
The Effect of Different Intensities of Treadmill Exercise on FGF23
Gene Expression in Gastrocnemius and Soleus Muscles of Wistar
Rats
Julia Windi Gunadi
1,2 a
, Diana Krisanti Jasaputra
2,3 b
, Decky Gunawan
1c
,
Ludovicus Edwinanto
4d
, Limdawati Kwee
5e
, Harijadi Pramono
1f
, Adrian Suhendra
6g
,
Ghita Sariwidyantry
4h
, Hanna Goenawan
7,8 i
and Ronny Lesmana
7,8 j
1
Department of Physiology, Faculty of Medicine, Maranatha Christian University, Jl. Surya Sumantri, Bandung, Indonesia
2
Maranatha Biomedical Research Laboratory, Maranatha Christian University, Jl. Surya Sumantri, Bandung, Indonesia
3
Department of Pharmacology, Faculty of Medicine, Maranatha Christian University, Jl. Surya Sumantri, Bandung,
Indonesia
4
Department of Biochemistry, Faculty of Medicine, Maranatha Christian University, Jl. Surya Sumantri, Bandung,
Indonesia
5
Department of Internal Medicine, Maranatha Christian University, Jl. Surya Sumantri, Bandung, Indonesia
6
Department of Clinical Pathology, Faculty of Medicine, Maranatha Christian University, Jl. Surya Sumantri, Bandung,
Indonesia
7
Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Jl. Raya Jatinangor, Bandung,
Indonesia
8
Division of Biological Activity, Central Laboratory, Universitas Padjadjaran, Jl. Raya Jatinangor, Bandung, Indonesia
limdawati@med.maranatha.edu, dokhar.1001tx@yahoo.co.id, suhendraadriansppk@gmail.com,
ghita.sariwidyantry@med.maranatha.edu, hanna@unpad.ac.id, ronny@unpad.ac.id
Keywords: FGF23, Treadmill Exercise, Gastrocnemius, Soleus.
Abstract: Fibroblast growth factor 23 (FGF23) acts as a hormone that regulates phosphate metabolism associated with
kidney function, and an inducer of left ventricle hypertrophy. But the role of FGF23 as a myokine has not
yet to be confirmed. This research aims to investigate the effect of different intensities of treadmill exercise
on FGF23 gene expression in gastrocnemius and soleus muscles of Wistar rats. Twenty male Wistar rats
were given different intensities of treadmill exercise (low, moderate, and high) for as long as 8 weeks.
FGF23 gene expression in gastrocnemius and soleus muscles was examined using semi-quantitative PCR.
In this study, we obtained no change of relative FGF23 mRNA expression in gastrocnemius muscles (p =
0.684) compared to control. But interestingly, we found a significant increase of relative FGF23 mRNA
expression in soleus muscles (p = 0.030). These results showed that different intensities of treadmill
exercise do not stimulate FGF23 gene expression in gastrocnemius muscles of Wistar rats. While the low
intensity of treadmill exercise does not increase FGF23 relative mRNA expression, moderate and high
intensities of treadmill exercise increase FGF23 gene expression in the Wistar rat’s soleus muscles.
a
https://orcid.org/0000-0003-3645-7486
b
https://orcid.org/0000-0001-5608-6112
c
https://orcid.org/0000-0001-6294-4590
d
https://orcid.org/0000-0002-7768-4047
e
https://orcid.org/0000-0001-7067-3567
f
https://orcid.org/0000-0002-2207-7143
g
https://orcid.org/0000-0003-4873-1673
h
https://orcid.org/0000-0003-1051-9773
i
https://orcid.org/0000-0002-1607-4843
j
https://orcid.org/0000-0002-7425-915X
Gunadi, J., Jasaputra, D., Gunawan, D., Edwinanto, L., Kwee, L., Pramono, H., Suhendra, A., Sariwidyantry, G., Goenawan, H. and Lesmana, R.
The Effect of Different Intensities of Treadmill Exercise on FGF23 Gene Expression in Gastrocnemius and Soleus Muscles of Wistar Rats.
