
The Physicochemical Characteristic and Inhibition Zone of
Eschericia coli in Ketepeng (Cassia alata L.) Leaf Extract
Transparent Soap
Encik Eko Rifkowaty
a
and Adha Panca Wardanu
b
Department of Plantation Product Management, Politeknik Negeri Ketapang, Ketapang, Indonesia
Keywords: Inhibition Zone of Eschericia coli, Ketepeng Leaf Extract, Physicochemical Characteristic, Transparent Soap.
Abstract: Ketepeng (Cassia alata) is a wild plant that is traditionally recognized as a medication for skin diseases. The
leaf of Ketepeng has a phytochemical content that plays a role to inhibit the growth of Eschericia coli. A
transparent soap with an addition of leaf extract is one of the innovations to increase the economic value of
the Ketepeng plant. This research aims to identify the effect of adding leaf extract on the quality of transparent
soap based on the Indonesian National Standards (SNI). The experiment design that used is Completely
Randomized Design (RAL) with a three-time repetition. It comprised three different treatments consist of the
addition of leaf extract as much as 2, 4, and 6 grams in 50-gram coconut oil as the basic material of soap. The
result of this research indicated that increasing the concentration of leaf extract might increase saponification
value, hardness, pH, inhibition zone of Eschericia coli ATCC 25922. It was also reported to increase the
attribute texture of the transparent soap, as well as to decrease water content, foam stability, free alkali, and
soap transparency. The best treatment in this study was the transparent soap which was added by 6 grams of
ketepeng leaf extract.
1 INTRODUCTION
The COVID-19 (coronavirus) pandemic requires
people to live clean. Washing hands with antiseptic
soap is one way to prevent COVID-19. Transparent
soap with the addition of ketepeng left extract is an
alternative to antiseptic soap. According Widiarto,
Janiarta, Intan and Hajiriah (2018) antiseptic soap is
a soap that contains chemical compounds that are
used to kill or inhibit the growth of microorganisms
on living tissues such as the surface of the skin and
mucous membranes. The use of antiseptics is highly
recommended when there is an epidermal disease
because it can slow the spread of the disease the
spread of the disease.
Ketepeng leaf extract has secondary metabolite
compounds for example alkaloid, flavonoid, tannin,
anthraquinone, and saponin have antibacterial
activities against Escherichia coli, Staphylococcus
aureus and Pseudomonas aeruginosa (Ekwenye and
Okorie, 2010; Lumbessya, Abidjulua, and Paendonga
a
https://orcid.org/0000-0003-2984-6977
b
https://orcid.org/0000-0002-4970-248X
2013). Ecoli is a gram-negative bacteria that can
cause skin diseases. However, it can be prevented by
the use of antibacterial soap (Stevens, Nicholas, and
David 2003). Generally, secondary metabolite
compounds have antibacterial. Thus, they can be used
as active materials in a soap-making process.
Meanwhile, saponin contains several properties like
the foaming agent, easily soluble, surfactant
compounds, and antibacterial (Tebogo, 2004).
Indonesia is home of tremendous natural
resources. One of the natural resources is Ketepeng
plant which is known as a cure and anti-bacteria for
skin problems such as ringworm and itching
(Kusmardi, Kumala, and Triana, 2007). Traditionally,
Ketepeng plant is applied by rubbing or sticking its
leaves to the affected skin. Esimone, Nworu, Ekong,
and Okereke (2007) discuss that this plant has an
excellent wound-healing characteristic. The results of
herbal soap which adding ketepeng leaves extract
with ethanol extract (95%) indicate has antibacteria
activities covering S. aureus, B. subtilis, E. coli, P.
786
Rifkowaty, E. and Wardanu, A.
The Physicochemical Characteristic and Inhibition Zone of Eschericia coli in Ketepeng (Cassia alata L.) Leaf Extract Transparent Soap.
DOI: 10.5220/0010953600003260
In Proceedings of the 4th International Conference on Applied Science and Technology on Engineering Science (iCAST-ES 2021), pages 786-793
ISBN: 978-989-758-615-6; ISSN: 2975-8246
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

aeruginosa, and C. Albicans. The soap with adding of
ketepeng extract on the market.
Ecoli is microorganism which can serve as an
indicator towards the possible existence of pathogen.
Pathogen is gram negative bacteria that can cause
skin diseases. However, it can be prevented by the use
of antibacterial soap (Stevens et al. 2003).
antibacterial properties such as saponin, tanin,
terpenoid (Ugbabe, Ezeunala, Edmond, Apev and
Salawu., 2010), alkaloid (Jasim, Hussein, Hameed
and Kareen 2015), flavonoid, tripenoid (Cushnie and
Lamb, 2005). Thus, they can be used as active
materials in a soap-making process. Meanwhile,
saponin contains several properties like foaming
agent, easily soluble, surfactant compounds, and
antibacterial (Widyasanti and Hasna, 2017 ; Tebogo,
2004).
