
Ensemble Feature Selection for Heart Disease Classification
Houda Benhar
1
, Ali Idri
1,2
and Mohamed Hosni
1,3
1
Software Project Management Research Team, ENSIAS, Mohammed V University, Rabat, Morocco
2
Complex Systems Engineering and Human Systems, Mohammed VI Polytechnic University, Ben Guerir, Morocco
3
Laboratory of Mathematical Modeling, Simulation and Smart Systems, ENSAM-Meknes, Moulay ISMAIL University,
Meknes, Morocco
Keywords: Heart Disease, Classification, Feature Selection, Ensemble Learning, Ensemble Feature Selection, Univariate
Filter.
Abstract: Feature selection is a fundamental data preparation task in any data mining objective. Deciding on the best
feature selection technique to use for a specific context is difficult and time-consuming. Ensemble learning
can alleviate this issue. Ensemble methods are based on the assumption that the aggregate results of a group
of experts with average knowledge can often be superior to those of highly knowledgeable individual ones.
The present study aims to propose a heterogeneous ensemble feature selection for heart disease classification.
The proposed ensembles were constructed by combining the results of five univariate filter feature selection
techniques using two aggregation methods. The performance of the proposed techniques was evaluated with
four classifiers and six heart disease datasets. The empirical experiments showed that applying ensemble
feature ranking produced very promising results compared to single ones and previous studies.
1 INTRODUCTION
Heart disease (HD) is considered the principal cause
of death worldwide and is, therefore, one of the main
priorities in medical research (Benhar et al., 2019).
Early and accurate diagnosis of cardiac disease is
crucial to start appropriate treatment immediately and
prevent early death. Data mining (DM) tools have
been of great help to researchers to assist physicians
and support patients in regard to heart disease
diagnosis (Kadi et al., 2017). DM is the mathematical
core in the process of knowledge discovery in
databases (KDD) which offers powerful tools that
allow the extraction of meaningful information,
patterns, associations, or relationships from huge
amounts of data. Classification is the DM task most
frequently used by researchers to diagnose heart
disease (Benhar et al., 2019). Classification and other
DM techniques are usually hindered by some data
imperfections such as missing values, outliers, noise,
imbalanced data, and high dimensionality (Benhar et
al., 2020). A data preprocessing step is, therefore,
mandatory to prepare data for the KDD process.
According to the systematic literature review
conducted in (Benhar et al., 2019), researchers were
mainly interested in feature selection (FS) as a
preprocessing task in order to improve the
performance of their DM techniques in HD
prediction. Researchers made use of different types of
feature selection techniques such as filters, wrappers,
embedded, and hybrids. However, according to the
authors’ knowledge, no work has investigated the use
of ensemble FS to predict HD. Ensemble methods are
based on the assumption that combining the outputs
of multiple learners can be significantly more
accurate than the output of a single one (Zhou, 2012).
In addition to classification problems, ensemble
learning can be applied to improve other machine
learning tasks such as FS (Seijo-Pardo et al., 2017).
Ensemble FS techniques can be classified as: (1)
heterogeneous ensembles which consist of using
different FS techniques (or base selectors) and the
same training data, and (2) homogeneous which
consist of using the same base selector and different
data subsets.
The present study aims to propose an
heterogeneous ensemble FS for heart disease
classification by combining the results of five
univariate filter FS techniques namely Linear
Correlation (Gooch, 2011), ReliefF (Urbanowicz et
al., 2018), Information Gain (Quinlan, 1986),
Symmetrical uncertainty (Hall & Smith, 1998), and
Chi-square (Jin et al., 2006). Univariate filters, also
known as feature rankers, consist of ranking features
Benhar, H., Idri, A. and Hosni, M.
Ensemble Feature Selection for Heart Disease Classification.
