
Effect of Stainless Steel Duplex Electrode Size on Hydrogen
Production through Electrolysis Process
Yohandri Bow
1a
, Rusdianasari
1b
, Anerasari Meidinariasty
1
and Muhammad Yori Pratama
2
1
Department of Chemical Engineering, Politeknik Negeri Sriwijaya, Jalan Srijaya Negara, Palembang, Indonesia
2
Department of Chemical Engineering, Institut Teknologi Bandung, Bandung, Indonesia
Keywords: Electrode, Duplex Stainless Steel, Hydrogen, Electrolysis.
Abstract: Renewable energy development is the focus of attention at this time, using environmentally friendly energy
sources and zero emission by utilizing water to make hydrogen through the electrolysis process. The electrode
material is selected from materials that have good electrical conductivity and corrosion resistance. Duplex
Stainless Steel (DSS) is a material with a combination of two phases, namely austenite and ferrite. The process
includes designing tools, manufacturing, and testing the performance of the electrolyzer against the variation
in the cross-sectional area with a voltage of 12V and variations in current and electrolytes used are brackish
water with a salinity of 8 ppt. The larger the cross-sectional area, the easier the electron transfer that occurs
during the electrolysis process, which causes the charge of electrons to react more, then the resulting current
will be more significant, this results in a large amount of power generated, while efficiency is inversely
proportional to power. Electrode size is also proportional to the current. Electrode 2 inch is more effective
than electrode 1.5 inch because, at current 35 A, an efficiency of 93.33% is obtained with the volume of gas
produced is 1.9087 Liters.
1 INTRODUCTION
Electrolysis of water is the decomposition of water
compounds (H
2
O) into hydrogen gas (H
2
) and oxygen
(O
2
) by using an electric current through water
(Ploetz et al., 2016; Rusdianasari et al., 2019).
Hydrogen is one of the new renewable energy sources
that can reduce exhaust emissions by using water as
fuel through the process of electrolysis of water
compounds (H
2
O) which is converted into its
constituent components, namely oxygen and
hydrogen (Kassaby et al., 2016; Imperiyka et al.,
2017; Irtas et al., 2021; Jannah et al., 2020).
The production of hydrogen gas from seawater
containing NaCl can take place quickly because NaCl
itself functions as a natural catalyst. The content of
the natural catalyst or commonly referred to as
salinity, affects the electrolysis process. In the study
of electrolysis made from water and seawater based
on variations in current and salinity, the salinity used
was 0.05 ppt, 15 ppt, and 35 ppt with the best gas
conversion at a salinity variation of 35 ppt
a
https://orcid.org/0000-0002-2741-7477
b
https://orcid.org/0000-0003-1955-396X
(Phalakornkule et al., 2020; Purnamasari et al., 2019;
Rusdianasari et al., 2020; Rusdianasari et al., 2020).
The production of H
2
gas by electrolysis of water
and seawater obtained the highest concentration of H
2
gas formed, namely the electrolysis of aqua DM,
which was added with NaCl and NaOH of 4500 ppm.
In this study, the concentration of NaOH catalyst was
not varied in the electrolysis process (Imperiyka et al.,
2017). The results of another study stated that in the
production of hydrogen gas with an H
2
SO
4
catalyst,
the greater the current and the greater the number of
electrodes provided with the same sulfuric acid
concentration, the greater the concentration of
hydrogen gas produced (Syakdani et al., 2019; Bow
et al., 2018; Amelia et al., 2021). The greater the
concentration of the catalyst, the greater the
precipitate formed during the electrolysis process and
causes obstruction of the process of forming gas
bubbles at the electrode (Hadi, 2020).
A catalyst is a substance that can accelerate the
rate of a chemical reaction which at the end of the
reaction is obtained in its original state or does not
Bow, Y., Rusdianasari, ., Meidinariasty, A. and Yori Pratama, M.
Effect of Stainless Steel Duplex Electrode Size on Hydrogen Production through Electrolysis Process.