DOI: 10.5220/0010744600003113
In Proceedings of the 1st International Conference on Emerging Issues in Technology, Engineering and Science (ICE-TES 2021), pages 81-86
ISBN: 978-989-758-601-9
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
81

1 INTRODUCTION
Fibroblast Growth Factor (FGF) could be divided
into three categories: intracrine, paracrine, and
endocrine (Kyrou, Weickert, Gharanei, Randeva, &
Tan, 2017; Ornitz & Itoh, 2015). The FGF family
consisted of 22 families, with FGF15/19 is being
ortholog in rodents and humans, while FGF23 is
included as an endocrine FGF (Ho & Bergwitz,
2021; Ornitz & Itoh, 2015). As a hormone produced
in osteoblasts and osteocytes, FGF23 is circulated
into many organs such as the kidney, heart, and
skeletal muscles (Faul et al., 2011; López-Otín,
Blasco, Partridge, Serrano, & Kroemer, 2013;
Vervloet, 2019). The action of FGF23 is mediated
by Fibroblast Growth Factor Receptor (FGFR)
together with the cofactor Klotho (Ornitz & Itoh,
2015).
FGF23 is an osteokine (a hormone produced in
bone), with the kidney as its main target, where it
inhibits calcitriol formation by suppressing 25-
hydroxyvitamin D-1α-hydroxylase (Bacchetta et al.,
2013; Ewendt, Feger, & Föller, 2021). Recent
researches have shown that FGF23 is also produced
by cardiomyocytes (Leifheit-Nestler & Haffner,
2018) and induces left ventricular hypertrophy (Faul
et al., 2011). These proofs ensuring the role of
FGF23 in the crosstalk between bone and muscle
(Ewendt et al., 2021; Lara-Castillo & Johnson,
2020). But it is still unclear whether FGF23 could
directly alter skeletal muscle function. A study by
Avin et al proved that FGF23 is indirectly
influenced the proliferation and differentiation of
skeletal muscle cells in an ex vivo experiment (Avin
et al., 2018).
Many factors might alter molecular mechanisms
in skeletal muscle, including the physiological
adaptation process to different intensities of exercise
(MacInnis & Gibala, 2017). Exercise has been well
known as a way to reduce chronic disease risk
(Anderson & Durstine, 2019). American College of
Sports Medicine (ACSM) recommended light to
moderate exercise that may progress gradually to
vigorous exercise, 30 minutes/day and for a
minimum of 3 days a week, for people without
cardiovascular, metabolic, or renal disease (Riebe et
al., 2015). Exercise induces numerous substances
secretion, known as myokines which conduce
positive effects to prevent metabolic disease and
sarcopenia (Son, Chae, Testroet, Du, & Jun, 2018).
It is still unclear whether FGF23 is a myokine or
just an osteokine induced by the change in
phosphate concentration (Lara-Castillo & Johnson,
2020; Takashi & Fukumoto, 2020). Some studies
found increased serum levels of FGF23 after
exercise in humans (Emrich, Dederer, et al., 2019;
Kerschan-Schindl et al., 2021; G Lombardi et al.,
2014). While in mice, a previous study has shown
that 1 week of treadmill exercise upregulated FGF23
in blood, mRNA expression, and mitochondrial
function in skeletal muscle (Li, Fu, Zhao, Ni, &
Shen, 2016). Therefore, in this study, we aim to
investigate the effect of different intensities of
treadmill exercise on gene expression of FGF23 in
the Wistar rat’s skeletal muscles (gastrocnemius and
soleus).
2 METHODS (AND MATERIALS)
2.1 Experimental Animals
We obtained twenty male rats, Wistar strain, aged
about 6-8 weeks, weight about 200-220 grams from
PT Biofarma, Bandung, Indonesia. Rats were
divided into four groups (N=5 for each group) and
put in a cage per group under constant photoperiod
(light/dark cycle every 12 hours), temperature
between 22-24°, and relative humidity. Standard
chow diet (47.3% carbohydrate, 4% fat, 20%
protein, 12% water, 4% fiber, 12% calcium, and
0/7% phosphorus) and water were provided ad
libitum. We experimented on animals based on the
use and care of laboratory animal guidelines
(Committee for the Update of the Guide for the Care
and Use of Laboratory Animals, Institute for
Laboratory Animal Research, Division on Earth and
Life Studies, 2011). The rats were treated following
an approved code of ethics from the Committee of
the Faculty of Medicine, Maranatha Christian
University- Immanuel Hospital Bandung Number
093/KEP/VI/2020.
