
Effect of Type of Activator and Ultrasonic Waves on the Chemical
Activation Process on the Characteristics of Activated Charcoal from
the Rubber Fruit Shell (Hevea Brasiliensis)
Sirajuddin, Harjanto and Inzirah
Department of Chemical Engeneering, Polytechnic State of Samarinda, East Kalimantan, Indonesia
Keywords: Activation, Shell for Rubber, Ultrasonic Waves, Type of Activator, Activated Carbon.
Abstract: The potential for rubber fruit shells in East Kalimantan Province in 2019 was 95.327 tons/year. Rubber fruit
shells contain 48.64% cellulose. The content of cellulose are organic compounds that can be used as activated
carbon. The purpose of this study was to determine the effect of the type of chemical activator with the help
of ultrasonic waves on the characteristics of activated carbon according to the SNI 06-3730-1995 standard.
Activated carbon is obtained through 4 stages, namely preparation of raw materials, carbonation at 500°C
for1 hour, chemical activation with variations in the types of activators H
3
PO
4
, H2SO4, KOH and Na2CO3
with a concentration of 10% b/v for 4 hours, then physically activated at a temperature of 600°C for 1 hour.
The best results were obtained in the variation of H
3
PO
4
activator types with I
2
absorption results of
771.3263mg/g, water content of 0.5745%, ash content of 0.4045%, and 9.8200% volatile matter content.
1 INTRODUCTION
Rubber shells are biomass-dense waste and currently
have no commercial value (Harun et al., 2010). The
solid waste is left and not utilized so that it is bad for
the environment (Ngah and Hanafiah, 2008). The
content of cellulose is relatively large so that rubber
shells are very potential to be processed into useful
and high-value activated carbon-making raw
materials (Ioannidou and Zabaniotou, 2007).
Activated carbon can be produced materials
containing cellulose such as wood, hazelnut shells,
peat, coal, bamboo and others. Activated carbon is
widely used in both large and small industries as
coagulation (Stephenson and sheldon, 1996),
chemical oxidation (Salem and El-Maazawi, 2008),
photo catalysts (Bukallah et al., 2008),
electrochemistry (Somasekhar et al., 2001), and
membrane separation (Porter and McKay, 1997).
Pyrolysis processes as one of the most reliable
thermochemical processes for the conversion of solid
waste raw materials into high-value activated carbon
(Somasekhar et al., 2001). Pyrolysis is usually done
in the absence of oxygen depending on the
temperature applied and the rate of heating (Chen et
al., 2018). However, slow pyrolysis often causes the
resulting activated carbon to contain a lot of oxygen
and affects a variety of applications and takes more
than 6 hours (Lee at al., 2017). Current methods that
can produce activated carbon that have high quality
use ultrasonic waves in the chemical activation
process. Ultrasonic is a process that aims to increase
mass transfer through the utilization of sound waves
to produce pressure fluctuations. The cavitation effect
of the wave can increase the temperature thus
accelerating the catalyst into the activated carbon to
remove impurities so that the activated carbon pores
enlarge and cause the active carbon absorption to
increase. Research on the utilization of ultasonic
waves on the manufacture of activated carbon has
been conducted using waste tobacco rods, waste tea,
wood and cotton rods (Ji.Y et al., 2007),(Wang et al.,
2009). The results showed that the process of making
activated carbon using ultrasonic waves in the
chemical activation process had advantages over
conventional pyrolysis methods such as low energy
use, easier to control, and shorter time.
In this study, variations in the type of activator
(H
3
PO
4,
H
2
SO
4,
Na
2
CO
3
and KOH) with a
concentration of 10% b/v each with the process of
carbonization using furnaces at a temperature of
500
o
C for 1 hour. The process of chemical activation
using the help of ultrasonic waves for 4 hours as well
as adding the physics activation process at a
temperature of 600℃ for 1 hour so that activated
carbon is obtained in accordance with the standard.
Sirajuddin, ., Harjanto, . and Inzirah, .
Effect of Type of Activator and Ultrasonic Waves on the Chemical Activation Process on the Characteristics of Activated Charcoal from the Rubber Fruit Shell (Hevea Brasiliensis).