DOI: 10.5220/0010946400003260
In Proceedings of the 4th International Conference on Applied Science and Technology on Engineering Science (iCAST-ES 2021), pages 393-397
ISBN: 978-989-758-615-6; ISSN: 2975-8246
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
393

react (Sellami and Loudiyi, 2017). In the electrolysis
of water, a catalyst is used to facilitate or accelerate
the decomposition of water into hydrogen and oxygen
because the catalyst ions are able to affect the stability
of water molecules into H
+
and OH
-
ions which are
easier to electrolyze due to a decrease in activation
energy (O’Neil et al., 2016).
Research using stainless steel electrodes produced
95.8 ml and 82.6 ml of HHO gas, respectively. The
electrolyte used is KOH and electrodes made of
Stainless Steel with variations in the cross-sectional
area of the electrode with sizes 9 cm x 11 cm and 9
cm x 14 cm (Meier, 2014).
The most important components of the electrolysis
process are electrodes and electrolyte solutions (Irena,
2020). One of the raw materials for electrolysis is
water. Electrolysis of water which has the chemical
formula H
2
O is the event of the decomposition of water
compounds (H
2
O) into its constituent elements,
namely hydrogen (H
2
) and oxygen (O
2
), by using an
electric current. Electrolysis is an event that occurs
when an electric current is passed through an ionic
compound and the compound undergoes a chemical
reaction (Abdel-AaL et al., 2020).
Hydrogen gas as the main product and oxygen
produced from the electrolysis of water form bubbles
at the electrodes and can be collected in a reservoir.
This principle is then used to produce hydrogen that
can be used as fuel for hydrogen vehicles. Michael
Faraday discovered the electrolysis process in 1820.
Factors that affect the electrolysis process include the
use of a catalyst, the immersed surface area, the
nature of the electrode raw material, the concentration
of the reactants, and the amount of external voltage
(Moulita et al., 2020).
The main product, hydrogen in the water
electrolysis process, is highly flammable and will
burn at a concentration of 4% in free air. The enthalpy
of combustion of hydrogen gas is -286 kJ/mol
(Yunsari et al., 2019). Based on this, safety is needed
when burning hydrogen gas. The arrestor is a tool
component that reduces the risk of work accidents
such as explosions due to flashback fires during the
hydrogen gas flame test (Bow et al., 2020). In this
study, electrolysis was carried out using a prototype
electrolysis reactor equipped with an arrestor in terms
of the effect of the size of Duplex Stainless Steel on
hydrogen production through the electrolysis process.
2 METHODOLOGY
The material used in this study is water with a salinity
of 8 ppt which was taken according to seawater
sampling standards (SNI 6964.8:2015). After
knowing the salinity that produces hydrogen gas
optimally, variations in the size of the stainless steel
duplex electrode are carried out. The seawater
electrolysis prototype consists of five main units,
namely, feed tank, electrolyzer tube, electrodes, H
2
and O
2
gas cylinders, flashback arrestor, and control
panel. The system is equipped with measuring
instruments, namely digital pressure detectors, digital
temperature detectors, H
2
smart sensors, and O
2
smart
sensors. The electrolysis equipment used can be seen
in Figure 1.
Figure 1: Electrolyzer Prototype.
The electrolysis process is carried out using the
Hydrogen Generator Using Water Electrolysis
Process method based on ISO 22734-1:2008 with
adjustments to the design and condition of the tool.
The electrolysis process in the electrolysis reactor
used is that the feed is pumped into the electrolyzer
as much as 17 liters. The electrolysis process is
carried out with a certain current. The electrolysis
results obtained are in the form of hydrogen and
oxygen gas in the electrolyzer, which will then be
temporarily accommodated in the gas holding tank.
During the electrolysis process, data is collected
every 2 minutes in the form of operational condition
data in the form of pressure, temperature, and voltage.
After hydrogen gas is obtained, then temperature data
is taken on the arrestor every 2 minutes.
