0036
b
https://orcid.org/0000-0002-8050-1707
c
https://orcid.org/0000-0002-7161-9762
d
https://orcid.org/0000-0003-0608-3516
e
https://orcid.org/0000-0002-5401-7794
f
https://orcid.org/0000-0002-7764-0482
g
https://orcid.org/0000-0002-3397-099X
h
https://orcid.org/0000-0003-4724-7548
i
https://orcid.org/0000-0001-9186-9841
j
https://orcid.org/0000-0003-2783-0672
*
Corresponding author
be caused by a several factors, including damaged
cells, pathogens, and toxic substances. These factors
can cause acute and/or chronic inflammatory
58
Kusuma, H., Tiono, H., Onggowidjaja, P., Obeng, S., Widowati, W., Wahyuni, C., Wijayanti, C., Maulana, M., Handayani, T. and Rizal, R.
Anti-inflammatory Activities of Pineapple (Ananas comosus) Core Extract in Lipopolysaccharide-induced RAW264.7 Cell Line.
DOI: 10.5220/0010744300003113
In Proceedings of the 1st International Conference on Emerging Issues in Technology, Engineering and Science (ICE-TES 2021), pages 58-64
ISBN: 978-989-758-601-9
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

responses in the pancreas, liver, heart, lung, brain,
kidney, reproductive system, and intestinal tract,
which could result in tissue damage or diseases (Chen
et al., 2018).
Inflammatory cytokines are produced by activated
macrophages, such as interleukins (ILs), tumor
necrosis factor (TNF-α), inflammatory mediators like
nitric oxide (NO), and prostaglandins (PGs), which
play a protective role for the host in inflammatory
conditions and also preserving normal cellular
conditions (Lee et al., 2017; Saanin et al., 2020). The
activation of macrophages causes the release of a
variety of chemicals, including Nitric Oxide (NO),
reactive oxygen species (ROS), prostaglandin E
(PGE), and Interleukin (IL)-1β, Interleukin (IL)-6,
cyclooxygenase-2 (COX-2), and
tumor necrosis factor
(TNF)-α (Widowati et al., 2016;
Novilla et al., 2017;
Lee et al., 2017; Laksmitawati et al., 2017). Many
chronic diseases have been linked to the inducible
forms of nitric oxide synthase (NOS) and
cyclooxygenase (COX), which are responsible for
raising NO and prostaglandins (PGs) levels,
respectively (Widowati et al., 2021). Thus, the
suppression of pro-inflammatory mediators can be an
effective indicator of anti-inflammatory drugs
(Saanin et al., 2020; Widowati et al., 2021).
Anti-inflammatory medications, both steroidal
and non-steroidal, are used in conventional treatment
for inflammatory diseases. However, the limitations
and risks associated with conventional therapy have
led people to explore alternative measures such as
medicinal plants for the treatment of inflammatory
diseases (Kargutkar and Brijesh, 2017).
Ananas comosus (L.) Merr. which is known as
pineapple is a species of tropical plant that belongs to
the Bromeliaceae family (Rahman et al., 2020). A.
comosus has a various compounds from several parts
of the plant, including alkaloids, anthraquinones,
bromelain, cardiac glycoside, coumarins, flavonoids,
glycoside, inulin, naphthoquinones, phenols,
phytosterols, polyphenols, quinine, saponin, steroids,
sterols, tacorins, terpenoids, tannins, and triterpenes
(Rahman et al., 2020). A. comosus can prevent
undesirable inflammatory processes and also has anti-
inflammatory activity (Yatoo et al., 2018). Bromelain
is a bioactive compound and as a major protease
enzyme found in A. comosus stems that demonstrate
anti-thrombotic, anti-inflammatory, and anti-
edematous (Ramli et al., 2018). Bromelain also has
anti-cancer properties and facilitates cell death by
apoptosis (Pavan et al., 2012). Pineapple core extract
(PCE) exhibit as antioxidant potent by 2,2-diphenyl-
1-picryl-hydrazyl-hydrate (DPPH) scavenging
activity (Vrianty et al., 2019).