DOI: 10.5220/0010950200003260
In Proceedings of the 4th International Conference on Applied Science and Technology on Engineering Science (iCAST-ES 2021), pages 633-637
ISBN: 978-989-758-615-6; ISSN: 2975-8246
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
633

The purpose of the study was to find out the types
of activators namely H
3
PO
4,
H
2
SO
4,
KOH and
Na
2
CO
3
with the help of ultrasonic waves against the
activated carbon characteristics of rubber fruit shells
resulting in activated carbon that conforms to the
standard of activated carbon quality that refers to the
standard (SNI, 1995).
2 METHODOLOGY
Tools used in this study include furnace, ultrasonic,
oven, desiccator, balance sheet analytics, hotplate and
magnetic stirrer, screening 100 mesh, 120 mesh,
crusher, porcelain cup, mortar stamper, beaker cup
clamp, spatula, funnel, rubber bulp, clamps and
statives, aquades bottle, buret 25 mL, drop pipette, 5
mL, and 10 mL measuring pipette, 10 mL, and 50 mL
volume pipette, erlenmeyer 100 mL and 250 mL,
pumpkin measuring 100 mL and 1000 mL, while the
material used rubber fruit shell, aquadest, solution
(H
3
PO
4,
H
2
SO
4,
Na
2
CO
3
and KOH) 10%, iodine
solution 0.1021 N, thiosulfat sodium solution 0.1033
N, amylum indicator 1% b/v, whatman filter paper
No.42, universal indicator.
The research began from the stage of collecting
dry old rubber shell raw materials. Dry old rubber
shell are cleaned and carbonated at a temperature of
500
o
C for 1 hour. Furthermore activated carbon is
screened and activated with H
3
PO
4,
H
2
SO
4,
Na
2
CO
3
and KOH with a concentration of 10% b/v each with
the help of ultrasonic waves with a frequency of 47
kHz for 4 hours and adds the physics activation
process at 60℃ temperatures for 1 hour. The Analysis
stage is conducted based on standards (SNI, 1995),
covering moisture content (ASTM D-3173), ash
content (ASTM D-3174), volatile matter (ASTM D-
3175), and iodine numbers (SNI 06-3730-1995).
Figure 1: Picture of the raw material and the resulting
activated carbon.
3 RESULT AND DISCUSSION
The purpose of the study was to determine the
influence of activator types H
3
PO
4,
H
2
SO
4,
KOH and
Na
2
CO
3
with the help of ultrasonic waves against the
activated carbon characteristics of rubber fruit shells
resulting in activated carbon that conforms to the
activated carbon quality standards that refer to the
standard (SNI, 1995).
In this study the raw material used is a rubber fruit
shell. The raw material of rubber fruit shells has a
yield of 24.17%, proximate analysis of water content
values of 4.73%, ash levels of 2.65%, volatile matter
levels of 17.62%, and iodine absorption of
469.72mg/g. Rubber fruit shells were previously
carbonated using furnaces with a temperature of
500℃ for 1 hour so that it becomes charcoal. After
the carbonization process is obtained charcoal is then
chemically activated for 4 hours with the help of
ultrasonic waves and without ultrasonic waves. Then
the activation of physics using the help of a furnace
with a temperature of 600℃ for 1 hour. Furthermore,
activated carbon obtained by proximate analysis
includes water content values, ash levels, volatile
matter, and analyzed the absorption of activated
carbon against iodine.
3.1 Moisture Content
Determination of water content aims to find out the
hygroscopic properties of activated charcoal and to
find out the water content in the cavity or cover the
pores in activated charcoal shown with a high water
content in charcoal. Low water content indicates many
cavities or gaps that can be occupied by adsorbate so
that the absorbs process will take place properly.
Figure 2: Graph of the relationship of activator types with
water content.
Based on the graph in figure 1 it can be seen that
the moisture content of activated carbon samples for
each type of activator substance has different values.
The type of activator substance is influential in the
activation process of activated carbon. The presence
of activator agents in relation to water content is as a
hydrating agent. How it works as a binder of water
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
634
molecules contained in raw materials so as to enlarge
the pores of activated carbon and expand the surface
of absorption. Low levels of activated carbon water
indicate the success of chemical activator agents in
binding to water molecules contained in the material
as well as the release of the free water content and
bound water contained in raw materials during the
carbonation process (Ahmed and Theydan, 2013).