This research uses coconut oil as a basic
ingredient in making soap. Coconut oil contains
saturated fatty acids which play a role in the foaming
properties of the soap. In particular, Lauric acid
(C12:0) in coconut oil produces soap with high
solubility and soft foam characteristics (Anggraini,
Ismanto, and Dahlia, 2015; Habib, Kumar, Sorowar,
Karmoker, Khatun and Al-Reza, 2016). This
becomes the basis of the research because it is
assumed that the addition of ketepeng leaf extract
with different concentrations can affect the
physicochemical characteristics of the resulted soap
and increase the inhibition zone of Escherichia coli.
This research could be antiseptic soap alternative in
the pandemic situation.
2 MATERIAL AND METHODS
2.1 Raw Material
This research was conducted in two phases. It started
by preparing sorting ketepeng leaves, extraction, and
a transparent soap making process. The ketepeng leaf
were taken into 5 parts from left to right of the stem
base of the Ketepeng. Prepare 50 g coconut oil. After
that, the soap involved chemical materials by using
NaOH (merck) 5 g, NaCl (merck) 0.2 g, citric acid 0.2
g, glycerin (brataco) 6.5 g, aquadest 5 ml, sugar
(brand: Gulaku) 7.5 g and ethanol (merck) 70% 7.5 g.
Pure inoculant of ATCC 25922 Escherichia coli.
2.1.1 Ketepeng Leaf Extraction
The process of ketepeng leaf extraction was
performed by refining 20 g ketepeng leaves which
had been sorted and dissolved with a 200 ml alcohol
solvent. The ratio of ingredients and solvents was
1:10. Having filtered the basic ingredients, then the
filtrate was evaporated by using a rotary evaporator
(RV-10, IKA-WERKE, Germany) at a 70⁰C
temperature, a speed of 160 rpm, and a pressure of
2.5. The process took place for 9 hours in order to
obtain thick extracts (Hernani, Bunasor and Fitriarti,
2010).
2.1.2 Making of Transparent Soap
The transparent soap was made by heating 50 g
coconut oil at 70⁰C temperature. After that, 5 g NaOH
was dissolved to 5 ml of water and stirred until
dissolved. Put the solution in the oil and then add 7.5
g of ethanol and 7.5 g of sugar. Prior to that, the
ethanol and sugar should be dissolved. Finally, add
glycerin by 6.5 g. All materials were stirred until the
stock of soap was formed. It was followed by adding
0.2 g of NaCl, 0.2 g of citric acid, and extract of
ketepeng leaf by 2, 4, 6 g until all the mixture was
immunized. After that, the mixture was poured into a
mold and it was cooled until the soap hardens. The
resulted soap was then examined in several tests like
free alkali test, water content test, hardness, pH test,
saponification value, foam stability, inhibition zone
test, and organoleptic test of color, texture,
transparency.
2.2 Statistical Analysis
This research used a completely randomized design
using 3 (three) repititions. After gathering the data, if
F table was smaller than F count, further test was
conducted namely the least significant different
(LSD) with 0.01 rate. The observation parameter
covered:
2.3 Parameter Analysis
2.3.1 Water Content
This research counted 5 gram of transparent soap in a
petri dish and put in the oven at 105⁰C temperature
for 4 hours. Then, the soap was cooled, desiccated,
weighted, and heated for 1 hour to obtain a constant
weight. After that, the water content was calculated
by using the following formula:
2.3.2 pH
The soap was weighted 1 g and dissolved in 10 ml
aquadest. When necessary, the mixture could be
heated to accelerate the dissolution process. After
that, a pH meter was dipped in the solution. Acidity
The Physicochemical Characteristic and Inhibition Zone of Eschericia coli in Ketepeng (Cassia alata L.) Leaf Extract Transparent Soap
787

degree (pH) was observed to obtain and take note the
results.
2.3.3 Hardness
To measure the level of hardness towards the resulted
soap, the research employed a penetrometer tool.
2.3.4 Foam Stability
1 g of sample was dissolved in 9 ml water and then
poured in a reaction tube. Then, the mixture was
shaken by using vortex for 30 seconds. This process
resulted foam and the height of the foam was
measured. The sample foam was left for 1 hour and
the foam was measured again. If the sample number
was more than one, the dimensions of all tubes must
be similar. To measure, the foam stability, the
following formula could be performed (1).
()
()
x 100 % (1)
2.3.5 Saponification Value
The soap was weighted 1.5 g in 250 ml of
Erlenmeyer. Add 50 ml NaOH solution in alcohol.
