
Activity Test of Temperature Variations in Cracking Process of Palm
Oil using Ni/Al2o3 to Green Diesel Viscosity
Sofiyan Adi Putra, Tiara Indah Damayanti, Faaiz Al Ghifari and Haris Puspito Buwono
Mechanical Engineering Department, State Polytechnic of Malang, Jl. Soekarno-Hatta No. 9, Malang City, Indonesia
haris.puspito@polinema.ac.id
Keywords: Green Diesel, Cracking Process, Vegetable Oil.
Abstract: Today petroleum reserves are running low along with the increasing demand of fuel transportation and
industries, therefore we are required to find alternative fuels, one of which is derived from vegetable oil,
namely Green Diesel. Green diesel is a product mixture of hydrocarbons such as diesel which is produced
through a cracking process using catalyst as reaction accelerator. The catalyst is Ni/Al
2
O
3
. Aimed to
understand the effect of temperature variations in palm oil cracking process using Ni/Al
2
O
3
catalyst to the
product viscosity. The cracking process is carried out using a batch reactor with temperature variations of
400
o
C and 460
o
C, for 10 minutes calculated from the target temperature achieved which is 400
o
C. The highest
amount of product acquired from 400
o
C which is 20.845 gram from the total of 30 grams raw material, the
product of 400
o
C is the only suitable density 0.8523 g/ml which is inside the range of green diesel standard
0.8150-0.8600 g/ml, and while the product of 400
o
C or 460
o
C does not have suitable viscosity 6,4563 cSt and
1,4305 cSt which are not inside the range of green diesel standard 2-4.5 cSt.
1
INTRODUCTION
Currently, the world's oil reserves are running low, at
the same time the human population continues to
grow and it is directly proportional to the increasing
demand for basic needs, one of which is the need for
motor vehicle fuel.
Currently, the majority of motor vehicle fuels use
petroleum derived from fossils such as pertalite,
diesel, and avtur. Petroleum is the result of the
evolution of fossils resulting from the extinction of
living things millions of years ago on earth and has
undergone a very long process to become crude oil
that is ready to be processed into fuel.
With various countries trying to turn into
industrial countries forcing fuel consumption to
increase from year to year, the average annual
increase in energy demand is 36 million barrels of oil
equivalent (BOE) from 2000 to 2014. Meanwhile,
energy reserves are not renewable energy sources,
such as oil, natural gas, and coal, are running low.
Based on the Strategic Plan (Renstra) of the Ministry
of Energy and Mineral Resources for 2015–2019,
Indonesia's oil reserves of 3.6 billion barrels are
estimated to be exhausted in the next 13 years.
(Sa'adah, et al, 2017).
Therefore, renewable fuels are required from
sources other than fossils, one of the product of
renewable source is Green diesel is a mixture of diesel
like hydrocarbons produced via catalytic reaction
involving hydroprocessing, decarbonylation, and
decarboxylation of trilicerides. (Auliastuti, et al,
2020)
Biofuel has a better characteristic than biodiesel
such as lower viscosity, better stability and produce
more energy. (Munir and Chumaidi 2019)
Green diesel does not produce any waste, very
efficient process, and all ready product can be use
directly after being produced. (Ristanti, et al, 2021)
Green diesel is produced using the
hydrodeoxygenation process of palm oil
(triglycerides) or animal fats through catalytic
treatment with hydrogen, produced a mixture of
straight and branched chain saturated hydrocarbons
which usually contain 15 to 18 carbon atoms per
molecule (C15 to C18) (Neunofa et al. 2017)
By using Ni/Al
2
O
3
catalyst as a reaction
accelerator with hydrotreating process with variations
in reaction temperature this research aimed to
determine the most suitable temperature that
produce a product with viscosity match with the
Green Diesel standard.
Putra, S., Damayanti, T., Al Ghifari, F. and Buwono, H.
Activity Test of Temperature Variations in Cracking Process of Palm Oil using Ni/Al2o3 to Green Diesel Viscosity.
DOI: 10.5220/0010940100003260
In Proceedings of the 4th International Conference on Applied Science and Technology on Engineering Science (iCAST-ES 2021), pages 65-68
ISBN: 978-989-758-615-6; ISSN: 2975-8246
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
65

2
RESEARCH METHODOLOGY
There are five steps to produce green diesel using
batch reactor, starting
with
the Al
2
O
3
Catalyst
Preparation, the Preparation of Active Metal
Ni,Catalyst Activation, Catalytic Cracking Process,
and Viscosity Measurement.