DOI: 10.5220/0010800500003123
In Proceedings of the 15th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2022) - Volume 5: HEALTHINF, pages 369-376
ISBN: 978-989-758-552-4; ISSN: 2184-4305
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
369

individually based on some performance measures
and the final features subset can be determined by
setting a cutoff threshold or specify how many
features to retain. The proposed ensemble combines
the features’ scores obtained with base rankers using
mean and median combination methods and the final
feature subsets are obtained by selecting 40% of the
top ranked features. The subsets selected with
ensemble rankers as well as single ones were
evaluated using four classifiers: K-Nearest Neighbors
(KNN) (Han et al., 2012), Decision Trees (DTs) (Han
et al., 2012), Support Vector Machines (SVM)
(Vapnik, 2000), and Multilayer Perceptron (MLP) for
heart disease diagnosis (Gardner & Dorling, 1998).
The motivation behind the choice of the aforesaid
classifiers is that they are the most frequently used
classifiers by researchers to predict heart disease
(Benhar et al., 2019)(Hosni et al., 2020). Moreover,
the reason for choosing the abovementioned FS
techniques is their popularity among researchers in
heart disease classification (Benhar et al., 2020) and
several other fields such as bioinformatics, software
development effort estimation, network intrusion
detection, and educational data mining. The
experiments were performed using Python’s Scikit-
learn and ITMO-FS libraries (Schlemmer et al.,
2014)(Pilnenskiy & Smetannikov, 2020). The
classifiers were evaluated using a 10-fold cross
validation method and accuracy rate. Overall, this
study evaluates 192 variants of classifiers: 192 = (4
classifiers) * (5 univariate-filters + 2 ensembles +
original features set) * (6 datasets); and aims at
addressing the following research questions:
RQ1: Is there any single ranking technique that
distinctly outperform other single ranking
techniques?
RQ2: Do ensemble feature rankers (EFR) outperform
single ones when used for heart disease
classification? Is there a combination method that
resulted in better ensembles?
The remainder of this paper is organized as
follows: Section 2 presents a brief review of ensemble
approaches and related work. The experimental
design is described in Section 3. Results are presented
and discussed in Sections 4. Finally, the conclusions
and future work are presented in Section 5.
2 ENSEMBLE LEARNING AND
RELATED WORK
It is well known that a machine learning technique
can perform well on some data and less accurately on
others. Ensemble methods were introduced to
overcome the weaknesses of single techniques and
consolidate their advantages (Zhou, 2012). Ensemble
learning has become a hot topic for the last three
decades and has been successfully applied to various
fields including heart disease classification (Hosni et
al., 2021).
According to the results of the systematic map
conducted in (Hosni et al., 2021), most of the studies
state that ensemble methods are able to perform better
than single ones. An overview of a set of selected
studies in (Hosni et al., 2021) is presented below.
Bashir and al. (Bashir, Qamar, & Javed, 2015)
developed a heterogeneous ensemble classification
technique by combining three base classifiers: Naïve
bayes (NB), SVM, and DT. The classifiers were
combined using majority vote aggregation rule. The
proposed technique achieved and accuracy of 81.82%
on Cleveland heart disease dataset and outperformed
single techniques. LO and al. (Lo et al., 2016)
proposed a majority voting heterogeneous ensemble
classifier by combining several base classifiers such
as SVM, KNN, NB, and DT, among others. The
proposed technique was evaluated using six heart
disease datasets and achieved an accuracy which
slightly outperformed those of single base classifiers.
Jadhav and al. (Jadhav et al., 2014) proposed a feature
selection-based homogeneous ensemble
classification technique to diagnose arrhythmia. The
proposed approach based on random subspace and
PART tree achieved an accuracy of 91.11%. In (Qin
et al., 2017), the authors suggested a novel ensemble
algorithm by combining seven classifiers to predict
arrhythmia. The proposed technique is based on
multiple feature selection techniques and a bagging
approach to increase data diversity which is an
important criterion to construct ensemble techniques.