After that, the solution was covered by a condenser
and boiled for 30 seconds. Then, add several drops of
phenolphthalein indicators and titrate with 0.5 N HCl
until its color changed from pink to transparent.
Saponification measure following formula (2).
(
)
,
,
𝑥 100% (2)
Description:
V
2
= The volume of the sample titration
V
1
= The volume Blanco titration
N = Normality 0,5 HCl
0,04 = Atom Weight NaOH
0,258 = The average saponification value of
coconut oil
W = Sample weight
2.3.6 The Number of Free Alkali
The transparent soap was weighted 10 g and
dissolved in 50 ml of hot alcohol. After that, drop
indicators pp 2-3 drops, refluxed for about 30
minutes. Then the soap was cooled and titrated by
using KOH 0.1% until it turned to pink. Take note the
titration volume and calculate the free alkali (3).
𝑥 100% (3)
Description:
V = Titration volume
BM = molecule weight
W = Sample weight
2.3.7 Bacteria Inhibition Zone Test
1) Microbe Rejuvenation Test
NA Media was weighted 5 g and dissolved by
aquadest by 250 ml. Sterilization was performed until
the temperature reached 121⁰C. Keep it still for 15
minutes then the media was put into reaction tubes
respectively to form titled NA. After the solid titled
NA was obtained from the Eschericia coli ATCC
25922 breed as much as one inoculating loop. Then,
they were inoculated in the titled NA media. The
tested bacteria were incubated for 24 hours at 37⁰C
and it could be used as tested bacteria.
2) Making test bacterial suspension
Test bacteria resulted from rejuvenation process went
through suspension process by using NaCl 0.9%
solution which was put into respective reaction tubes.
After that, it was mixed with a sterilized NA media
and then immunized.
3) Bacteria inhibition zone test
NA media was turned to a solid form in a petri dish.
After being solid, prepare a bacteria suspension test.
Dip a stick with cotton at the end on the bacteria
suspension test than swab vertically and horizontally
on the NA surface which had been solidified until all
surfaces were covered. Leave it until all surfaces
dried. Meanwhile, each transparent soap sample was
melted on a hot plate. Soak disc paper for 1 hour in
the soap sample which was melted and then dry the
disc paper.
After all NA surfaces dried, the disc paper which
was soaked in the transparent soap was put on the NA
surface by using pin set. It was then incubated at 37⁰C
for 24-48 hours. The petri dish which was incubated
for 24-48 hours then was observed and measured to
obtain its inhibition diameter.
2.3.8 Organoleptic Test
Organoleptic test was performed to score the attribute
of transparency and texture. Panelists in this research
were 25 semi-trained panelists. The assessment score
was based on the level of quality where 1 (one)
indicated the lowest score and 7 (seven) meant the
highest.
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
788

3 RESULT AND DISCUSSION
3.1 Water Content
High water content in the soap may cause a hydraulic
reaction between water and fat which is not
sapofinied to form free fat acid and glycerol. This
may cause the decrease of quality during storage
(Vivian, Nathan, Osano, Mesopirr and Omwoyo,
2014). The high water content may lead the soap to
easily dissolve in water, shrink, and have a soft
texture (Hambali, Bunasor, Suryani and Kusumah
2005). In addition, it may cause a short span of the
soap storage (Habib, et.al., 2016). Table 1 shows that
the water content kept decreasing as the addition of
ketepeng leaf extract. The water content of soap
which was added by ketepeng leaf extract by 2, 4,
and 6 gram decreased by 18.15, 12.09, and 10.78%.
According to Indonesian National Standard (SNI),
the maximum water content of soap is 15%.
Table 1: Water content of Transparent Soap.
Ketepeng Leaf
Extract Treatmen
t
Water Content
(%)
2
g
ra
m
18.15
a
±0.23
4
g
ra
m
12.09
b
±0.41
6
g
ra
m
10.78
c
±0.28
The decrease of water content in the soap which
was added by ketepeng leaf extract was resulted from
active compound of saponin contained in the plant.
This compound possessed a characteristic to absorb
water which could decrease the percentage of water
in the soap. Widyasanti and Hasna (2017) in their
study on transparent soap with white tea extract claim
that the more white tea extract added to the soap, the
more water will be absorbed by sugar so that the
water in the soap decreases. Saponin is a glycoside
compound which will produce glycone (sugar) and
glycone (non-sugar) if hydrolyzed. Sugar has
hygroscopic properties and causes the soap to
solidify.
3.2 Saponification Value
The saponification value gives information about the
solubility in water and soap formation (Ohimain,
Izah, and Fawari, 2013). The degree of saponification
depends on the type of ion, its ionic strength, the
temperature of the solution, and above all on the pH
of the solution (Briscoe, Evans, and Tabor, 1976).