However, the previous studies were focused on
the fruit, peels, and leaves of pineapple (A. comosus)
but there were limited studies on the core of the fruit.
This study was purpose to evaluates the anti-
inflammatory activity of PCE through the inhibitory
activity of pro-inflammatory mediators including
PGE-2, IL-1β, and TNF-α on LPS-induced RAW 264
cells.
2
METHODS AND MATERIALS
2.1 Extract Preparation
A. comosus plants were obtained from Tambaksari
village, Cagak district, Subang, West Java, Indonesia.
Plant identification was done at Herbarium Bandung
Laboratory, School of Biological Sciences, Bandung
Institute of Technology. The preparation of A.
comosus ethanolic extract based on Vrianty et al.
(2019) method. A.
comosus
were sorted, washed,
weighed in wet weight,
dried in a food dehydrator,
weighed in dry weight, and then crushed into powder
form (core crude drug). And then, the core crude drug
was extracted using maceration techniques with a
70% ethanol solvent. Every 24-hour, the filtrate was
until the ethanol filtrate turned colorless. Before being
used for assays, PCE was stored at 20 ºC (Vrianty et
al., 2019).
2.2 Raw264.7 Cells Culture
The RAW 264.7 (ATCC®TIB-71™) murine
macrophage cell line was obtained from the
Biomolecular and Biomedical Research Center,
Aretha Medika Utama. RAW 264.7 cells were grown
in Dulbecco's Modified Eagle Medium (DMEM)
(Biowest, L0104) supplemented with 10% fetal
bovine serum (FBS) (Biowest, S1810) and 1%
antibiotic–antimycotic (Gibco, 15240062). The cells
were incubated at 37 ºC and 5% CO
2
in the
humidified atmosphere until confluent (80%–90%).
Trypsin-EDTA 0.25% (Gibco, 25200072) was used
to harvest the cells which were then seeded on plates
for the assays (Sandhiutami et al., 2017;
Laksmitawati et al., 2016; 2017; Saanin et al., 2020;
Widowati et al., 2016; 2021).
2.3 Viability Assay
The cytotoxicity of PCE was determined by the
viability of RAW 264.7 cells using MTS assay
(Promega, G3580). This method is used to assess the
sample concentration that is both safe and non-toxic
Anti-inflammatory Activities of Pineapple (Ananas comosus) Core Extract in Lipopolysaccharide-induced RAW264.7 Cell Line
59

for the next assay. The cells density of 5 × 10
3
cells
per well were plated in medium (DMEM
supplemented with 10% FBS and 1% antibiotic–
antimycotic in a 96-well plate and incubated for 24
hours at 37
o
C in a humidified atmosphere incubator
with 5% CO
2
. The medium was washed and replaced
with 99 μL of fresh medium and 1 μL of PCE in two
concentrations (4, 20, 100 µg/mL), and 1% DMSO,
then the plate was incubated for 24 hours. The
negative control group consisted of cells that had not
been treated. The twenty microlitres of MTS were
added to each well. For 4 hours, the plate was
incubated in 5% CO
2
at 37
o
C at an incubator. The
absorbance was quantified at 490 nm using a
microplate reader (Multiskan Go, Thermoscientific)
(Widowati et al., 2016; 2021; Laksmitawati et al.,
2016; 2017; Saanin et al., 2020).
2.4 Pro-inflammatory Activation of
RAW264.7 Cells
The cells were seeded at a density of 5 × 10
5
cells per
well in a 6 well-plate and incubated for 24 hours at
37
o
C in a humidified atmosphere with 5%
CO
2
. The
DMEM was washed and supplemented
with a 1,600
μL growth medium and 200 μL PCE (4, 20 µg/mL)
after being supplemented with 10% FBS and 1%
antibiotic–antimycotic. After 1-2 hours, 200 μL
lipopolysaccharide (1 μg/mL) from Escherichia coli
(Sigma Aldrich, L2880) was added to the
medium
and incubated for 24 hours at 37
o
C, 5% CO
2
.