3 RESULT AND DISCUSSION
In this study, we want to get the effect of Duplex
Stainless Steel as an electrode and the electrolyte
used is brackish water on the production of
hydrogen gas produced. The prototype for making
hydrogen was carried out by varying the size of the
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
394
electrode with the respective electrode diameters (2
inches and 1.5 inches), the thickness was 0.33 mm,
and the electrode height was 40 cm, and the current
variation with a voltage of 12 Volts for 480 seconds
and using an electrolyte, namely water. brackish
with a salinity of 8 ppt. The production of hydrogen
gas produced by varying the size of the electrodes
can be seen in Table 1.
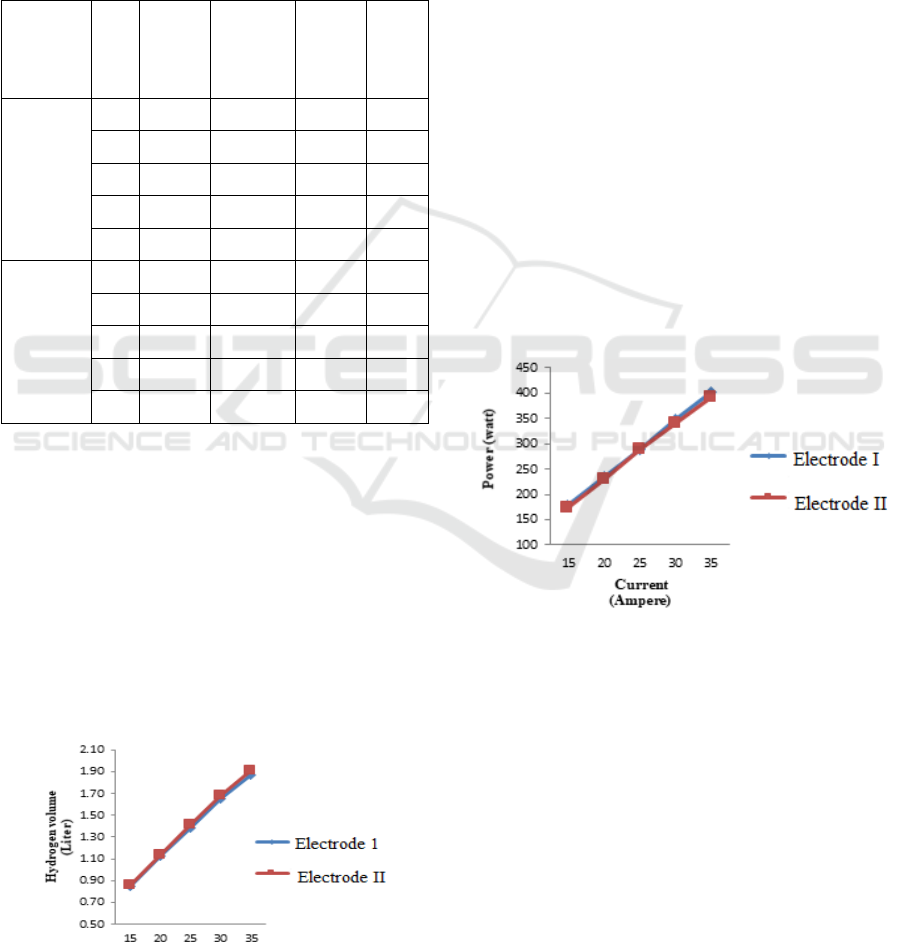
Table 1: Hydrogen production based on electrode cross-
sectional area.
Electrode
Size
I
(A)
V
(Volt)
H
2
Gas
Volume
(Liter)
P
(Watt)
η (%)
Electrode
I
(D= 2 inch,
t= 40cm)
15 11.9 0.8512 178.5 99.17
20 11.7 1.1251 234 97.50
25 11.6 1.3877 290 96.67
30 11.6 1.6538 348 96.67
35 11.5 1.8719 402.5 95.83
Electrode
II
(D= 1,5
inch,
t=40cm)
15 11.6 0.8539 174 96.67
20 11.5 1.1407 230 95.83
25 11.5 1.4179 287.5 95.83
30 11.3 1.6769 339 94.17
35 11.2 1.9087 392 93.33
3.1 Effect of Current and Electrode
Size on the Volume of Hydrogen
Produced
The formation of hydrogen gas in the electrolysis
process has a directly proportional relationship
between gas yield, time, and current strength. The
results of measuring the volume of hydrogen gas
produced at different electric currents and the cross-
sectional area of the duplex stainless steel electrodes
used during the electrolysis process can be seen in
Figure 2.