2.2 Different Intensities of Treadmill
Exercise
After two weeks of the adaptation period, the rats
were trained to run on a treadmill with a gradual
increase of speed and time for another two weeks for
the initial adaptation. The protocol of treadmill
exercise intensities was modified from the previous
protocol, with lactate threshold as the basis for
defining the intensities. The sub-lactate threshold is
categorized as low intensity (10 meters per minute),
lactate threshold as moderate intensity (20 meters
per minute), and supra-lactate threshold as high
intensity (30 meters per minute) (Lesmana et al.,
2016). The treadmill exercise was conducted 5 times
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
82

a week, from Monday to Friday, 30 minutes per day,
for 8 consecutive weeks. The sedentary group was
used as a control group. At the end of the
experiment, all the animals were sacrificed under
inhalation anesthesia (5% isoflurane). We separated
skeletal muscle tissues (gastrocnemius and soleus
muscles), froze them at -80°C for RNA extraction.
2.3 RNA Extraction, Semi-quantitative
PCR
RNA Trisure isolation reagent (Bioline, BIO-38032,
London, UK) was used according to the protocol of
RNA extraction from the manufacturer. After the
extraction, RNA concentration and purity were
quantified by measuring its absorbance in 260/280
nm (Tecan Infinite M200 Pro, No. 30050303,
Switzerland). For the analysis of FGF23 and
GAPDH gene expression, semi-quantitative PCR
was conducted using a One-Step RT PCR Kit
(Bioline, BIO-65409, London, UK). The process
then continued with electrophoresis (Mupid Exu
Submarine Electrophoresis System, Mupid Exu,
Japan), and the result is visualized using Blupad
Dual LED Blue/White Light Transilluminator (Bio-
Helix, GeneDirex BP001CU, Taiwan).
Quantification of the band was conducted using
Image J. This procedure was adapted from the
previous study (V. M. Tarawan et al., 2019). The
primer sequences of FGF23 and GAPDH have been
summarized in Table 1 (V. Tarawan, Gunadi,
Subekti, Widowati, & Goenawan, 2019; Wang et al.,
2017).
Table 1: Primer Sequence Used in This Study.
Gene
Primer Sequence
Upper strand: sense
Lower strand: antisense
Product
Size (bp)
FGF23
CCTTCCTCTGCACTCGGTAG
301
TGCCAGCTGCCAAGACGGTG
GAPDH
GTTACCAGGGCTGCCTTCTC
GATGGTGATGGGTTTCCCGT 177
2.4 Statistical Analysis
Data are expressed as mean ± SEM. Differences
between groups were evaluated by Analysis of
Variance (ANOVA), followed by an LSD post hoc
test. Statistical significance was set at p<0.05.
3 RESULTS AND DISCUSSION
As a result of this study, we found no difference in
relative ratio of gastrocnemius’s FGF23 mRNA
expression in different intensities of treadmill
exercise were: 0.628 ± 0.05 (low); 0.593 ± 0.03
(moderate); 0.647 ± 0.02 (high); compared to 0.602
± 0.04 (control). This result is presented in figure 1.
Figure 1: Relative Ratio of FGF23 mRNA Expression in
Gastrocnemius Muscle of Wistar Rats After 8 weeks of
Treadmill Exercise with Different Intensities. (Control =
Sedentary, Low =low intensity (10 m/minute), Mod =
moderate intensity (20 m/minute), High = high intensity
(30 m/minute).
We found a significant increase of FGF23
mRNA expression in soleus muscles of Wistar rats
(p = 0.030). Relative ratio of soleus’s FGF23 mRNA
expression in different intensities of treadmill
exercise were: 0.583 ± 0.06 (low); 0.624 ± 0.03
(moderate); 0.648 ± 0.05 (high); compared to 0.552
± 0.02 (control). This result is presented in figure 2.
In this study, we found no difference of FGF23
in gastrocnemius muscle of Wistar rats after 8 weeks
of treadmill exercise with different intensities. But
interestingly, we found a significant increase of
FGF23 gene expression in soleus muscles of Wistar
rats after 8 weeks of treadmill exercise with
moderate and high intensities, while low intensity
did not change FGF23 gene expression compared to
control.