Based on the analysis data to find out the effect of
the addition of ultrasonic wave assistance in the
analysis of water content, chemical activation is
carried out with the same treatment without the help
of ultrasonic waves. Based on figure 1 it is known that
the water content is better obtained from activation
with the help of ultrasonic.
On the graph can be seen the lowest percent water
content value obtained by activator substance H
2
SO
4
with and without the help of ultrasonic waves,
respectively, which is 0.32% and 0.42 percent
respectively. The highest water content obtained
KOH activator substances with and without the help
of ultrasonic waves, respectively, is 1.65% and 1.62%
respectively. Based on the data, it is known that acid
activators H
2
SO
4
are more able to dehydrate water
bound in activated carbon when compared to
activators H
3
PO
4
, Na
2
CO
3
and KOH.
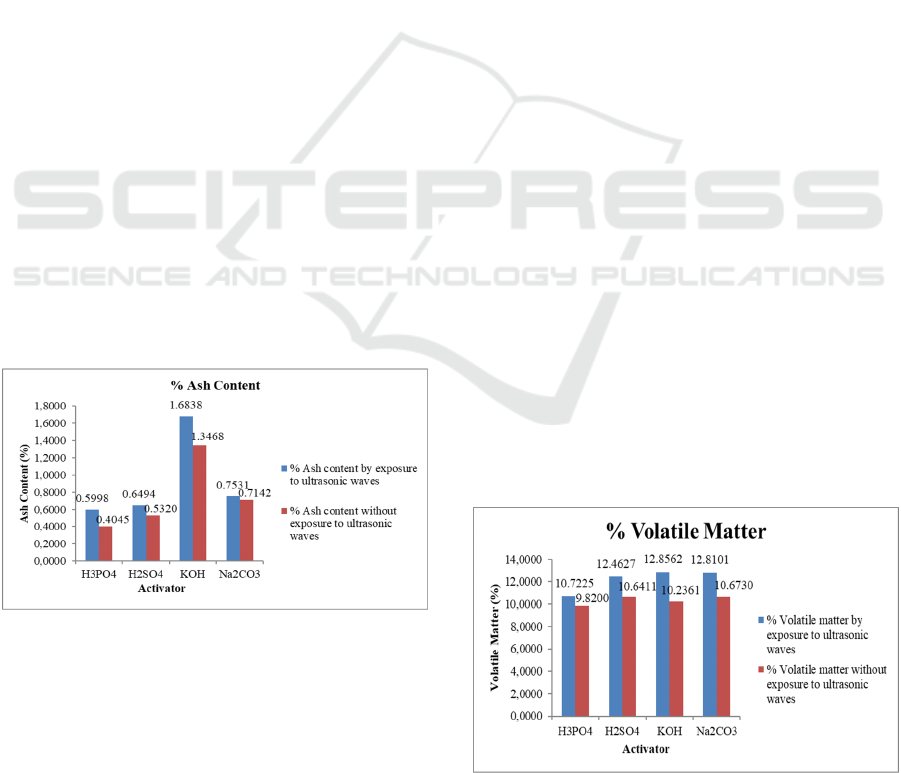
3.2 Ash Content
Determination of ash levels aims to find out the content
of metal oxides in activated carbon. Ash levels are
assumed to be residual minerals left behind from the
rubber shell carbonization process. The results of the
analysis of ash level can be seen at figure 3.
Figure 3: Graph of the relationship of type of activator with
ash levels.
Based on the graph in figure 3 it can be seen that
the ash levels of activated carbon in each type of
activator substance have different values. Activated
carbon consists of layers stacked against each other
that make up pores. Where in the pores of charcoal
there are usually impurities in the form of inorganic
minerals and metal oxides that cover the pores. Ash
levels can occur due to the formation of mineral salts
during the process of authoring which when
continued will form fine particles of the mineral salts.