Saponification value indicates the neutralization
process between fatty acid and base in the process of
soap stock. If the saponification is not perfect, it may
increase the percentage of free alkali or unsaponified
fat acid. As a result, it can decline the quality of the
soap.
The addition of leaf extract increased significantly
the value of saponification (Tabel 2). The increase of
this value is a result of alkaloid content in the leaf
extract. Alkaloid constitutes a hydrogen base so that
the addition of leaf extract will affect the solution's
basicity. Cotte, Checroun, Susini, Dumas, Tchoreloff,
Besnard and Walter (2006) explain that the process of
triolein saponification with various types of lead salts
on pH base can increase the percentage of soap
amount. Sears and Schulman (1964) argue that pH 13
saponification is essentially complete. At a lower pH
8.5 the degree of association between the cation and
the fatty acid could be less than complete.
Table 2: Saponification Value of Transparent Soap.
Ketepeng Leaf
Extract Treatmen
t
Saponification Value
(M
g
/NaOH)
2
g
ra
m
155.5
c
± 0.81
4
g
ra
m
164.8
b
± 0.18
6
g
ra
m
178.2
a
± 0.46
3.3 Hardness
Level of hardness test aims at understanding the
efficiency of the soap when used. Hard soap is
considered higher resistance towards damage or form
changing as a result of physical disturbances.
Table 3: Hardness of Transparent Soap.
Ketepeng Leaf
Extrac
t
Treatmen
t
Hardness
2
g
ra
m
0.5
c
±0.04
4
g
ra
m
1.1
b
±0.05
6
g
ra
m
1.4
a
±0.02
Level of hardness on the soap correlated with the
percentage of water content and saponification value.
The water content decreases when the leaf extract is
added and the level of hardness increases too.
Similarly, the increase also occurs to saponification
value that can affect the hardness of the soap.
Saponifaction value provides information about the
soap forming process (Ohimain et al. 2013). A high
value of saponifation that has a lot of greased fat
indicates a high quality of the soap (Ketaren, 1986
cited in Kusumaningsih and Hastuti, 2014). Soap
texture can be affected by the length of hydrocarbon
chain and oil doubled bond.
The Physicochemical Characteristic and Inhibition Zone of Eschericia coli in Ketepeng (Cassia alata L.) Leaf Extract Transparent Soap
789

3.4 Foam Stability
Foam stability is measured by esthetic value of a soap
product. Customers perceive that good soap is those
which produce much foam. In fact, the amount of
foam is not necessarily correlated positively with its
ability to clean dirt. There is no requirement regarding
the minimum or maximum of foam height for soap.
The results of this research revealed that the
addition of leaf extract in the soap would make the
foam stability low. Based on LSD (0.01) soap that
was added 4 and 6 grams of the leaf extract was
significantly different from a 2-gram addition. The
concentration of leaf extract increases significantly
decrease the stability of the foam.
Table 4: Foam Stability of Transparent Soap.
Ketepeng Leaf
Extract Treatmen
t
Foam Stability
2
g
ra
m
55.5
a
±1.08
4
g
ra
m
38.4
b
±0.79
6
g
ra
m
35.7
b
±0.92
Ketepeng’s leaf which is extracted by using
ethanol contains alkaloid, saponin, flavonoid, tannin,
and antraquinon compounds (Lumbessya et al. 2013).
Jin-Young Park et al. (2005) argue that saponin has
several properties such as easy to dissolve in water,
surfactant, and the ability of form stable foam in
solution. For these reasons, saponin is often used as
an agent of foaming, emulsifier, and detergent in meal
and non-meal products. However, as the
concentration of leaf extract increases, the stability of
the foam decreases. This is in line with a study by
Widyasanti and Hasna (2017) in transparent soap
with white tea extract. Adding white tea extract would
make the foam stability low. This occurs because the
foam stability is influenced by ethanol content in the
white extract. Putri (2017) supports that methanol and
ethanol in the extracts play a role as antifoaming
agent in the soap.
Beside saponin, alkaloid also affects the stability
of foam in soap. Saunders (1935) widely claims that
the tension of soap surface is affected by pH. pH itself
is influenced by the amount of organic base like
alkaloid. Widyasanti and Hasna (2017) posit that
alkaloid has base properties causing it to increase the
degree of acidity in soap. According to Gwi-Taek
Jeong, Hwa-Won Ryu, Yung-Il Joe, Don-Hee Park
and Tanner (2002) base pH can potentially decrease
the foam stability. Hence, it is assumed that the
addition of leaf extract will increase alkaloid content
in the soap that it can decrease the foam stability.