The approach achieved an accuracy of 93.7%.
Although some of the studies applied feature
selection, the focus was on applying ensemble
learning during the classification phase. This
motivates us to conduct the present study.
3 EXPERIMENTAL DESIGN
This section describes the heart disease data used and
the methodology followed to conduct the
experiments.
3.1 Heart Disease Dataset
Table 1 summarizes the number of features (the class
attribute is not included) with their types, the number
HEALTHINF 2022 - 15th International Conference on Health Informatics
370

of instances, number of classes, and missing values
for each dataset.
Our purpose is to distinguish between the absence
and presence of a heart disease, and thus, all class
values indicating the presence of heart disease in the
multi-class datasets were replaced by 1 while class 0
indicates the absence of heart disease.
3.2 Methodology Used
The aim of this work is to apply ensemble FS on heart
disease datasets for the classification task. The
heterogeneous ensembles will combine different
feature ranking techniques based on different
measures for diversity. In this study the 10-fold cross
validation strategy is used (Witten et al., 2011). KNN,
SVM, MLP and DT classifiers were applied using the
default parameters of the Scikit-learn library.
The methodology performed on each dataset is as
follows:
Step 1: Apply single feature ranking techniques
Step 2: Combine the results of the 5 rankers using
mean and median combination methods
Step 3: Apply the 40% threshold for single and
ensemble rankers. This will result in 7 subsets for
each dataset in addition to the original feature set.
Step 4: Classify the 8 obtained subsets using
KNN, SVM, MLP and DT classifiers. Evaluate, by
means of accuracy score, the four classifiers using a
10-fold cross validation method. In total we obtain 32
classifiers for each dataset.
Step 5: Cluster the classifiers using Scott-Knott
test (Scott & Knott, 1974) based on their accuracy
scores to assess the statistical significance of the
classification results.
For the sake of simplicity, we used the following
abbreviations to name the constructed classifiers:
LC, RF, IG, SU, and CHI2 denote Linear
Correlation, ReliefF, Info gain, Symmetrical
uncertainty, and Chi-square univariate filter FS
techniques respectively. EME and EMD are the
abbreviations of the ensemble rankers constructed
with mean and median combination methods
respectively. Furthermore, the entire feature set was
denoted ORG.
Example: SVMEME refers to SVM classifier
trained on a subset selected with the ensemble ranker
using mean combination method.
4 EMPIRICAL RESULTS AND
DISCUSSION
The results of the empirical experiments are presented
and discussed in this section. Feature selection and
classification were performed using ITMO-FS and
Scikit-learn python libraries respectively, while the
Scott-Knott (SK) statistical test was performed using
R Software. Thereafter, we present a comparison of
our results with those from the literature.
4.1 Data Cleaning and Transformation
Before tackling the feature selection process, the
datasets were checked for missing values and
irrelevant features. Therefore, a total of thirty-eight
attributes of the Unprocessed Cleveland dataset were
removed since they contained high percentages of
missing values (more than 20%), were irrelevant, or
had the same values over all instances. Moreover, one
attribute containing 83% of missing values was
deleted from the Arrhythmia dataset. Thereafter,
instances containing missing values were deleted.
Afterwards, all attributes were transformed using the
Min-Max normalization technique. The performance
of the four classifiers before and after applying
normalization was verified. The transformation
process did not hurt the classification accuracy; on the
contrary, it significantly improved it in the majority
of cases.
4.2 Single and Ensemble Feature
Selection Results
The application of single and ensemble feature
selection resulted in the selection of different feature
subsets with the sizes of 5, 4, 5, 14, 22, and 111
features for processed Cleveland, Hungarian, Statlog,
unprocessed Cleveland, Z-Alizadeh Sani, and
Arrhythmia datasets respectively.
4.3 Classification Results
For each dataset, a total of 32 classifiers were
evaluated. The SK test results in terms of accuracy
score for the six selected datasets are illustrated in
Fig. 1.