Recent research has proven that the effect of
FGF23 on skeletal muscles was mediated by other
endogenous substances that might act in concert
with FGF23 (Avin et al., 2018). Our result (figure 2)
suggested the increase of FGF23 in soleus muscles
might be associated with exercise alteration of
skeletal muscle metabolism, particularly with
parathyroid hormone (PTH). PTH and FGF23 are
modulating each other’s secretion, FGF23 decreases
The Effect of Different Intensities of Treadmill Exercise on FGF23 Gene Expression in Gastrocnemius and Soleus Muscles of Wistar Rats
83
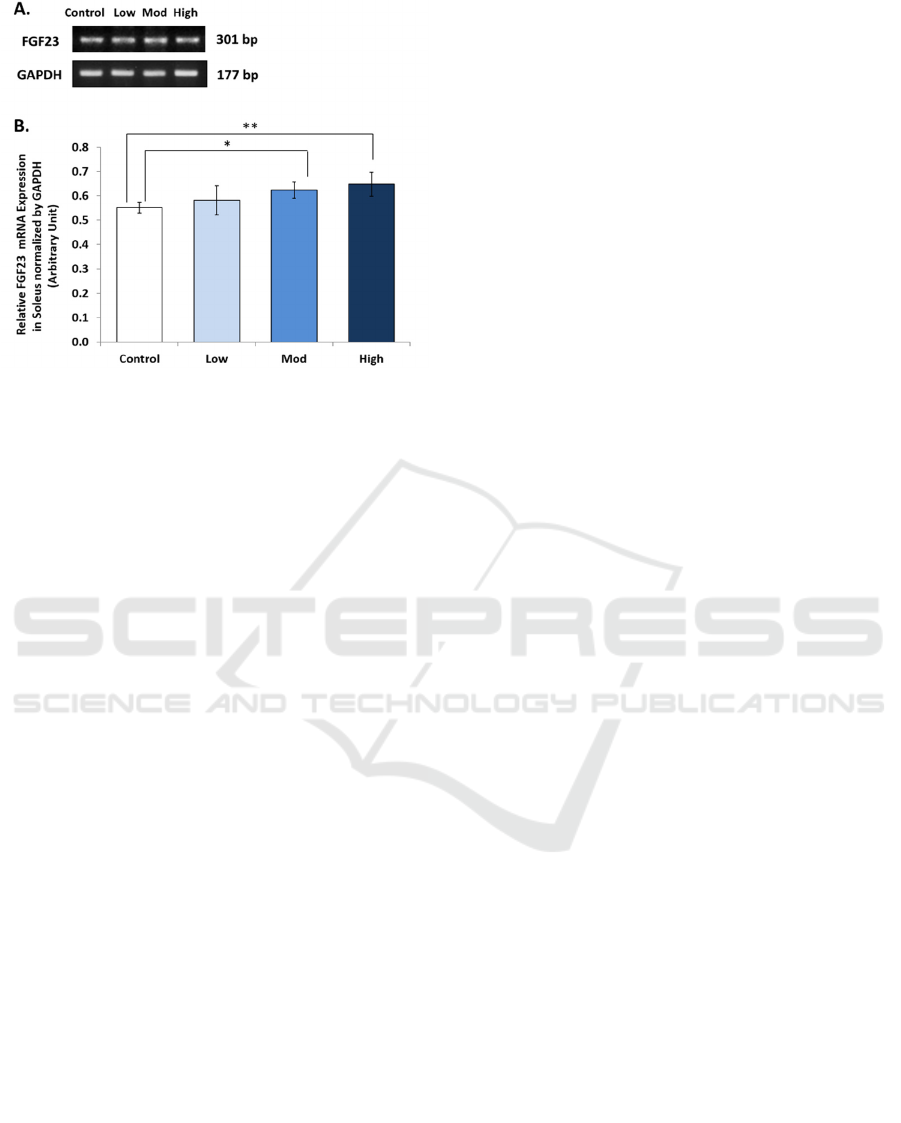
Figure 2: Relative Ratio of FGF23 mRNA Expression in
Soleus Muscle of Wistar Rats After 8 weeks of Treadmill
Exercise with Different Intensities. (Control = Sedentary,
Low =low intensity (10 m/minute), Mod = moderate
intensity (20 m/minute), High = high intensity (30
m/minute). * = significant (p<0.05), ** = very significant
(p<0.01).