Ketepeng extract contains pigment chlorophyll,
the pigment can arise in the solution can increase
surface tension, thereby reducing foam stability. The
presence of dissolved substances in the liquid will
increase viscosity which in turn will increase surface
tension and result in reduced foaming ability.
3.5 pH
Incomplete hydrolysis from saponification process
produced high pH values so that acidity degree (soap
pH) should be measured. Normal soap pH is around
9.0-10.8 (Gusviputri, Meliana, Aylianawati and
Indraswati 2013). pH 10 has base properties and is
good for skin. If the pH is <9, it might cause the skin
dry. Meanwhile, pH values which are >10.8 may
cause skin irritations. Table shows that the addition of
leaf extract by 2, 4, and 6 gram has acidity degree or
pH around 9.33-9.67. It indicates that pH of
transparent soap which is added by the leaf extract has
met SNI 06 3532 1994.
Table 5: pH of Transparent Soap.
Ketepeng Leaf
Extract Treatmen
t
pH
2
g
ra
m
9.33
a
±0.94
4
g
ra
m
9.67
a
±0.47
6
g
ra
m
9.67
a
±0.47
3.6 Free Alkali
Free Alkali in soap is caused by the presence of alkali
which does not react with fat acid during a
saponification process (Zulkifli and Estiasih, 2014).
The increase of concentration of leaf extract
significantly raises the saponification values. It leads
to the decline of free alkali because of the lack of
alkali which reacts with fatty acid. Based on SNI 06
3532 1994 regulations, the value of free alkali in soap
should be a maximum of 0.1%. In this research, the
LSD (0.01) test of free alkali towards the three
treatments was not significantly different. It shows us
that the tested soap is safe. High free alkali (>0.1%)
will lead to skin irritations (Hernani et al. 2010) and
dry skin (Widyasanti and Hasna, 2017).
Table 6: Free Alkali of Transparent Soap.
Ketepeng Leaf
Extract Treatmen
t
Free Alkali (%)
2
g
ra
m
0.0060
a
±0.006
4
g
ra
m
0.0056
a
±0.006
6
g
ra
m
0.0026
a
±0.003
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
790
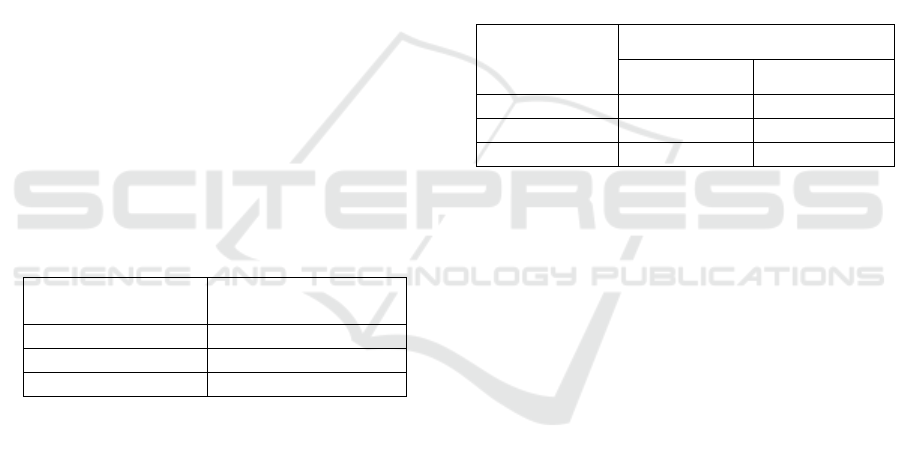
3.7 Inhibition Zone
Escherichia coli is bacteria that cause diarrhea,
urinary tract infection, meningitis, peritonitis,
mastitis, septicemia, pneumonia, and food poisoning
(Brooks, Butel and Morse 2008). Inhibition zone is an
active substance’s or compound’s ability to inhibit the
growth of microbe. It is marked by the existence of
transparent zone on agar media. Based on LSD (1%)
test, the inhibition zone of transparent soap towards
the three treatments was significantly different. The
more the addition of leaf extract on the soap would be
able to inhibit the growth of Escherchia Coli ATCC
25922. The difference of the inhibition zone size from
each concentration stemmed from the difference of
active compound contents. It was shown by the size
of the inhibition zone which increasingly widespread.
Juliansyah and Paotonan (2017) support that the
higher the concentration of the extract, the more
active compounds which potentially serve as
antibacterial. The ketepeng leaf extract contains
secondary metabolite compounds like alkaloid,
flavonoid, anthraquinone, and saponin. These
compounds constitute as phenolic compounds which
has a phenol group. Hernani et al. (2010) argue that
the phenol group has bacteriacide properties because
it may cause protein coagulation and bacteria cell
membranes may become lysis.
Table 7: Inhibition Zone of Transparent Soap.