Figure 2: The volume of hydrogen gas produced is based on
the curren t and the size of the electrode.
Figure 2 shows that the transfer of electrons
during electrolysis results in an increase in the
production rate of hydrogen gas produced. This is
indicated by an increase in current and a different size
of the electrode. The size of electrode I (2 inches) is
obtained with a current of 35A, the volume of gas
produced is 1.8719 liters, while the size of the
electrode II (1.5 inches) is that the volume of gas
produced is 1.9087 liters. The increase in the volume
of gas produced at different electrode sizes is not
significant, this occurs because the electrode sizes are
2 inches and 1.5 inches respectively, with the same
electrode height. The increase in current and voltage
causes the electrolysis process to occur quickly
because the movement of the molecules is also
increasing. The temperature in this process also
increases due to the faster movement of electrons
during the electrolysis process (Ploetz, R.
Rusdianasari and Eviliana, 2016).
3.2 Effect of Current and Electrode
Size on the Electric Power
Generated
The relationship between current and power
generated by using different sizes of electrodes can be
seen in Figure 3.
Figure 3: The electric power generated is based on the
current and the size of the electrode.
Figure 3 shows that the relationship between the
current and the supplied power is less significant for
different electrode sizes, this happens because the
electrode sizes are 2 inches and 1.5 inches,
respectively with the same electrode height. At the
size of the electrode I obtained with a current of 35A,
the power supplied was 402.5 Watts, while the size of
the electrode II obtained a power supply of 392 Watts.
However, if the cross-sectional area affects the power
supply, the larger the size of the electrode, the easier
it will be for the transfer of electrons that occurs
during the electrolysis process, causing more
electrons to react, so the current generated will be
Effect of Stainless Steel Duplex Electrode Size on Hydrogen Production through Electrolysis Process
395
even greater, this results in a large amount of power
being generated directly proportional to the current
(Purnamasari, I., Yerizam, M., Hasan, A., Junaidi, R.,
2019).
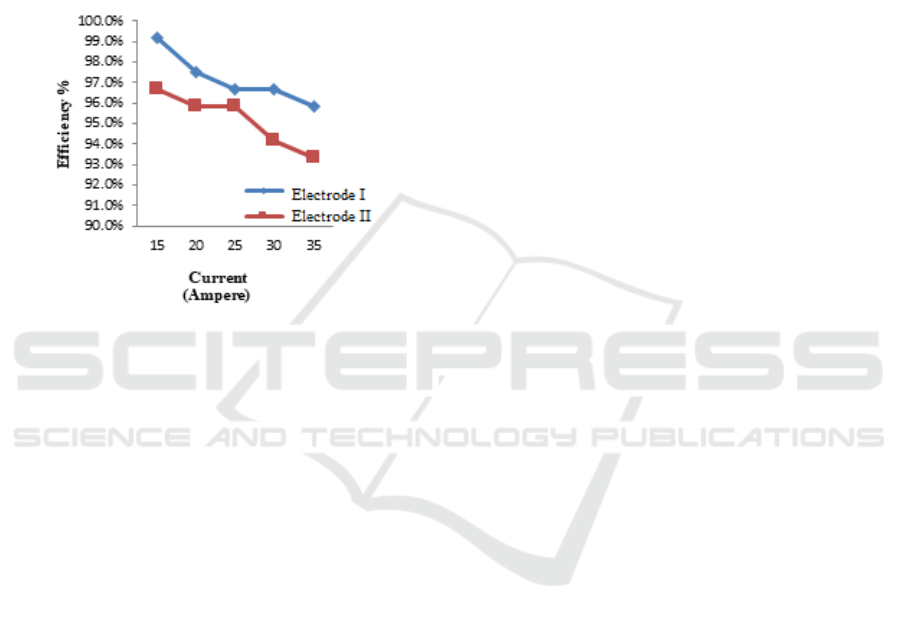
3.3 Electrolyzer Efficiency
The performance of the electrolyzer prototype that
converts seawater into hydrogen gas with variations
in electric current and different sizes of stainless steel
duplex electrodes can be seen in Figure 4.