PTH secretion, and PTH increases FGF23 secretion
(Giovanni Lombardi, Ziemann, Banfi, & Corbetta,
2020; Peacock, 2021). PTH responds to acute and
chronic exercise, and its response might be
influenced by some factors, such as VO
2
max,
vitamin D status, and hormonal status (Giovanni
Lombardi et al., 2020). A study by Gardinier et al
proved that PTH has a role in bone adaptation to
exercise, and they found an increase of FGF23
mRNA expression in the tibia after 6 days of
treadmill training (Gardinier, Al-Omaishi, Morris, &
Kohn, 2016). But the crosstalk between PTH and
FGF23 in bone and skeletal muscles needs further
investigation. Effects exerted by PTH in muscle
cells might be secondary to the effects on other
tissue. (Lombardi, Ziemann, Banfi, & Corbetta,
2020).
The different results between gastrocnemius
(figure 1) and soleus muscles (figure 2) might be
associated with different properties of their
mitochondria. Gastrocnemius and soleus muscles are
two muscles that differ in their fiber types, while
gastrocnemius muscle is fast type muscle fiber, the
soleus muscle is slow type muscle fiber (Qaisar,
Bhaskaran, & Van Remmen, 2016). Soleus muscles
have a slow contraction speed and predominantly
use oxidative metabolism for energy production,
while gastrocnemius is fast-twitch and uses
glycolytic metabolism (Qaisar et al., 2016).
Therefore, soleus muscles have more and bigger
mitochondria than gastrocnemius muscles (Sanchez,
Li, Bragos, & Rutkove, 2014).
Different properties of gastrocnemius and soleus
muscles could explain the different results of this
study. A previous study concluded that exercise
promotes FGF23 mRNA expression in skeletal
muscle tissue by controlling mitochondrial function
(Li et al., 2016). This is consistent with other studies
that stated endurance training improved the function
of muscle mitochondria which is essential for the
homeostasis of energy in skeletal muscles (Zoladz,
Koziel, Woyda-Ploszczyca, Celichowski, &
Jarmuszkiewicz, 2016). These findings might
explain why the increase of FGF23 was only found
in soleus muscles (figure 2), but not in
gastrocnemius muscles (figure 1).
The effect of FGF23 after exercise is still under
debate because some studies have shown different
results. Some studies claimed that FGF23 increased
after exercise (Emrich, Dederer, et al., 2019;
Kerschan-Schindl et al., 2021; Li et al., 2016; G
Lombardi et al., 2014), while other studies found no
change/decrease ((Buskermolen et al., 2019; Emrich,
Baier, et al., 2019; Keshavarzi, Daryanoosh,
Kooshki Jahromi, & Mohammadi, 2017; Neves et
al., 2021). Different results of those studies might be
affected by intensities, duration, and type of
exercise, high altitude, and phosphate intake. There
is a possibility that FGF23 would increase more
after a long duration, high intensity, over strenuous
exercise, and high phosphorus diet. The limitation of
this study is we have no data regarding phosphate
and PTH concentration which might be correlated
with the increase of FGF23 in soleus muscles. For a
better understanding of the FGF23 mechanism in the
adaptation of skeletal muscles to exercise, we
recommend a more detailed study, especially by
investigating the crosstalk between FGF23 and PTH,
the function of skeletal muscles mitochondria after
exercise, and longer duration of exercise.
4 CONCLUSIONS
In summary, FGF23 gene expression in
gastrocnemius muscles is not upregulated after 8
weeks of treadmill exercise with different intensities
but upregulated in soleus muscles after 8 weeks of
treadmill exercise with moderate and high
intensities. But the role of FGF23 in skeletal muscle
after exercise still needs further investigation.
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
84

ACKNOWLEDGEMENTS
We would like to thank Susianti, Nurul Ihsani,
Meita, Lydia for the technical assistance for
laboratory experiments. And we also would like to
thank dr Yuni, dr. Nova, dr. Teresa, dr. Cherry for
their assistance in studying the Wistar rats.