The preparation of Al
2
O
3
catalyst begins with
mixing 470
grams of Al2(SO4)3 and 400 ml of
distilled water at a temperature of 70oC and dripping
with NH3 to pH 9, stirred until it becomes a gel and
filtered using filter paper, the filtered gel must be
dried at a temperature of 70oC for 24 hours until it
can be mashed with a mortar, after becoming a dry
powder it is calcined at a temperature of 600oC with
holding time of 3 hours and then removed when the
furnace temperature has dropped to 70oC.
The preparation for the active metal Ni begins by
lowering the commercial Ni concentration from
100% to 19% by dissolving 20 grams of Ni with 20
ml of distilled water. Then prepared 5 grams of
Al2O3 which has been calcined in a 600oC furnace
then dissolve it with 5 ml of distilled water and
dripped with 19% Ni with the desired amount of the
variable with M impregnated 2.5859 grams which is
assumed to be 10% Ni concentration if required 5%
of Ni then the M impregnated would be 1.2929
grams, dripping the Ni while stirring the catalyst
using a magnetic stirrer, then waiting for it to dry for
approximately 3 hours and mash it in a mortar and
then calcined in a furnace with a temperature of
400
o
C and holding time for 2 hours.
The calculation of M impregnated is carried out
using the following formula:
𝑀
𝑖
𝑚
𝑝
𝑟
𝑒
𝑔
𝑛
𝑎
𝑡
𝑒
𝑑
(%)=
𝑀
𝑀
𝐷
𝑒𝑠
𝑐𝑟
𝑖
𝑝
𝑡𝑖𝑜
𝑛
𝑠
∶
𝑀
= 𝑐𝑜𝑛𝑐𝑒𝑛𝑡𝑟𝑎𝑡𝑖𝑜𝑛 (%)
𝑚
=
𝑚𝑎𝑠𝑠
(
𝑔𝑟𝑎𝑚
)
𝑀
𝑖
𝑚
𝑝
𝑟
𝑒
𝑔
𝑛
𝑎
𝑡
𝑒
𝑑
=
10%
𝑀
𝑖
𝑛
𝑖
𝑡
𝑖
𝑎
𝑙
=
19%
𝑚
𝑏𝑢𝑓𝑓𝑒𝑟
= 5 𝑔𝑟𝑎𝑚
After being calcined, the catalyst powder is
formed into solid pills using a small pipe mold with
the help of a press machine, the pressure used in the
press machine is 100 kg/cm2 for a minute.
The catalyst is activated by putting it into the
reactor, then close it tightly using a wrench, then
attach helium gas hose, this process is carried out to
ensure that the oxygen in the reactor has been
removed and replaced by helium, with the method of
inputting 10 bar of helium gas into the reactor and
then slowly discharge it at a rate of 20 ml/min
repeated twice. After the drain has been completed,
input 1 atm of hydrogen gas and heated to a
temperature of 200
o
C for an hour, then the gas is
discharged when the reactor temperature has dropped
to room temperature.
The catalytic cracking reaction begins by putting
30 grams of palm oil and 1,57 grams of Ni/Al
2
O
3
catalyst into the reactor. After that, the heating
process begins with temperature settings to 400
o
C.
Wait until the temperature is reached then start
counting the time using a stopwatch for 10 minutes.
After the time is reached, the heater is turned off
and the reactor is cooled with a fan and then wait for
the temperature to drop to the room temperature then
gas is released at a flow of 20 ml/min. After the
temperature and pressure decreased the product is
saved in a container jar.
Before measuring the viscosity and density, the
product is first filtered with filter paper, and weighed
to determine the amount of product that becomes gas
and the amount of product that becomes liquid.
Then the density measurements were carried out
using a pycnometer and the density was explained as
follows.
The mass of the pycnometer was measured in an
empty state, namely 11.0546 grams. Then the
product is put into the pycnometer until it is full and
the fluid rises to the top hole, after that it is weighed
in the analytical balance, the mass of the pycnometer
with the specimen is reduced by the empty mass
with the following results.