The
medium was then taken for PGE-2, TNF-α, and IL-1β
levels quantification, centrifuged at 2000 g for 10
minutes, and the supernatant was stored at -80
0
C.
(Widowati et al., 2016; 2021; Laksmitawati et al.,
2016; 2017; Sandhiutami et al., 2017; Novilla et al.,
2017; Saanin et al., 2020).
2.5 Quantification of PGE-2, TNF-α,
IL-1β Levels in RAW264.7 Cells
The measurement of PGE-2, TNF-α, and IL- 1β levels
were conducted based on the ELISA method, using
PGE-2 ELISA Kit (E-EL-M0052), TNF-α ELISA
Standard Kit (Elabscience, E-EL- M0049), and IL-1β
ELISA Kit (Elabscience, E-EL- M0037), respectively
according to the manufacturer’s instructions. The
inhibition activity was calculated based on the
percentage of a PGE-2, TNF-α, and IL- 1β levels
(Widowati et al., 2016; 2021; Laksmitawati et al.,
2016; 2017; Saanin et al., 2020).
2.6 Statistical Analysis
All data were obtained after doing it in triplicate. The
data were presented as mean ± standard
deviation. The
data were analyzed using ANOVA and
Tukey HSD
Post Hoc Test with p < 0.05 using SPSS software
(version 20.0).
3
RESULTS AND DISCUSSION
3.1 Viability RAW264.7 Cells
The viability assay was performed to determine
the
safe and nontoxic concentration for the next assay,
which was assessed using the MTS assay (Widowati
et al., 2016; 2021). The viability of RAW264.7 cell
lines has been presented in Table 1.
Table 1: The effect of various concentrations of PCE
towards the viability of RAW264.7 cells.
Sample Viability cells (%)
Negative Control 100.00 ±3.54
c
PCE 4 µg/mL 95.03 ±2.07
b
PCE 20 µg/mL 92.94 ±1.04
b
PCE 100 µg/mL 76.86 ±4.09
a
*The data was presented as mean ± standard deviation from
3 replications. Different superscript letters in the same
column (a, b, c) showed significant differences among
treatments at p <0.05 (Tukey HSD post hoc test).
The increased concentration was correlated with
increased toxicity (<90% viability cells). Table 1
shows the cytotoxicity of PCE concentration on
RAW264.7 cell lines. The viability of cells was
decreased in a concentration-dependent manner. In
concentration 100 µg/mL demonstrated the lowest
viability of cells by PCE with a value of 76.86 ±
4.09%. Based on the data (Table 1), the safe and un-
toxic of PCE in murine macrophage cells were 4, 20
µg/mL, these concentrations were used for the
treatment in LPS-induced RAW264.7 cells as
inflammation cells model.