Ketepeng Leaf
Extract Treatmen
t
Inhibition Zone
(mm)
2
g
ra
m
1.8
c
±0.24
4
g
ra
m
4.0
b
±0.47
6
g
ra
m
6.0
a
±0
In this research, the inhibition zone of transparent
soap which was added leaf extract by 2 gram (1.8
mm) was categorized weak. The addition of the
extract by 4 gram (4 mm) and 6 gram (6 mm) was
grouped medium. Magdalena and Kusnadi (2015)
explain the level of resistance of negative Gram
bacteria (Escherichia coli ATCC 25922) was high
One of the reason was because the structure of
bacteria cell walls of the negative gram bacteria was
complex. It had three layers, outside layers
lipoprotein, middle layer lipopolysaccharide which
served to block antibacterial bioactive materials, and
inside layer peptidoglycan which had high lipid
contents (11-12%). This made the active compound
of leaf extract difficult to penetrate the nonpolar lipid
layer on Escherichia coli ATCC 25922.
3.8 Organoleptic Test
An organoleptic test for transparent soap with leaf
extract covered attribute of transparency and texture
ranging from 1 – 7. Soap texture assessment is
performed by pressing samples using fingers. In the
other side, transparency test is done by using the
senses of vision by observing samples with the help
of light (Meilgaard, civele and Carr, 1999).
Table 8 show that the texture attributes of the
three treatments were not significantly different. It
can be seen from the results of the panelist's
assessment which pointed out that the three
treatments had the same texture (from neutral to
rather hard). Meanwhile, the attributes of
transparency in the three different treatments were
very significant (neutral to transparent).
Table 8: Organoleptic Test of Transparent Soap.
Ketepeng Leaf
Extract
Treatmen
t
Organoleptic Test
Texture Transparency
2
g
ra
m
4,32 5,48
a
4
g
ra
m
4,40 5,36
a
6
g
ra
m
4,72 4,20
b
The increased concentration of leaf extract could
improve the texture of the soap. This result is
correlated with the decrease of the soap water content.
Low water content in soap increases the hardness of
the soap (Tabel 1). Based on the organoleptic test,
The texture attributes of the three treatments were not
significantly different. It indicates that the addition of
leaf extract does not affect consumer perceptions
towards texture attributes.
Meanwhile, adding 2 and 4 grams of ketepeng leaf
extracts was significantly different from 6 grams on
the attribute of transparency (Tabel 8). It shows that
the addition of ketepeng extract affects consumer
perceptions of the attributes of transparency. The
more addition of leaf extract to transparent soap, the
browner and less transparent soap is. According to
Anggraini et al. (2015) a heating process causes
chlorophyll (green) to become a compound of
pheophytin (olive green). Therefore, if the soap is
added by more leaf extract, the soap will turn to
dominantly brown. The flavonoid content contained
in the extract also affects transparency. According to
Ahmad, Hasan, Muhamad, Bilal, Yusof and Idris
(2018) the brightness level of transparency decreases
with the increased content of flavonoids and phenol
compounds.
Based on the Indonesian National Standards
(SNI) for solid bath soap, the treatment of the addition
The Physicochemical Characteristic and Inhibition Zone of Eschericia coli in Ketepeng (Cassia alata L.) Leaf Extract Transparent Soap
791

of 6-gram ketepeng leaf extract had a better quality
soap than the two treatments. The treatment also had
the largest ATCC 25922 Escherichia coli inhibition
zone as much as 6 mm despite having low foam
stability by 35.7. Finally, the organoleptic score of
texture attribute was 4.72 (rather hard) and the
transparency value was 4.20 (from neutral to rather
transparent).
4 CONCLUSION
In conclusion, increasing the concentration of leaf
extract could increase saponification, hardness, pH,
Escherichia coli inhibition zone ATCC 25922, and
the texture attributes of transparent soap. In addition,
it is capable of reducing water content, foam stability,
free alkali and soap transparency.
The best treatment in this study was transparent
soap which was added by leaf extract as much as 6
grams, although it produced soap which its
transparency attributes ranged from neutral to rather
transparent.
REFERENCES
Ahmad, N., Hasan, Z.A.A., Muhamad, H., Bilal, S.H.,
Yusof, N.Z., and Idris, Z., (2018). Determination of
Total Phenol, Flavonoid, Antioxidant Activity Of Oil
Palm Leaves Extracts and Their Application in
Transparent Soap. Journal of Oil Palm Research
Vol.30(2) June 2018 p.315-325.
Anggraini, T., Ismanto, S.D. and Dahlia, (2015). The
making of Transparent Soap From Green Tea Extract.
International Journal on Advanced Science
Engineering Information Technology vol.5 (2015)
No.4.