𝜌
𝑠𝑎
𝑚
𝑝
𝑙
𝑒
=
𝜌
𝐷
𝑒𝑠
𝑐𝑟
𝑖
𝑝
𝑡𝑖𝑜
𝑛
𝑠
∶
𝜌
𝑠𝑎
𝑚
𝑝
𝑙
𝑒
=
𝑠
𝑎𝑚𝑝𝑙
𝑒
𝑑𝑒𝑛𝑠𝑖𝑡𝑦
(𝑔/𝑐𝑚
3
)
𝜌
𝑤𝑎𝑡𝑒𝑟 =
𝑤𝑎𝑡𝑒𝑟
𝑑𝑒
𝑛
𝑠
𝑖
𝑡𝑦
(
𝑔
/
𝑐
𝑚
3
)
𝑚
𝑠𝑎
𝑚
𝑝
𝑙
𝑒
=
𝑠
𝑎𝑚𝑝𝑙
𝑒
𝑚
𝑎𝑠
𝑠
(
𝑔𝑟
𝑎𝑚
)
𝑚
𝑤𝑎𝑡𝑒𝑟 =
𝑤𝑎𝑡𝑒𝑟 𝑚𝑎𝑠𝑠 (𝑔𝑟𝑎𝑚)
Furthermore, the measurement of product
viscosity is carried out by inserting the specimen into
the viscometer then the liquid is raised to the top line
on the viscometer using a suction device, then the
suction device is removed and waits for the specimen
to drop to the bottom of the viscometer with a two-
line indicator, when the specimen begins to descend
from the top line timed until the specimen reaches the
bottom line.
The results of the time calculation are entered
into the following formula:
𝜇
𝑑𝑖𝑛
𝑠𝑎𝑚𝑝𝑙𝑒
=
𝜇
𝑑𝑖𝑛
𝑤𝑎𝑡𝑒𝑟
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
66
𝐷
𝑒𝑠
𝑐𝑟
𝑖
𝑝
𝑡𝑖𝑜
𝑛
𝑠
:
𝜇
𝑑𝑖𝑛
𝑤𝑎𝑡𝑒𝑟
=
𝑠
𝑎𝑚𝑝𝑙
𝑒
𝑠
𝑑𝑦
𝑛
𝑎𝑚𝑖
𝑐
𝑣𝑖
𝑠
𝑐𝑜
𝑠
𝑖
𝑡𝑦
(
𝑚
𝑃
𝑎
.
𝑠
)
𝜌
𝑠𝑎
𝑚
𝑝
𝑙
𝑒
=
𝑠
𝑎𝑚𝑝𝑙
𝑒
𝑠
𝑑𝑒
𝑛
𝑠
𝑖
𝑡𝑦
(
𝑘
𝑔
/
𝑐
𝑚
3
)
𝜇
𝑑𝑖
𝑛
𝑤
𝑎𝑡𝑒
𝑟
=
𝑤
𝑎𝑡𝑒𝑟
𝑑𝑦
𝑛
𝑎𝑚𝑖𝑐
𝑣𝑖
𝑠
𝑐𝑜
𝑠
𝑖
𝑡𝑦
(
𝑚
𝑃
𝑎
.
𝑠
)
𝜌
𝑤𝑎𝑡𝑒𝑟
= 𝑤𝑎𝑡𝑒𝑟 𝑑𝑒𝑛𝑠𝑖𝑡𝑦 (𝑘𝑔/𝑐𝑚
3
)
𝑡
=
𝑓𝑙𝑜𝑤
𝑡𝑖𝑚𝑒
(
𝑠
)
𝜇
𝑘𝑖𝑛𝑒
𝑚
𝑎
𝑡
𝑖𝑐
=
𝜇
𝑘𝑖
𝑛
𝑒
𝑚
𝑎
𝑡
𝑖
𝑐
=
𝑘𝑖𝑛𝑒𝑚
𝑎𝑡𝑖
𝑐
𝑣𝑖
𝑠
𝑐𝑜
𝑠
𝑖
𝑡𝑦
(
𝑚
2
/
𝑠
)
𝜇
𝑑𝑦
𝑛
𝑎
𝑚
𝑖
𝑐
=
𝑑𝑦
𝑛
𝑎𝑚𝑖
𝑐
𝑣𝑖
𝑠𝑐
𝑜𝑠
𝑖
𝑡𝑦
(
𝑁
𝑠
/
𝑚
2
)
𝜌 = 𝑑𝑒𝑛𝑠𝑖𝑡𝑦 (𝑘𝑔/𝑚
3
)
Viscosity of water is seen from the reference
table, sample flow time and water flow time are
calculated from the time the fluid passes the top line
and bottom line on the viscometer.