3.2 Effect of PCE toward PGE-2 Level
in LPS-induced RAW264.7 Cells
Inhibitory activity of PGE-2 generation from COX-2
also causes an anti-inflammatory effect (Mahesh et
al., 2021). The RAW 264.7 murine macrophage cell
line is commonly used as an in vitro inflammatory
model (Widowati et al., 2016; 2021). The
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
60
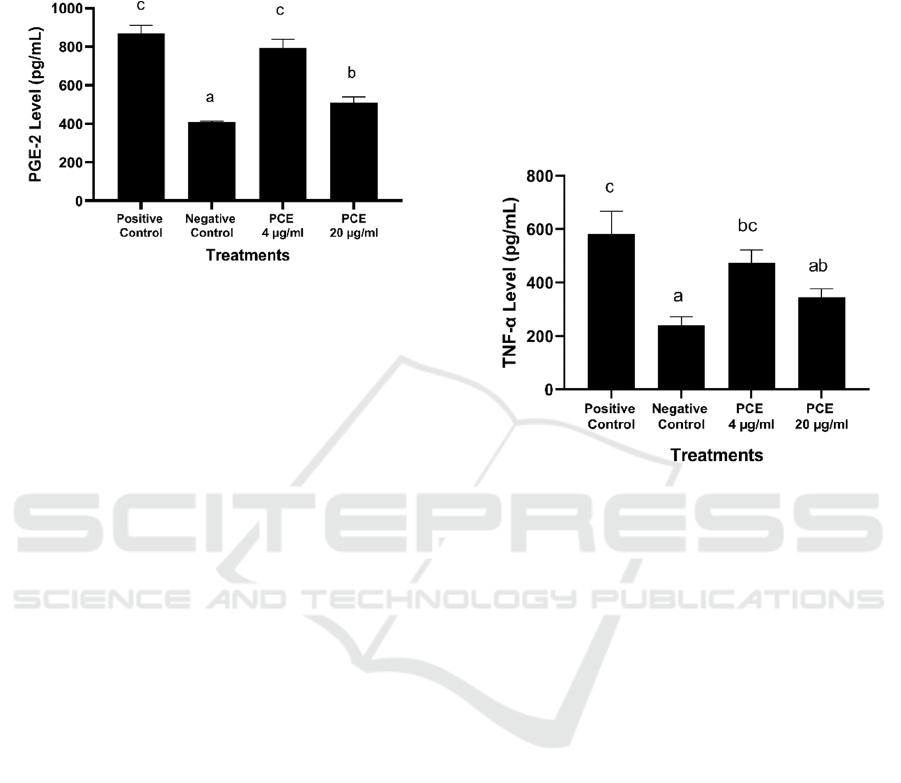
inflammatory response is marked releasing of the
PGE-2 level. The anti-inflammatory activity was
showed by decreasing PGE-2 level in LPS-induced
RAW264.7 cells as inflammatory cells model.
*The data was presented as mean ± standard deviation from 3
replications. Negative control: untreated cell; Positive control:
LPS-induced RAW264.7 cells. Different superscript letters (a, b,
c) showed significant differences among treatments at p <0.05
(Tukey HSD post hoc test).
Figure 1: Effects of PCE toward PGE-2 level in LPS-
induced RAW264.7 cells.
PCE 20 µg/mL has a better ability to suppress
PGE-2 level (507.68 pg/mL) compared to PCE 4
µg/mL (795.37 pg/mL) and positive control (LPS-
induced cells) with value 870.48 ± 39.54 pg/mL
(Figure 1). Our findings provide evidence that PCE
significantly inhibits the production of PGE-2
without affecting the cell viability in LPS-induced
RAW 264.7 cells.
A. comosus leaf extract has an ability to inhibit
PGE2 production in a dose-dependent manner with a
value of 2198.83 ± 280.87 pg/mL in the highest
concentration (500 µg/ml). The inhibition of COX-2
enzyme induction and subsequent inhibition of COX-
2 mRNA expression may be the mechanism behind
some phytoconstituents anti-inflammatory activity
(Yatoo et al., 2018). It has also been discovered that
inhibiting COX-2 development results in a lower level
of PGE (Kasemsuk et al., 2018).
The methanol extract of fruit peel (MEFP) of A.
comosus has anti-inflammatory potential through
decreasing the level of PGE-2, so it has the potential
to protect cartilage from damage caused during
rheumatoid arthritis (Kargutkar and Brijesh, 2016).
The anti-inflammatory effect of bromelain also
was correlated with reduced LPS-induced nuclear
factor-kappaB (NF-κB) activity and cyclooxygenase
2 (COX-2) mRNA expression in rat livers (Kasemsuk
et al., 2018). The roles of bromelain are also well
recognized in activating the healthy immune system
with the rapid response to cellular stress (Rathnavelu
et al., 2016).