FUNDING
This study was funded by Universitas Kristen
Maranatha under Hibah Internal Skema Tambahan
(034/SK/ADD/UKM/VI/2021) to JWG, DKJ, DG,
LE, LK, HP, AS, and GS.
REFERENCES
Anderson, E., & Durstine, J. L. (2019). Physical activity,
exercise, and chronic diseases: A brief review. Sports
Medicine and Health Science, 1(1), 3–10.
https://doi.org/https://doi.org/10.1016/j.smhs.2019.08.
006
Avin, K. G., Vallejo, J. A., Chen, N. X., Wang, K.,
Touchberry, C. D., Brotto, M., … Wacker, M. J.
(2018). Fibroblast growth factor 23 does not directly
influence skeletal muscle cell proliferation and
differentiation or ex vivo muscle contractility.
American Journal of Physiology. Endocrinology and
Metabolism, 315(4), E594–E604.
https://doi.org/10.1152/ajpendo.00343.2017
Bacchetta, J., Sea, J. L., Chun, R. F., Lisse, T. S.,
Wesseling-Perry, K., Gales, B., … Hewison, M.
(2013). Fibroblast growth factor 23 inhibits extrarenal
synthesis of 1,25-dihydroxyvitamin D in human
monocytes. Journal of Bone and Mineral Research :
The Official Journal of the American Society for Bone
and Mineral Research, 28(1), 46–55.
https://doi.org/10.1002/jbmr.1740
Buskermolen, J., van der Meijden, K., Furrer, R., Mons,
D.-J., van Essen, H. W., Heijboer, A. C., …
Bravenboer, N. (2019). Effects of different training
modalities on phosphate homeostasis and local
vitamin D metabolism in rat bone. PeerJ, 7, e6184.
https://doi.org/10.7717/peerj.6184
Committee for the Update of the Guide for the Care and
Use of Laboratory Animals, Institute for Laboratory
Animal Research, Division on Earth and Life Studies,
& N. R. C. (2011). Guide for the care and use of
laboratory animals (8th ed.). Washington (DC).
https://doi.org/10.17226/12910
Emrich, I. E., Baier, M., Zawada, A. M., Meyer, T., Fliser,
D., Scharhag, J., & Heine, G. H. (2019, March).
Plasma FGF23 does not rise during physical exercise
as a physiological model of sympathetic activation.
Clinical Research in Cardiology : Official Journal of
the German Cardiac Society, Vol. 108, pp. 341–343.
Germany. https://doi.org/10.1007/s00392-018-1347-7
Emrich, I. E., Dederer, J., Kircher, A., Klemis, V.,
Lennartz, C. S., Untersteller, K., … Heine, G. H.
(2019). Does a rise in plasma erythropoietin after
high-altitude exposure affect FGF23 in healthy
volunteers on a normal or low-phosphorus diet?
Nutrition, Metabolism, and Cardiovascular Diseases :
NMCD, 29(12), 1361–1367.
https://doi.org/10.1016/j.numecd.2019.09.002
Ewendt, F., Feger, M., & Föller, M. (2021). Myostatin
regulates the production of fibroblast growth factor 23
(FGF23) in UMR106 osteoblast-like cells. Pflugers
Archiv : European Journal of Physiology.
https://doi.org/10.1007/s00424-021-02561-y
Faul, C., Amaral, A. P., Oskouei, B., Hu, M.-C., Sloan, A.,
Isakova, T., … Wolf, M. (2011). FGF23 induces left
ventricular hypertrophy. The Journal of Clinical
Investigation, 121(11), 4393–4408.
https://doi.org/10.1172/JCI46122
Gardinier, J. D., Al-Omaishi, S., Morris, M. D., & Kohn,
D. H. (2016). PTH signaling mediates perilacunar
remodeling during exercise. Matrix Biology : Journal
of the International Society for Matrix Biology, 52–54,
162–175. https://doi.org/10.1016/j.matbio.2016.02.010
Ho, B. B., & Bergwitz, C. (2021). FGF23 signalling and
physiology. Journal of Molecular Endocrinology,
66(2), R23–R32. https://doi.org/10.1530/JME-20-0178
Kerschan-Schindl, K., Skenderi, K., Wahl-Figlash, K.,
Gelles, K., Föger-Samwald, U., Thalmann, M., …
Pietschmann, P. (2021). Increased serum levels of
fibroblast growth factor 23 after an ultradistance run.