Figure 4: Electrolysis efficiency based on the electrode size.
Figure 4 shows that the greater the electric
current, the greater the power produced because
power is directly proportional to current, while
efficiency is inversely proportional to power. So that
the highest efficiency obtained at electrode I is
99.17% with an electrode size of 2 inches at a current
of 15A, while the lowest efficiency obtained at
electrode II is 93.33% with an electrode size of 1.5
inches at a current of 35A. The relationship between
current and efficiency generated, this happens, when
an increase in current occurs, the power generated is
more significant and the efficiency of the electrolyzer
is getting smaller. The efficiency of the electrolyzer
is not only affected by the increase in current and
power but is also affected by the heat energy
produced, which is directly proportional to hydrogen
gas. The type of electrode greatly affects the
efficiency of the electrolyzer. The electrode used is
Duplex Stainless Steel. Besides being able to produce
a larger current, this type also reacts and produces a
constant and relatively large gas in the long term.
4 CONCLUSIONS
The production of hydrogen has been carried out by
the electrolysis method using a prototype electrolyzer
which has an electrolysis cell with a capacity of 15-
20 liters. The electrodes used are Duplex Stainless
Steel with varying diameter sizes, namely 1.5 inches
and 2 inches with a height of 40 cm and an electrode
thickness of 0.3 mm. In this process, the highest
volume of gas produced with different electrode sizes
is found in the size of electrode II (D = 1.5 inches, t =
40cm) at a current of 35A of 1.9087 Liters. The power
generated is more significant because the power is
directly proportional to the current so that the highest
power is obtained at 402.5 Watt with a current of 35A
at the electrode size I (D = 2 inches, t = 40cm). The
highest efficiency was obtained at the size of the
electrode I (D = 2 inches, t = 40cm) with a current of
15A of 99.17%.
ACKNOWLEDGEMENTS
The authors would like to thank the Ministry of
Education, Culture, Research, and Technology; The
Directorate General of Vocational Studies has funded
this applied research for the 2021 fiscal year.
REFERENCES
Abdel-AaL, H.K., Zohdy, K.M.., Kareem, M.A. (2010).
Hydrogen Production using Sea Water Electrolysis. The
Open Fuel Cell Journal, Vol. 3, pp. 1-7.
Amelia, I., Rohendi, D., Rachmat, A., Syarif, N. (2021).
Hydrogen Adsorption/Desorption on Lithium alanat
Catalyzed by Ni/C for Sustainable Hydrogen Storage.
Indonesian Journal of Fundamental and Applied
Chemistry, Vol. 6(2), pp. 59-63.
Bow, Y., Dewi, T., Taqwa, A., Rusdianasari, Zulkarnain.
(2018). Power Transistor 2N3055 as a Solar Cell
Device. In International Conference on Electrical
Engineering and Computer Science (ICECOS), IEEE.
Bow, Y., Rusdianasari, Yunsari, S. (2020). CPO based
Biodiesel Production using Induction Heating Assisted.
Oil Palm Research and Review, Vol. 1(1), pp. 1-6.
Hadi, S. (2020). Effect of Electrodes, Electric Current, and
NaHCO
3
Concentration against HHO Pressure
Generator. Int. Journal of Engineering Science, Vol. 10
(4), pp. 1-3.
Imperiyka, MH., Rahuna, MN., Iman, EA. (2017).
Hydrogen Production using Mediterranean Sea Water
of Benghazi Shore and Synthetic Sea Water
Electrolysis. Academic Journal of Chemistry, Vol. 2
No. 1, pp. 8-15.