3.3 Effect of PCE towards TNF-α
Level in LPS-induced RAW264.7
Cells
TNF-α is one of the most important pro- inflammatory
cytokines and is mainly produced by monocytes and
macrophages. These pro- inflammatory cytokines
influence the proliferation and death of cells and it is
secreted during the early phase of acute and chronic
inflammatory diseases such rheumatoid arthritis
(Wang and He, 2018; Saanin et al., 2020).
*The data was presented as mean ± standard deviation from 3
replications. Negative control: untreated cell; Positive control:
LPS-induced RAW264.7 cells. Different superscript letters (a, ab,
bc, c) showed significant differences among treatments at p <0.05
(Tukey HSD post hoc test).
Figure 2: Effects of PCE toward TNF-α level in LPS-
induced RAW264.7 cells.
Treatment with PCE 20 µg/mL potentially inhibit
TNF-α production (345.90 pg/mL) compared to PCE
4 µg/mL (474.19 pg/mL) and also positive control
with value 581.71 pg/mL (Figure 2). This treatment
indicates that PCE had anti-inflammatory activity.
Based on another study, A. comosus extract has a
significant antioxidant activity, as well as anti-
inflammatory ability. It is thought that molecules with
both an anti-oxidative effect and an anti-
inflammatory effect should be effective in treating
diseases caused by oxidative stress as a result of ROS
generation as well as in inflammatory diseases (Lee
et al., 2018; Vrianty et al., 2019).
Kargutkar and Brijesh’s (2017) study showed that
A. comosus leaf extract (ALE) has anti-inflammatory
activity due to significantly decreasing the release of
TNF-α, IL-1β, PGE-2, and ROS by LPS-stimulated
macrophages in a dose-dependent manner. ALE can
decrease the secretion of TNF-α at a concentration of
500 µg/mL with a maximum reduction of 409.89
pg/mL (Kargutkar and Brijesh, 2017).
Anti-inflammatory Activities of Pineapple (Ananas comosus) Core Extract in Lipopolysaccharide-induced RAW264.7 Cell Line
61

The phytoconstituents prevent inflammation and
apoptosis by inhibiting TNF-α in human
macrophages (Wee et al., 2020). Bromelain in A.
comosus potentially inhibits the pro-inflammatory
mediators productions of NFкB, IL-1β, IL-6, TNF-α,
PGE-2 (Bakare et al., 2021). In another study was
reported that Bromelain can inhibit the secretion of
IL-1, IL-6, and TNF by peripheral blood mononuclear
cells (PBMCs) and modulate surface adhesion
molecules on T cells, macrophages, and natural killer
cells (Pavan et al., 2012). Bromelain inhibits the Raf-
1/extracellular-regulated-kinase- (ERK-) 2 pathways
by inhibiting T cell signaling (Kwatra, 2019).
Bromelain also suppressed the LPS-activated
extracellular signal-regulated kinase (ERK), c-Jun N-
terminal kinase (JNK), and p38 mitogen-activated
protein kinase (MAPK) (Insuan et al., 2021).
However, the anti-inflammatory effects of the
bromelain preparation in vitro and in vivo studies
suggest its therapeutic potentials (Kasemsuk et al.,
2018).
3.4 Effect of PCE towards IL-1β
Level in LPS-induced RAW264.7
Cells
IL-1β is a potent immunomodulator that regulates a
variety of immune and inflammatory responses,
including B and T cells activation (Rapsinski et al.,
2015).
PCE 20 µg/mL decreased the IL-1β level (217.63
pg/mL) compared to PCE 4 µg/mL (350.78 pg/mL)
and also positive control with value 433.53 pg/mL.
PCE has anti-inflammatory properties through
inhibitory of IL-1β level among treatments.