The SK test identified two clusters for the
processed Cleveland dataset. The best cluster
contains 23 classifiers. All SVMs, MLPs, and KNNs
appeared in the best cluster, with the exception of
those based on SU single ranker. On the contrary, all
Ensemble Feature Selection for Heart Disease Classification
371

DTs belonged to the second cluster with the exception
of the one based on SU single ranker.
It can be noticed that the best SK cluster of Statlog
dataset contains three clusters. A total of 18 classifiers
belong to the best cluster. With the exception of
MLPRF, all MLP classifiers appear in the best
cluster. No DT classifier appears in the best cluster,
except for DTEMD. Furthermore, the best cluster
include all SVM and KNN classifiers trained with the
original feature set and subsets selected with CHI2,
LC, EME, and EMD.
The SK test for Hungarian dataset identified three
clusters. With the exception of SVMRRF, DTIG,
DTORG, DTRF, and KNNSU, all the classifiers
belong to the best SK cluster.
A total of 22 classifiers are present in the best SK
cluster for the unprocessed Cleveland dataset. As can
be observed, with the exception of SVMORG,
SVMCHI2 and DTIG, all DTs, SVMs, and MLPs
belong to the best cluster. Moreover, only one KNN
classifier is present in the best cluster (KNNIG).
For Z-Alizadeh Sani dataset, the SK test identified
two clusters. The best SK cluster includes a total of
19 classifiers. With the exception of MLPSU and
SVMSU, all SVMs and MLPs are present in the best
cluster. None of DT classifiers appear in the best
cluster while for KNN, only three appeared in the best
cluster.
The SK test for the Arrhythmia dataset resulted in
two clusters. It is to be noted that, with the exception
of MLPSU and SVMSU, all SVMs and MLPs belong
to the best cluster. None of KNN classifiers appear in
the best SK cluster for this dataset. For DT classifiers,
only DTRF, DTORG, DTIG and DTLC are present in
the best cluster.
4.4 Discussion
The empirical results are discussed in this section
according to the RQs from Section 1.
RQ1: Is there any single ranking technique
that distinctly outperform other single ranking
techniques? The SK test results are summarized in
Table 2. to answer this RQ. Table 2. presents the
number of occurrences of each feature selection
technique present in the best SK cluster for each
dataset regardless of the classifier used. We can
conclude that LC gives very satisfying results over
different datasets since in total 19 out of 24 LC
techniques were present in the best SK clusters. The
number of occurrences of RF, IG, and CHI2 is
acceptable over different datasets. Nonetheless, SU
single ranker seem to perform worse than other single
rankers and fail to select the most relevant features
since its total number of occurrence in the best
clusters is very low. In fact, the main difference
between LC, which seems to be the best performing
single ranker, and SU, the worst performing one, is
that LC is based on linear relationships while SU is
based on non-linear ones (Saikhu et al., 2019). This
suggests that the most relevant features to predict
heart disease have a linear relationship with the class
attribute and SU failed to identify them.
RQ2: Do ensemble feature rankers (EFR)
outperform single ones when used for heart
disease classification? Is there a combination
method that resulted in better ensembles?
Taking into consideration the initial number of
single and ensemble rankers used, 61% of single
rankers and 81% of ensemble rankers were present in
the best SK clusters over all datasets. This shows that
promising results can be achieved by applying
ensemble feature selection for heart disease
classification. However, some poor performing single
techniques such as SU in this case, may influence the
performance of ensemble techniques, and thus,
investigating multiple ensembles of different sizes
might be required. Besides, using the features ranks
instead of their scores should be investigated.
As regards the combination methods, there is only
a difference of three occurrences between the
presence frequency of ensembles constructed with
mean and those constructed with median, therefore, it
is difficult to draw conclusions.