Irtas, D., Bow, Y., Rusdianasari. (2021). The Effect of
Electric Current on the Production of Brown’s Gas
using Hydrogen Fuel Generator with Seawater
Electrolytes. In IOP Conf. Ser., Earth Environ. Sci.
79(012001).
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
396

IRENA. (2020). Green Energy: A Guide to Policy Making,
International Renewable Energy Agency, Abu Dhabi.
Jannah, Z., Susilo, S.H. (2020). Effect of Electrodes,
Electric Currents, and NaHCO3 Concentration Against
HHO Pressure Generator. International Journal of
Engineering Science, Vol. 10, pp. 1-3.
Kassaby, E., Eldrainy, MM., Khidr, EA., Khidr, K. I.
(2016). Effect of Hydroxy (HHO) Gas Addition on
gasoline Engine Performance and Emissions.
Alexandria Engineering Journal, Vol. 55, pp. 243-251.
Meier, K. (2014). Hydrogen Production with Sea Water
Electrolysis using Norwegian offshore Wind Energy
Potentials. Int. J. Energy Environ. Eng. Vol 5(104).
Moulita, RAN., Rusdianasari, Kalsum, L. (2020).
Biodiesel Production from Waste Cooking Oil using
Induction Heating Technology. Indonesian Journal of
Fundamental and Applied Chemistry, Vol. 5 (1), pp.
13-17.
O’Neil, G.D., Christian, C.D., Brown, D.E., Esposito, D.U.
(2016). Hydrogen Production with a Simple and
Scalable Membraneless Electrolyzer. Journal of the
Electrochemical Society, Vol. 163(4), pp. 3012-3019.
Phalakornkule, C., Sukkasem, P., Mutchimsattha, C.
(2020). Hydrogen Recovery from the
Electrocoagulation Treatment of Dye-Containing
Wastewater. International Journal of Hydrogen
Energy, Vol. 35, pp. 10934-10943.
Ploetz, R. Rusdianasari, Eviliana. (2016). Renewable
Energy: Advantages and Disadvantages. In Proceeding
Forum in Research, Science, and Technology (FIRST).
Purnamasari, I., Yerizam, M., Hasan, A., Junaidi, R. (2019).
Oxygen Adsorption Kinetics Study used Pressure
Swing Adsorber (PSA) for Nitrogen Production. J.
Phys.: Conf. Ser., 1167(012049),
Rusdianasari, Bow, Y., Dewi, T. (2019). HHO Gas
Generation in Hydrogen Generator using Electrolysis.
In IOP Conference Series: Earth and Environmental
Science, 258 (012007). https://doi.org/10.1088/1755-
1315/258/1/012007
Rusdianasari, Bow, Y., Dewi, T., Risma, P. (2020).
Hydrogen Gas Production using Water Electrolyzer as
Hydrogen Power. In 2019 International Conference on
Electrical Engineering and Computer Science
(ICECOS), IEEE.
Rusdianasari, Bow, Y., Dewi, T., Taqwa, A., Prasetyani, I.
(2020). Effect of Sodium Chloride Solution
Concentartion on Hydrogen Gas Production in Water
Electrolyzer Prototype. In 2019 International
Conference on Technologies and Policies in Electric
Power and Energy, IEEE.
Sellami, M.H., Loudiyi, K. (2017). Electrolytes Behavior
during Hydrogen production by Solar Energy.
Renewable and Sustainable Energy Reviews, Vol. 70,
pp. 1331-1335.
Syakdani, A., Bow, Y., Rusdianasari, Taufik, M. (2019).
Analysis of Cooler Performance in Air Supply Feed for
Nitrogen Production Process using Pressure Swing
Adsorption (PSA) Method. In J. Phys.: Conf. Ser.,
1167(012055).
Yunsari, S., Husaini, A., Rusdianasari. (2019). Effect of
Variation of Catalyst Concentration in the Producing of
Biodiesel from Crude Palm Oil using Induction Heater.
Asian Journal of Applied Research and Community
Development and Empowerment, Vol. 3(1), pp. 24-27.
Effect of Stainless Steel Duplex Electrode Size on Hydrogen Production through Electrolysis Process
397