Bromelain is a crude, aqueous extract derived
from the stem and fruit of the pineapple plant, which
contains a variety of proteolytic enzymes and has
anti-inflammatory and analgesic properties (Cai et al.,
2017). Bromelain from A. comosus also has ability to
decreases NO, PGE-2, TNF-α, IL-1β and IL-6
production by downregulation of the NF-κB, AP-1,
and JAK/ STAT signaling pathways in LPS-induced
RAW 264.7 macrophages (Lee et al., 2017;
Kargutkar and Brijesh, 2016).
*The data was presented as mean ± standard deviation from 3
replications. Negative control: untreated cell; Positive control:
LPS-induced cell. Different superscript letters (a, b, c, d) showed
significant differences among treatments at p <0.05 (Tukey HSD
post hoc test).
Figure 3: Effects of PCE toward IL-1β level in LPS-
induced RAW264.7 cells.
Bromelain has the ability to modulate the immune
response to reduce the allergic reaction and to
modulate macrophages, NK cells, and T cells. It also
increases the secretion of IL-1β, IL-6, and TNF- α
(Cai et al., 2017). Flavonoids and tannins compounds
in A. comosus can be attributed in anti- inflammatory
properties (Jiang et al., 2014).
4 CONCLUSIONS
PCE has the potential as an anti-inflammatory by
decreasing PGE2, TNF-α, and IL-1β levels. However,
PCE protects against LPS-induced RAW264.7 cells
via inhibiting oxidative stress and inflammation.
ACKNOWLEDGEMENTS
This research was funded by Aretha Medika Utama
Biomolecular and Biomedical Research Center
(AMU-BBRC), Bandung, Indonesia. The authors
also would like to thank Ervi Afifah and Seila
Arumwardana from AMU-BBRC for their valuable
assistance.
REFERENCES
Bakare, A. O., Owoyele, B. V. (2021). ‘Bromelain reduced
pro-inflammatory mediators as a common pathway that
mediate antinociceptive and anti-anxiety effects in
sciatic nerve ligated Wistar rats’. Sci. Rep. 11(1), 1-13.
Cai, T., Verze, P., La Rocca, R., Palmieri, A., Tiscione, D.,
Luciani, L. G., Mazzoli, V., Malossini, G. (2017). ‘The
clinical efficacy of pollen extract and vitamins on
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
62

chronic prostatitis/chronic pelvic pain syndrome is
linked to a decrease in the pro-inflammatory cytokine
interleukin-8’. World J. Mens Health. 35(2), 120- 128.
Chen, L., Deng, H., Cui, H., Fang, J., Zuo, Z., Deng,
J., Li, Y., Wang, X., Zhao, L. (2018).
‘Inflammatory responses and inflammation- associated
diseases in organs’. Oncotarget, 9(6), 7204.
Insuan, O., Janchai, P., Thongchuai, B., Chaiwongsa, R.,
Khamchun, S., Saoin, S., Insuan, W., Pothacharoen, P.,
Apiwatanapiwat, W., Boondaeng, A., Vaithanomsat, P.
(2021). ‘Anti- Inflammatory effect of pineapple
rhizome bromelain through downregulation of the
NFκB- and MAPKs-signaling pathways in
Lipopolysaccharide (LPS)-stimulated RAW264. 7
cells’. Curr. Issues Mol. Biol. 43(1), 93-106.
Jiang, J., Yuan, X., Wang, T., Chen, H., Zhao, H., Yan,
X., Wang, Z., Sun, X., Zheng, Q. (2014).
‘Antioxidative and cardioprotective effects of total
flavonoids extracted from Dracocephalum moldavica
L. against acute ischemia/reperfusion- induced
myocardial injury in isolated rat heart’. Cardiovasc.
Toxicol. 14(1), 74-82.
Kargutkar, S., Brijesh, S. (2016). ‘Anti-rheumatic activity
of Ananas comosus fruit peel extract in a complete
Freund’s adjuvant rat model’. Pharm. Biol. 54(11),
2616-2622.