Journal of Science and Medicine in Sport, 24(3), 297–
300. https://doi.org/10.1016/j.jsams.2020.09.010
Keshavarzi, Z., Daryanoosh, F., Kooshki Jahromi, M., &
Mohammadi, M. (2017). The effect of 12 weeks of
aerobic exercise on plasma levels of fibroblast growth
factor 23, Angiotensin converting enzyme and left
ventricular hypertrophy in hypertensive elderly
women TT - ﯽﺳﺭﺮﺑ ﺮﻴﺛﺄﺗ 12 ﻪﺘﻔﻫ ﺖﻴﻟﺎﻌﻓ ﯽﺷﺯﺭﻭ ﺮﺑ ﺡﻮﻄﺳ
ﯽﻣﺮﺳ ﺭﻮﺘﮐﺎﻓ ﺪﺷﺭ ﻞﺑﻭﺮﺒﻴﻓ . SSU_Journals, 25(3), 222–229.
Retrieved from http://jssu.ssu.ac.ir/article-1-3998-
en.html
Kyrou, I., Weickert, M. O., Gharanei, S., Randeva, H. S.,
& Tan, B. K. (2017). Fibroblast growth factors: new
insights, new targets in the management of diabetes.
Minerva Endocrinologica, 42(3), 248–270.
https://doi.org/10.23736/S0391-1977.16.02536-0
Lara-Castillo, N., & Johnson, M. L. (2020). Bone-Muscle
Mutual Interactions. Current Osteoporosis Reports,
18(4), 408–421. https://doi.org/10.1007/s11914-020-
00602-6
Leifheit-Nestler, M., & Haffner, D. (2018). Paracrine
Effects of FGF23 on the Heart. Frontiers in
Endocrinology, 9, 278.
https://doi.org/10.3389/fendo.2018.00278
Lesmana, R., Iwasaki, T., Iizuka, Y., Amano, I.,
Shimokawa, N., & Koibuchi, N. (2016). The change in
thyroid hormone signaling by altered training intensity
The Effect of Different Intensities of Treadmill Exercise on FGF23 Gene Expression in Gastrocnemius and Soleus Muscles of Wistar Rats
85

in male rat skeletal muscle. Endocrine Journal, 63(8),
727–738. https://doi.org/10.1507/endocrj.EJ16-0126
Li, D. J., Fu, H., Zhao, T., Ni, M., & Shen, F. M. (2016).
Exercise-stimulated FGF23 promotes exercise
performance via controlling the excess reactive
oxygen species production and enhancing
mitochondrial function in skeletal muscle.
Metabolism: Clinical and Experimental, 65(5), 747–
756. https://doi.org/10.1016/j.metabol.2016.02.009
Lombardi, G, Corsetti, R., Lanteri, P., Grasso, D.,
Vianello, E., Marazzi, M. G., … Banfi, G. (2014).
Reciprocal regulation of calcium-/phosphate-
regulating hormones in cyclists during the Giro
d’Italia 3-week stage race. Scandinavian Journal of
Medicine & Science in Sports, 24(5), 779–787.
https://doi.org/10.1111/sms.12080
Lombardi, Giovanni, Ziemann, E., Banfi, G., & Corbetta,
S. (2020). Physical Activity-Dependent Regulation of
Parathyroid Hormone and Calcium-Phosphorous
Metabolism. International Journal of Molecular
Sciences, 21(15).
https://doi.org/10.3390/ijms21155388
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M.,
& Kroemer, G. (2013). The hallmarks of aging. Cell,
153(6), 1194.
https://doi.org/10.1016/j.cell.2013.05.039
MacInnis, M. J., & Gibala, M. J. (2017). Physiological
adaptations to interval training and the role of exercise
intensity. The Journal of Physiology, 595(9), 2915–
2930. https://doi.org/10.1113/JP273196
Neves, R. V. P., Corrêa, H. L., Deus, L. A., Reis, A. L.,
Souza, M. K., Simões, H. G., … Rosa, T. S. (2021).