The use of activator materials can dissolve substances
still present in charcoal such as hydrocarbons, ash,
nitrogen and sulfur. The process of washing on
chemical activation can dissolve metals or minerals
present in activated charcoal so that the ash levels
become relatively lower.
Based on the analysis data to find out the effect
of adding ultrasonic wave assistance to the analysis
of ash levels, chemical activation is carried out with
the same treatment without the help of ultrasonic
waves. Based on the graph in figure 3 it is known that
ash levels are better obtained from activation with the
help of ultrasonic for each type of activator. This is
because ultrasonic waves cause mechanical effects
that can increase reaction speed (Kentish and
Ashokkumar, 2011)
On the graph can be seen the lowest percent ash
levels obtained by activator substance H
3
PO
4
with and
without the help of ultrasonic waves of 0.40% and
0.60% respectively. The highest ash content obtained
KOH activator substances with and without the help of
ultrasonic waves by 1.35% and 1.68% respectively.
Based on the analysis data it is known that acid
activator H
3
PO
4
has the lowest ash levels when
compared to activators H
2
SO
4,
Na
2
CO3
and KOH.
3.3 Volatile Matter
The level of vaporizing substances in carbon is the
amount of substance that evaporates from a material,
which evaporates consisting of flammable gases, such
as hydrogen and carbon monoxide and a small portion
of vapor that can condense. The results of the analysis
of vaporized substance levels using activators
H
3
PO
4,
H
2
SO
4,
KOH and Na
2
CO3 can be seen in
figure 4.
Figure 4: Graph of the relationship of activator types with
volatile matter levels.
Effect of Type of Activator and Ultrasonic Waves on the Chemical Activation Process on the Characteristics of Activated Charcoal from the
Rubber Fruit Shell (Hevea Brasiliensis)
635
Based on the graph in figure 4 it can be seen that
the volatile matter levels of the activated carbon
sample for each type of activator substance have
different values. The high levels of the resulting
flying substance indicate that the surface of activated
charcoal is still covered by non-carbon compounds,
affecting its absorption. The levels of volatile matter
obtained are better with the help of ultrasonic waves
for each type of activator. Ultrasonic waves give rise
to cavitation energy that can increase pores more in
activated charcoal (Hamdaoui et al., 2008).
On the graph can be seen the lowest volatile
matter levels obtained by activator substance H
3
PO
4
with and without the help of ultrasonic waves of
9.82% and 10.72%, respectively. Volatile matter
levels is highest obtained by activator substance
Na
2
CO
3
with and without the help of ultrasonic waves
by 10.67% and 12.81% respectively. Based on the
data of the analysis it is known that acid activator
H
3
PO
4
has the lowest volatile matter levels when
compared to other activators. This suggests that acid
activators are more able to reduce non-carbon
compounds attached to the surface of activated
carbon when compared to activators H
2
SO
4,
Na
2
CO
3
and KOH.
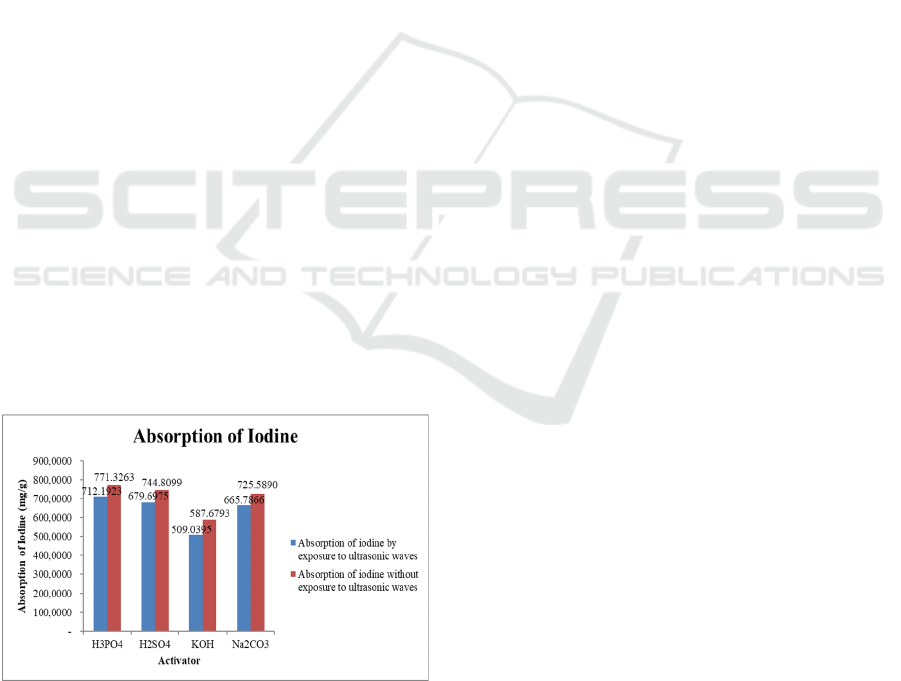
3.4 Absorption of Iodine
The absorption of Iodine solution in activated carbon
is a parameter to determine the ability of activated
charcoal to absorb molecules of small molecular
weight and substances in the liquid phase. The
determination of activated carbon adsorption power
to iodine has a correlation with the surface area of
activated charcoal. The results of the analysis for
iodine absorption are shown in figure 5 below.