Kargutkar, S., Brijesh, S. (2017). ‘Anti-inflammatory
evaluation and characterization of leaf extract of
Ananas comosus’. Inflammopharmacol. 26(2), 469-
477.
Kasemsuk, T., Vivithanaporn, P., Unchern, S. (2018). ‘Anti-
inflammatory effects of bromelain in LPS- induced
human U937 macrophages’. Chiang Mai J. Sci. 45(1),
299-307.
Kwatra, B. (2019). ‘A review on potential properties and
therapeutic applications of bromelain’. World J. Pharm.
Pharm. Sci, 8, 488-500.
Laksmitawati, D. R., Prasanti, A. P., Larasinta, N., Syauta,
G. A., Hilda, R., Ramadaniati, H. U., Widyastuti, A.,
Karami, N., Afni, M., Rihibiha, D. D., Kusuma, H. S.
W., Widowati, W. (2016). ‘Anti-Inflammatory potential
of gandarusa (Gendarussa vulgaris Nees) and soursoup
(Annona muricata L) extracts in LPS stimulated-
macrophage cell (RAW264. 7)’. J. Nat. Remed.
16(2), 73-81.
Laksmitawati, D. R., Widyastuti, A., Karami, N., Afifah,
E., Rihibiha, D. D., Nufus, H., Widowati, W. (2017).
‘Anti-inflammatory effects of Anredera cordifolia and
Piper crocatum extracts on lipopolysaccharide-
stimulated macrophage cell line’. Bangladesh J.
Pharmacol. 12(1), 35- 40.
Lee, J. H., Lee, J. B., Lee, J. T., Park, H. R., Kim, J. B.
(2018). ‘Medicinal effects of bromelain (Ananas
comosus) targeting oral environment as an anti-oxidant
and anti-inflammatory agent’. J. Food Nutr. Res.
6(2018), 773-784.
Lee, S. B., Lee, W. S., Shin, J. S., Jang, D. S., Lee, K. T.
(2017). ‘Xanthotoxin suppresses LPS-induced
expression of iNOS, COX-2, TNF-α, and IL-6 via AP-1,
NF-κB, and JAK-STAT inactivation in RAW 264.7
macrophages’. Int. Immunopharmacol. 49, 21-29.
Mahesh, G., Kumar, K. A., Reddanna, P. (2021). ‘Overview
on the discovery and development of anti-inflammatory
drugs: Should the focus be on synthesis or degradation
of PGE2?’. J. Inflamm. Res. 14, 253-263.
Novilla, A., Djamhuri, D. S., Nurhayati, B., Rihibiha, D. D.,
Afifah, E., Widowati, W. (2017). ‘Anti- inflammatory
properties of oolong tea (Camellia sinensis) ethanol
extract and epigallocatechin gallate in LPS-induced
RAW 264.7 cells’. Asian Pac. J. Trop. Biomed. 7(11),
1005-1009.
Pavan, R., Jain, S., Kumar, A. (2012). ‘Properties and
therapeutic application of bromelain: a review’.
Biotechnol. Res. Int., 1-6.
Rahman, I. A., Mohamed, E., Camalxaman, S. N., Haron,
N., Rambely, A. S. (2020). ‘Ananas comosus (L.)
Merr.: A mini review of its therapeutic properties:
Medicinal benefits of pineapple plant’. Healthscope:
The Official Research Book of Faculty of Health
Sciences, UiTM, 3(2), 54-59.
Ramli, A. N. M., Manas, N. H. A., Hamid, A. A. A., Hamid,
H. A., Illias, R. M. (2018). ‘Comparative structural
analysis of fruit and stem bromelain from Ananas
comosus’. Food Chem. 266, 183-191.
Rathnavelu, V., Alitheen, N. B., Sohila, S., Kanagesan, S.,
Ramesh, R. (2016). ‘Potential role of bromelain in
clinical and therapeutic applications’. Biomed. Rep.
5(3), 283-288.