Briscoe, B.J., Evans, D.C.B., Tabor, D., (1976). The
Influence of Contact Pressure and Saponification on the
Sliding Behavior of Stearic Acid Monolayers. Physics
and Chemistry of Solids, Cavendish Laboratory,
Cambridge, England. Journal of Colloid and Interface
Science, VoL 61, No. 1, August 1977.
Brooks, G.F., Butel, J.S. & Morse, S.A. (2008).
Mikrobiologi Kedokteran. Jakarta: Salemba Medika.
Cotte, M., Checroun, E., Susini, J., Dumas, P., Tchoreloff,
P., Besnard, M., Walter, Ph., (2006). Kinetics of Oil
Saponification By Lead Salts In Ancient Preparation Of
Pharmaceutical Lead Plasters And Painting Lead
Mediums. Talanta 70 (2006) 1136-1142.
www.elsevier.com/locate/talanta.
Cushnie, T.P.T., and Lamb, A..J., (2005). Antimicrobial
Activity of Flavonoid. Internationl Journal of
Antmicrobial Agents 26 (2005) 343-356.
Ekwenye, U.N., and Okorie, C.F., (2010). Antibacterial
Activity of Terapleura Taub.pod Extracts. International
Journal of Pharma and Bio Sciences Vol.1/Issue-4/Oct-
Dec.2010.
Esimone, C., Nworu, C., Ekong, U., Okereke, B., (2007).
Evaluation of the antiseptic properties of Cassia
alatabased herbal soap. The Internet Journal of
Alternative Medicine Volume 6 Number 1.
Gusviputri, A., Meliana, N., Aylianawati, Indraswati, N,
(2013). Pembuatan Sabun Dengan Lidah Buaya
(Aloevera) Sebagai Antiseptik Alami. WIDYA TEKNIK
Vol. 12, No. 1, 2013 (11-21)
Gwi-Taek Jeong, Hwa-Won Ryu, Yung-Il Joe, Don-Hee
Park, and Tanner, R.D., (2002). Effect of pH on the
Foam Fractionation of Seed Proteins. Theories and
Applications of Chem. Eng., 2002, Vol. 8, No. 2.
Habib, A., Kumar, S., Sorowar, S., Karmoker, J., Khatun,
K., Al-Reza, S., (2016). Study on the Physicochemical
Properties of Some Commercial Soaps Available in
Bangladeshi Market. International Journal of
Advanced Research in Chemical Science (IJARCS)
Volume 3, Issue 6, June 2016, PP 9-12.
Hambali, E. T. K., Bunasor, A. Suryani and Kusumah, G.
A. (2005). Aplikasi Dietanolamida Dari Asam Laurat
Minyak Inti Sawit Pada Pembuatan Sabun Transparan.
Jakarta: Penebar Swadaya.
Hernani, Bunasor, T.K., Fitriati, (2010). Formula Sabun
Transparan Antijamur Dengan Bahan Aktif Ekstrak
Lengkuas ( Alpinia galanga L.Swartz.). Bul. Littro. Vol.
21 No. 2, 2010, 192 – 205.
Jasim, H., Hussein, A.O., Hameed, I.H., and Kareen, M.A.,
(2015). Characterization of Alkaloid constitution and
Avaluation of Antimicrobial Activity of Solanum
nigrum Using Gas Chromatography Mass
Spectrometry (GC-MS). Journal of Pharmacognosy
and Phytotherapy Vol 7(4), pp.56-72, April 2015.
Jin-Young Park, Minerva A Plahar, Yen-Con Hung, Kay H
McWatters and Jong-Bang Eun, (2005). Effect of
saponins on the foam/flow properties of paste and
physical characteristics of akara made from
decorticated black-eyed cowpeas. J Sci Food Agric
85:1845–1851(2005).
Juliansyah dan Paotonan (2017). Uji Daya Hambat Sediaan
Sabun Transparan Ekstrak Jarak Pagar (Jatropha
curcas) Terhadap Pertumbuhan Bakteri Uji
Propionibacterium acnes. Jurnal Mandala Pharmacon
Indonesia, Vol 3.No.2.
Kusmardi., S. Kumala dan E.E Triana. (2007). Efek
Imunolodulator Ekstrak Daun Ketepeng Cina
(Cassia alata) terhadap Aktifitas dan Kapasitas
Fagositosis Makrofag. Makara Kesehatan. Vol.11, No
2.