Measurement of the viscosity of the sample and
water must be in the same temperature conditions
and made a comparison with the table above. Then it
is calculated by the formula and the viscosity value
of the sample is known, with the principle that the
greater the viscosity value, the thicker the sample.
In this study, we tried at a temperature of 400
and 460, because we had tried it at a temperature of
300 and the result was a solid product not liquid, so
we used a higher temperature of 400 and 460, because
at this temperature we found that the result was a
liquid.
3
RESULT AND DISCUSSION
Green diesel products are said to meet the standards
if the values indicated are 0.8150 gr/ml and 0.8600
gr/ml. And the density value of the raw materials
used is 0.9112 gr/ml. While the resulting product is a
solid or solid whose density value is > 0.9112 gr/ml.
Therefore the resulting product does not meet the
standards of green diesel fuel.
Table 1: Cracking Process Parameters.
Cracking Process Parameters
Ni/Al
2
O
3
mass
5% = 1.57 gram
RBDPO weight 30 gram
Reaction time 10 minutes
Temperature 400
o
C and 460
o
C
Pressure 1 atm
In this study, the viscosity test was carried out
using an Ostwald viscometer. Green diesel products
are said to meet the standards if the kinematic
viscosity values shown are 2 cSt and 4.5 cSt. While
the dynamic viscosity of the raw materials used is
0.39 Ns/m2. The density value of the raw materials
used is 0.9112 gr/ml. Then the value of its kinematic
viscosity is 428 cSt.
3.1 Effect of Temperature Variations
Mass
21
20,5
20
19,5
19
18,5
18
17,5
400˚C 460˚C
Reaction Temperature (°C)
Figure 1: Effect of temperature variations in palm oil
cracking process using 5% mass of Ni/Al2O3 catalyst in
400oC temperature and 1 atm pressure for 10 minutes to
the product mass.
From this graph it is known that the most amount of
product is produced using 400
o
C temperature which
is 20,845 gram from the total raw material of 30
gram.
3.2 Effect of Temperature Variations
on Density
0,86
0,84
0,82
0,8
0,78
0,76
400˚C 460˚C
Reaction Temperature (˚C)
Figure 2: Effect of temperature variations in palm oil
cracking process using 5% mass of Ni/Al2O3
catalyst in 400oC temperature
and 1 atm pressure for
10 minutes to the product density.
From this graph it is known that only the 400
o
C
product has the value of density that qualify the green
diesel product standard, the 400
o
C product is 0,8523
g/ml which is between 0,8150-0,8600 g/ml.
Density (g/ml)
Product Mass (gram)
Activity Test of Temperature Variations in Cracking Process of Palm Oil using Ni/Al2o3 to Green Diesel Viscosity
67