4.5 Accuracy Comparison with
Previous Studies
Compared to previous works, the classification
results achieved in our study are very encouraging as
shown in Table 3. For example, the accuracy rate
achieved for Cleveland dataset with MLP and five
attributes selected with ensemble ranking feature
selection is very promising compared to that of more
complex models such as: (1) BagMOOV (Bashir,
Qamar, & Hassan, 2015), an ensemble technique
based on five heterogeneous classifiers, or (2) RF
ensemble based on CFS and PCA (Ozcift & Gulten,
2011).
For Hungarian dataset, it can be noticed that there
is not a significant difference between the accuracy
achieved in our study and that achieved in (Kadam &
Jadhav, 2020) which used ensemble classification,
hyper-parameter optimization and the entire feature
set.
Very competitive results are achieved for Statlog
and unprocessed Cleveland datasets compared with
the previous studies, with only 5 and 14 attributes.
HEALTHINF 2022 - 15th International Conference on Health Informatics
372
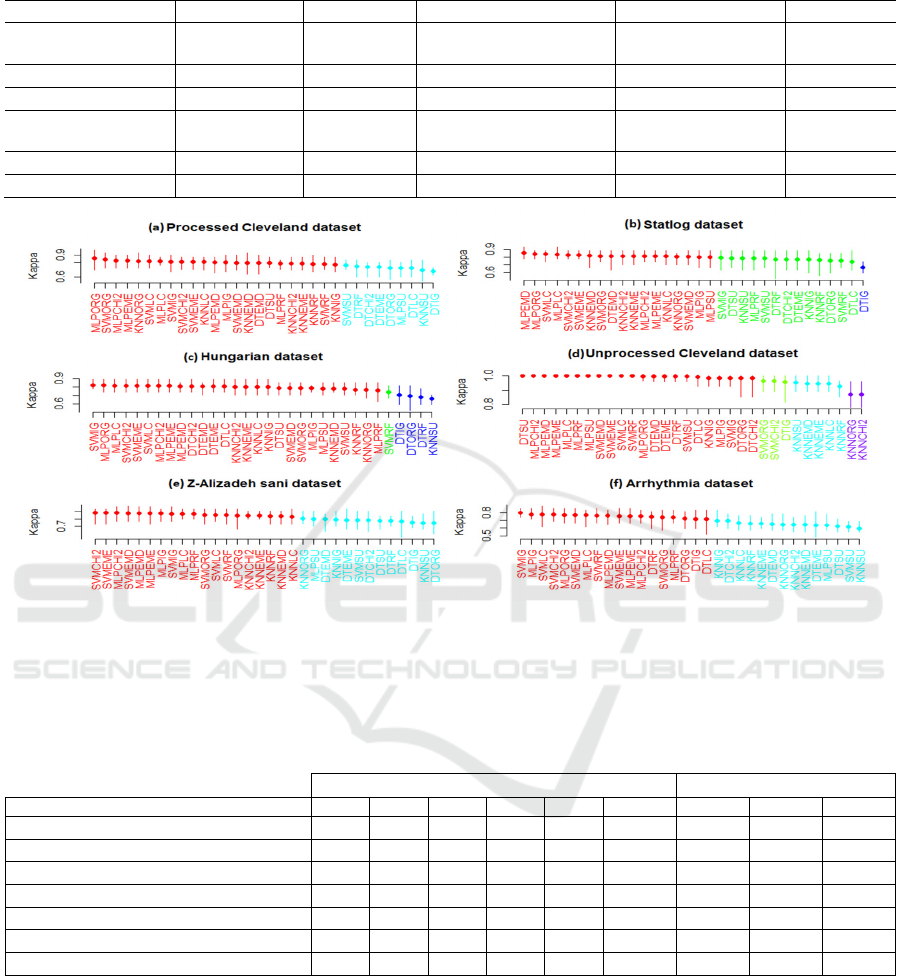
Table 1: Datasets descriptions.