Rapsinski, G. J., Wynosky-Dolfi, M. A., Oppong, G. O.,
Tursi, S.A., Wilson, R. P., Brodsky, I. E., Tukel, C.
(2015). ‘Toll-like receptor 2 and NLRP3 cooperate to
recognize a functional bacterial amyloid, curli’. Infect.
Immun. 83, 693- 701.
Saanin, S. N., Wahyudianingsih, R., Afni, M., Afifah, E.,
Maesaroh, M., Widowati, W. (2020). ‘Suppression of
pro-inflammatory cytokines and mediators production
by ginger (Zingiber officinale) ethanolic extract and
gingerol in lipopolysaccharide-induced RAW264. 7
murine macrophage cells’. Indian J Nat. Prod.
Resour. 11(4), 260-266.
Sandhiutami, N. M. D., Moordiani, M., Laksmitawati, D.
R., Fauziah, N., Maesaroh, M., Widowati, W. (2017).
‘In vitro assesment of anti- inflammatory activities of
coumarin and Indonesian cassia extract in RAW264. 7
murine macrophage cell line’. Iran. J. Basic. Med.
Sci. 20(1), 99-106.
Vrianty, D., Qodariah, R. L., Widowati, W., Sinaga, A. P.
F., Fibrina, D., Fachrial, E. (2019).‘Comparison of
antioxidant and anti-tyrosinase activities of pineapple
(Ananas comosus) core extract and luteolin compound’.
JKB. 30(4), 240- 246.
Wang, T., He, C. (2018). ‘Pro-inflammatory cytokines:
The link between obesity and
osteoarthritis’. Cytokine Growth F. R., 44, 38-50.
Wee, H. N., Neo, S. Y., Singh, D., Yew, H. C., Qiu,Z. Y.,
Tsai, X. R. C., How, S. Y., Caleb Yip, K.
Y., Tan, C. H.,
Koh, H. L. (2020). ‘Effects of
Vitex
trifolia production in
human U937 macrophages’. BMC Complement. Altern.
Anti-inflammatory Activities of Pineapple (Ananas comosus) Core Extract in Lipopolysaccharide-induced RAW264.7 Cell Line
63

Med. Ther. 20(1), 1-15. L. leaf extracts and
phytoconstituents on cytokine
Widowati, W., Darsono, L., Suherman, J., Fauziah, N.,
Maesaroh, M., Erawijantari, P. P. (2016). ‘Anti-
inflammatory effect of mangosteen (Garcinia
mangostana L.) peel extract and its compounds in LPS-
induced RAW264. 7 cells’. Nat. Prod. Sci. 22(3),
147-153.
Widowati, W., Murti, H., Widyastuti, H., Laksmitawati, D.
R., Rizal, R., Kusuma, H. S. W., Sumitro, S. B., Widodo,
M. A., Bachtiar, I. (2021b). ‘Decreased inhibition of
proliferation and induction of apoptosis in breast cancer
cell lines (T47D and MCF7) from treatment with
conditioned medium derived from hypoxia- treated
Wharton’s jelly MSCs compared with normoxia-
treated MSCs’. Int. J. Hematol. Oncol. Stem Cell Res.
15(2), 77-89.
Widyanto, R. M., Nafi'Halimah, R., Rahmi, Y., Utomo, B.,
Proborini, W. D., Yunimar, Y. (2020). ‘Antioxidant
and cytotoxic effect of water
extract of
Ananas comosus
in human breast cancer
cell line’. J. Islamic Med. 4(2),
123-130.
Yatoo, M., Gopalakrishnan, A., Saxena, A., Parray, O. R.,
Tufani, N. A., Chakraborty, S., Tiwari, R., Dhama, K.,
Iqbal, H. (2018). ‘Anti-inflammatory drugs and herbs
with special emphasis on herbal medicines for
countering inflammatory diseases and disorders-a
review’. Recent. Pat. Inflamm. Allergy Drug. Discov.
12(1), 39-58.
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
64