Dynamic not isometric training blunts osteo-renal
disease and improves the sclerostin/FGF23/Klotho
axis in maintenance hemodialysis patients: a
randomized clinical trial. Journal of Applied
Physiology (Bethesda, Md. : 1985), 130(2), 508–516.
https://doi.org/10.1152/japplphysiol.00416.2020
Ornitz, D. M., & Itoh, N. (2015). The fibroblast growth
factor signaling pathway. Wiley Interdisciplinary
Reviews: Developmental Biology, 4(3), 215–266.
https://doi.org/10.1002/wdev.176
Peacock, M. (2021). Phosphate Metabolism in Health and
Disease. Calcified Tissue International, 108(1), 3–15.
https://doi.org/10.1007/s00223-020-00686-3
Qaisar, R., Bhaskaran, S., & Van Remmen, H. (2016).
Muscle fiber type diversification during exercise and
regeneration. Free Radical Biology & Medicine, 98,
56–67.
https://doi.org/10.1016/j.freeradbiomed.2016.03.025
Riebe, D., Franklin, B. A., Thompson, P. D., Garber, C.
E., Whitfield, G. P., Magal, M., & Pescatello, L. S.
(2015). Updating ACSM’s Recommendations for
Exercise Preparticipation Health Screening. Medicine
and Science in Sports and Exercise, 47
(11), 2473–
2479.
https://doi.org/10.1249/MSS.0000000000000664
Sanchez, B., Li, J., Bragos, R., & Rutkove, S. B. (2014).
Differentiation of the intracellular structure of slow-
versus fast-twitch muscle fibers through evaluation of
the dielectric properties of tissue. Physics in Medicine
and Biology, 59(10), 2369–2380.
https://doi.org/10.1088/0031-9155/59/10/2369
Son, J. S., Chae, S. A., Testroet, E. D., Du, M., & Jun, H.-
P. (2018). Exercise-induced myokines: a brief review
of controversial issues of this decade. Expert Review
of Endocrinology & Metabolism, 13(1), 51–58.
https://doi.org/10.1080/17446651.2018.1416290
Takashi, Y., & Fukumoto, S. (2020). Phosphate-sensing
and regulatory mechanism of FGF23 production.
Journal of Endocrinological Investigation, 43(7), 877–
883. https://doi.org/10.1007/s40618-020-01205-9
Tarawan, V., Gunadi, J., Subekti, T., Widowati, W., &
Goenawan, H. (2019). Effect of Acute Physical
Exercise with Moderate Intensities on FGF23 Gene
Expression in Wistar Rat Heart. Majalah Kedokteran
Bandung, 51, 221–225.
https://doi.org/10.15395/mkb.v51n4.1844
Tarawan, V. M., Gunadi, J. W., Setiawan, Lesmana, R.,
Goenawan, H., Meilina, D. E., … Supratman, U.
(2019). Alteration of Autophagy Gene Expression by
Different Intensity of Exercise in Gastrocnemius and
Soleus Muscles of Wistar Rats. Journal of Sports
Science & Medicine, 18(1), 146–154.
Vervloet, M. (2019). Renal and extrarenal effects of
fibroblast growth factor 23. Nature Reviews.
Nephrology, 15(2), 109–120.
https://doi.org/10.1038/s41581-018-0087-2
Wang, K., Wang, F., Bao, J.-P., Xie, Z.-Y., Chen, L.,
Zhou, B.-Y., … Wu, X.-T. (2017). Tumor necrosis
factor α modulates sodium-activated potassium
channel SLICK in rat dorsal horn neurons via p38
MAPK activation pathway. Journal of Pain Research,
10, 1265–1271. https://doi.org/10.2147/JPR.S132185
Zoladz, J. A., Koziel, A., Woyda-Ploszczyca, A.,
Celichowski, J., & Jarmuszkiewicz, W. (2016).
Endurance training increases the efficiency of rat
skeletal muscle mitochondria. Pflügers Archiv -
European Journal of Physiology, 468(10), 1709–1724.
https://doi.org/10.1007/s00424-016-1867-9
ICE-TES 2021 - International Conference on Emerging Issues in Technology, Engineering, and Science
86