Kusumaningsih, W.S., Hastuti, S. (2014). Pengaruh Ekstrak
Etanol Dan Dekokta Kulit Manggis (Garcinia
Mangostana L.) Sebagai Pewarna Terhadap Kualitas
Sabun Organik Transparan Berbasis Minyak Jelantah
Yang Dimurnikan Dengan Ekstrak Mengkudu Dengan
Pengaroma Minyak Atsiri Kulit Jeruk Purut (Citrus
Hystrix). Indonsian Journal on Medical Science
Volume 1 No 2 – 2014
iCAST-ES 2021 - International Conference on Applied Science and Technology on Engineering Science
792

Lumbessya, M., Abidjulua, J., Paendonga, J., (2013). Uji
Total Flavonoid Pada Beberapa Tanaman Obat
Tradisonal Di Desa Waitina Kecamatan Mangoli Timur
Kabupaten Kepulauan Sula Provinsi Maluku Utara.
JURNAL MIPA UNSRAT ONLINE 2 (1) 50-55.
Magdalena, N.V., dan Kusnadi, J., (2015). Antibakteri Dari
Ekstrak Kasar Daun Gambir (Uncaria gambir var
Cubadak) Metode Microwave-Assisted Extraction
Terhadap Bakteri Patogen. Jurnal Pangan dan
Agroindustri Vol. 3 No 1 p.124-135, Januari 2015.
Meilgaard M, GV civele & BT Carr. (1999). Sensory
Evalution Technique. New York: CRC Press.
Ohimain, E. I., Izah, S.C. and Fawari, A.D. (2013). Quality
Assessment of Crude Palm Oil Produced by Semi-
Mechanized Processor in Bayelsa State, Nigeria.
Journal of Agriculture and Food Sciences
www.resjournals.org/JAFS ISSN: 2346-7002 Vol.
1(11): 171-181, November, 2013.
Putri, W.E.S., (2017). The Quality of Transparent Soap
with Addition of Moringa Leaf Extract. Advances in
Social Science, Education and Humanities Research
(ASSEHR), volume 112. 1st International Conference
on Social, Applied Science and Technology in Home
Economics (ICONHOMECS 2017).
Saunders, L., (1935). Review Article: Surface And Colloid
Chemistry. University of London: School of Pharmacy.
Sears, D.F and Schulman, J.H., (1964). Influence of Water
Structures on the Surface Pressure, Surface Potential,
and Area of Soap Monolayers of Lithium, Sodium,
Potassium, and Calcium. The Journal of Phusical
Chemietry, Volume 68, Number 12 December, 1964.
SNI 06 3532, (1994). Sabun Mandi. Badan Standarisasi
Nasional, Indonesia.
Stevens, M., Nicholas, A., David, C., (2003). Review of
Coliforms as Microbial Indicators of Drinking Water
Quality. Australian Government National Health and
Medical Research Council.
Tebogo, M.O., (2004). Thesis: Separation and
Characterization of Saponins From The Bark Extract of
The South American Soap Bark Tree; Quillaja
Saponaria Molina (Potentialimmuno-Adjuvant Active
Compounds). School of Pharmacy Memorial
University of Newfoundland St John's, Newfoundland.
Canada.
Ugbabe, G.E, Ezeunala, M.N, Edmond, I.N, Apev, J and
Salawu, O.A. (2010). Preliminary Phytochemical,
Antimicrobial and Acute Toxicity Studies of the Stem,
bark and the Leaves of a cultivated Syzygium cumini
Linn. (Family: Myrtaceae) in Nigeria. African Journal
of Biotechnology Vol. 9(41), pp. 6943-6747, 11
October, 2010.
Vivian, O.P., Nathan, O., Osano, A., Mesopirr, L.,
Omwoyo, W.N., (2014). Assessment of the
Physicochemical Properties of Selected Commercial
Soaps Manufactured and Sold in Kenya. Department of
Chemistry, Maasai Mara University, Narok, Kenya.
Open Journal of Applied Sciences, 2014, 4, 433-440.
Widiarto, M., Janiarta, M. A., Intan, P. K. and Hajiriah, T.
L. 2018. Analisa Kandungan Antiseptik Getah
Tumbuhan Patikan Kebo (Euphorbia hirta) Sebagai
Dasar Pembuatan Brosur Penanganan Luka Ringan
Pada Masyarakat. Bioscientist : Jurnal Ilmiah Biologi
vol. 6, no. 1, p. 7, 2018.
Widyasanti, A. dan Hasna, A.H., 2017. The study of
transparent soap making from virgin coconut oil-based
with the addition of white tea extract as an active
ingredients. J. Penelit. Teh Dan Kina, vol. 19, no. 2,
Jan. 2017.
Zulkifli, M., dan Estiasih, T., (2014). Sabun dari Distilat
Asam Lemak Minyak Sawit: Kajian Pustaka. J. Pangan
Dan Agroindustri, 2: 170–177.
The Physicochemical Characteristic and Inhibition Zone of Eschericia coli in Ketepeng (Cassia alata L.) Leaf Extract Transparent Soap
793