Figure 5: Graphic of activator type relationship with iodine
absorption.
Based on figure 4 it can be seen that the iodine
absorption of the activated carbon sample for each
type of activator has different values. The activated
carbon absorption of rubber fruit shells activated with
and without the help of ultrasonic waves in the study
ranged from 575.57mg/g-755.13mg/g and
498.45mg/g-697.38mg/g, respectively. Activated
carbon with the highest iodine absorption capability
is activated carbon with activation using activator
H
3
PO
4
with iodine absorption of 755.13mg/g. A
solution of phosphoric acid (H
3
PO
4
)
as anactivator
also affects the surface area because it is a strong acid
capable of lifting hydrocarbon compounds or
impurities causing the formation of pores on the
surface of carbon. Iodine absorption is affected by
water content, ash content and volatile matter of
activated carbon. The lower the water content, the
water molecules that fill the activated carbon pores
the less so that, the pores are not closed and the ability
of activated carbon in absorbing iodine will be better.
Volatile matter the presence of volatile substances
attached to the surface of activated carbon affects
iodine power. The lower the levels of volatile
substances that cover activated carbon so that
activated carbon can absorb iodine more effectively.
Low water content will increase the effectiveness of
activated carbon in absorbing iodine. The addition of
ultrasonic wave assistance to the absorption of iodine
absorption is better obtained from activation with
ultrasonic assistance for each type of activator.
ultrasonic waves gives the effect of the phenomenon
of cavitation, namely the formation of small bubbles
in the intermediate medium, which over time the
bubbles that get bigger and burst and release large
forces that can be used for chemical processes in
ultrasonic. It is this bubble breakdown that then forms
more pores within the activated carbon, resulting in a
greater surface area of activated
carbon. (Ahmed and
Theydan, 2013),
Hamdaoui et al., 2008)
. This
increased surface area results in increasing adsorption
capabilities from activated carbon. The increasing
adsorption capability of activated carbon, the better
the quality of the activated carbon.
4 CONCLUSIONS
In this study it can be concluded that all types of
activators used for the manufacture of activated
carbon from rubber shells meet the standards (SNI.,
1995) for proximate analysis while for iodine
absorption only H
3
PO
4
activators meet technical
activated charcoal quality standards namely H
3
PO
4
activators at 0.57% moisture content, 0.40% ash
content, 9.82% volatile matter and iodine absorption
of 755.13mg/g. Exposure of ultrasonic waves at the
time of chemical activation for all types of activators
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
636

indicates the effect of ultrasonic waves seen from the
comparative data of proximate analysis and iodine
absorption in each activator with and without the help
of ultrasonic waves.
The use of rubber shells as raw material for
making activated charcoal can reduce environmental
pollution and increase the economic value of rubber
shell.
ACKNOWLEDGEMENTS
The author would like to acknowledge the Center for
Research and Community Service at Polytechnic
State of Samarinda which has provided funding for
this research as well as to the Chemical Engineering
Laboratory of Polytechnic State of Samarinda as a
place for the research to be carried out.
REFERENCES
Bukallah, S.B., M.A. Rauf and S.S. Ashraf, 2007.