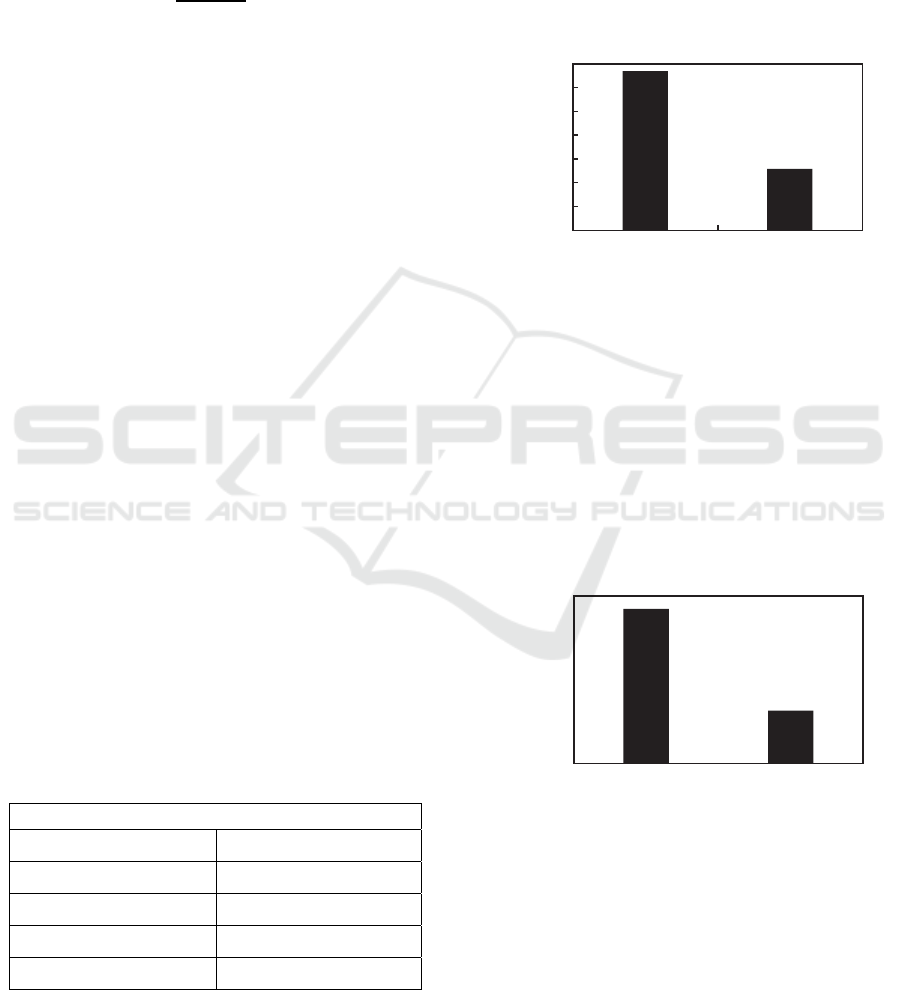
3.3 Effect of Temperature Variations
on Viscosity
7
6
5
4
3
2
1
0
400˚C 460˚C
Reaction Temperature (˚C)
Figure 3: Effect of temperature variations in palm oil
cracking process using 5% mass of Ni/Al2O3 catalyst in
400oC temperature and 1 atm pressure for 10 minutes to
the product viscosity.
From this graph it is known that none of product has
the value of viscosity that qualify the green diesel
product standard, namely the 400
o
C product is 6,4563
cSt and the 460
o
C is 1,4305 cSt which both are not
between 2-4,5 cSt.
4
CONCLUSIONS
The effect of temperature variations in the palm oil
cracking process using Ni/Al
2
O
3
catalyst on the
response of product mass, density, and viscosity, it
was found that the higher the temperature, the lower
the value of these responses. The mass response of the
product obtained the most is 20.845 grams, and the
highest density response is 0.8523 g/ml, and the
highest viscosity response is 6.4563 cSt.
For the product that best meets the Green Diesel
standard specifications, it is analyzed from density
data, namely only the 400
o
C product of which have
met the standard, namely 0.8523 g/ml and of which is
in the standard range of 0.8150-0,8600 g/ml.
Meanwhile, the product analysis was based on
the viscosity value, none of the product met the
standard, namely the 400
o
C product 6,4563 cSt and
460
o
C 1,4305 cSt which were not in the standard
range of 2-4.5 cSt.
ACKNOWLEDGEMENTS
The author would like to thank UPT P2M Politeknik
Negeri Malang DIPA No. SP DIPA
023.18.2.677606/2021 with contract no. 5439/PL2
.1/HK/2021 for financial support.
REFERENCES
Sa’adah, A. F., Fauzi, A., & Juanda, B. (2017). Peramalan
Penyediaan dan Konsumsi Bahan Bakar Minyak
Indonesia. Jurnal Ekonomi dan Pembangunan
Indonesia Vol. 17 No. 2, 118–137.
Auliastuti, M. A., Munir, M. B., & Chumaidi, A. (2019).
PENGARUH PENAMBAHAN KATALIS Mg-Zn
Terhadap Komposisi Green Diesel Dengan Metode
Dekarboksilasi. Distilat Jurnal Teknologi Separasi, 58-
62.
Munur, M. B, & Chumaidi, M. (2019). Pengaruh Waktu
Proses Terhadap Komposisi Green Diesel Dalam
Reaksi Dekarboksilasi. Distilat Jurnal Teknologi
Separasi, 147- 151.
Ristanti, R. A., Sagara, B. P., Murti, S. S., & Redjeki, S.
(2020). Pembuatan Green Diesel dari Minyak Biji
Kapuk (ceiba pentandra) Menggunakan Katalis
NiMo/y-Al2O3 dengan Proses Hidrogenasi dan
Fraksinasi. Jurnal Teknik Kimia, Vol. 15 No. 1.
Neonufa, G. F., Soerawidjaja, T. H., & Prakoso, T. (2017).
Catalytic and Thermal Decarboxylation of Mg-Zn
Basic. J. Eng. Technol. Sci., Vol. 49, No. 5, 575-586.
Viscosity (cSt)
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
68