Photocatalytic decoration of coomass ie brilliant blue
with titanium oxide. Dyes Pig., 2: 353-356.
Chen, H., Chen, Z., Zhao, G., Zhang, Z., Xu, C., Liu, Y.,
Chen, J., Zhuang, L., Haya, T., Wang, X., 2018.
Enhanced adsorption of U(VI) and241Am (III) from
wastewater using Ca/Al layered double
hydroxide@carbon nanotube composites. J. Hazard.
Mater. 347, 67–77,
Cheung, C.W., J.F. Porter and G. McKay, 1997. Sorption
kinetics for the removal of copper and zinc from
effluents using bone char. Sep. Purf. Technol., 19: 55-
64.
Hamdaoui, O., Chiha, M. and Naffrenchoux, E.,
“Ultrasound assisted removal of malachite green from
aqueous solution by dead pine needles”, Ultrasonic
Sonochemistry, Vol.15, No. 5, (2008), 799-807.
Ioannidou, O. and A. Zabaniotou, 2007. Agricultural
residues as precursors for activated carbon production-
A review. Renewable Sustainable Energy Rev., 11:
1966-2005.
Kentish, S. and Ashokkumar, M., “Physical and chemical
effects of ultrasound”, Ultrasound Technology for Food
and Bioprocessing, (2011), 1-12.
Lee, J., Kim, K.H., Kwon, E.E., 2017. Biochar as a catalyst.
Renew. Sustain. Energy Rev. 77,
MJ Ahmed, SK Theydan, Micro-porous activated carbon
from Siris seed pods by microwave-induced KOH
activation for metronidazole adsorption, J. Anal. Pirole
app., 99 (2013) 101-109
Mouni, L., Belkhiri, L., Bollinger, J.C., Bouzaza, A.,
Assadi, A., Tirri, A., Dahmoune, F., Madani, K.,
Remini, H., 2018. Removal of methylene blue from
aqueous solutions by adsorption on Kaolin: kinetic and
equilibrium studies. Appl. Clay Sci. 153, 38–45.
Noorfidza Yub Harun, MT Afzal, Mohd Tazli Azizan,
Rubber Seed Kernel TGA Analysis, International
Journal of Engineering, 3 (2010) 639-641
Rao, N.N., K.M. Somasekhar, S.N. Kaul and L.
Szpyrkowicz, 2001. Electochemical oxidation of
tannery waste water. J. Chem. Technol. Biotechnol., 76:
1124-1131.
Salem, I.A. and M. El-Maazawi, 2000. Kinetics and
mechanism of color removal of methylene blue with
hydrogen peroxide catalyzed by some supported
alumina surfaces. Chemosphere, 41: 1173-1180.
SNI. (1995). Technical Activated Charcoal (06-3730th–
19th ed.).
Sodeifian, G., Ali, S., 2018. Utilization of ultrasonic-
assisted RESOLV (US-RESOLV) with polymeric
stabilizers for production of amiodinearone
hydrochloride nanoparticles: optimization of the
process. Chem. Eng. Res. Des. 142, 268–284,
Stephenson, R.J. and J.B. Sheldon, 1996. Coagulation and
precipitation of mechanical pueffluent. 1. Removal of
carbon and turbidity. Water Res., 30: 781-792.
T. Wang, S. Tan, C. Liang, Preparation and characterization
of activated carbon from wood via microwave-induced
ZnCl2 activation, Carbon. 47 (2009) 1880-1883.
Wan Ngah, W.S. and M.A.K.M. Hanafiah,
2008.Adsorption of copper on rubber (Hevea
brasiliensis) leaf powder: Kinetic, equilibrium and
thermodynamic studies. Biochem. Eng. J., 39: 521-530.
Y. Ji, T. Li, L. Zhu, X. Wang, Q. Lin, Preparation of
activated carbon by microwave heating activated KOH,
Appl. Surfing. science. 254 (2007) 506-512.
Effect of Type of Activator and Ultrasonic Waves on the Chemical Activation Process on the Characteristics of Activated Charcoal from the
Rubber Fruit Shell (Hevea Brasiliensis)
637