Dataset No. of instances No. of features Types of features No. of missing values No. of classes
Processed Cleveland
dataset
303 13 6 numeric, 7 nominal 6 5
Hungarian dataset 294 13 6 numeric, 7 nominal 782 5
Statlog Heart data 270 13 6 numeric, 7 nominal 0 2
Unprocessed Cleveland
dataset
282 75 42 numeric, 33 nominal 5968 5
Z-Alizadeh Sani dataset 303 55 22 numeric, 33 nominal NA 2
Arrhythmia dataset 452 279 206 numerical, 73 nominal 407 16
Figure 1: SK test results on each dataset. The x-axis represents the classifiers generated where the better positions start from
the left side. The y-axis represents the accuracy values. Each vertical line represents the 10-fold cross validation values for
each variant and the small dots represent the mean accuracy values. Lines (classifiers) with the same color belong to the same
cluster.
Table 2: Number of occurrence for each FS technique present in the best cluster regardless of the classifier used over all
datasets.
Single rankers Ensemble rankers
Dataset LC RF IG SU CHI Total EME EMD Total
Processed Cleveland dataset 3 3 3 1 3
13
3 4
7
Hungarian datase
t
4 2334
16
4 4
8
Statlog Heart data 3 0 1 1 3
8
3 4
7
Unprocessed Cleveland dataset 3 4 3 3 2
15
3 3
6
Z-Alizadeh Sani dataset 3 3 2 0 3
11
3 4
7
Arrhythmia dataset 3 3 3 0 2
11
2 2
4
Total 19 15 15 8 17 74 18 21 39
For Z-Alizadeh Sani dataset, SVMEMD and
KNNEME achieved good results compared to the HE
classification technique proposed by Cuvitoglu and
al. (Cüvitoǧlu & Işik, 2018). Moreover, although the
Bagging SMO-SVM outperformed our classifiers, it
used a higher number of features (Alizadehsani et al.,
2013).
While the classifiers constructed in this study
showed promising results for Arrhythmia dataset
compared with those proposed in (Xu et al., 2017),
the ensemble technique proposed by Jadhav and al.
(Jadhav et al., 2014) achieved a remarkably higher
accuracy. Nevertheless, we believe that our results
can be improved by building ensembles of different
sizes, using other combination methods, and
optimizing the hyper-parameters of the classifiers.
Ensemble Feature Selection for Heart Disease Classification
373

Table 3: Accuracy comparison with previous works.
Dataset Study Technique No. of features Accuracy
Processed
Cleveland
dataset
Our study MLPEME 5 82.17%
(Bashir, Qamar, & Hassan, 2015) BagMOOV _ 84.16%
(Ozcift & Gulten, 2011) CFS + PCA + RF 7 80.49%
Hungarian
dataset
Our study SVMEME 4 81.48%
MLPEME 4 81.11%
KNNEME 4 80%
(Kadam & Jadhav, 2020) DT- based AdaBoost + RS 13 83%
Statlog Heart
data
Our study MLPEMD 5 85.55%
SVMEME 5 82.96%
KNNEMD 5 81.85%
(Kadam & Jadhav, 2020) DT- based AdaBoost BO 13 84.81%
(Bashir, Qamar, & Hassan, 2015) BagMOOV _ 84.07%
Uprocessed
Cleveland
dataset
Our study MLPEMD and MLPEME 14 100%
SVMEMD and SVMEME 14 100%
DTEMD and DTEME 14 99.66%
(H. et al., 2016) AdaBoost 29 80.14%
(Gárate-Escamila et al., 2020) Gradient-boosted Tree 75 98.7%
Z-Alizadeh
Sani dataset
Our study
SVMEMD 22 87%
KNNEME 22 84.17%
(Cüvitoǧlu & Işik, 2018) t-test + PCA + HE 25 86%
(Alizadehsani et al., 2013) Bagging SMO 33 92.74%
Arrhythmia
dataset
Our study SVMIG 111 80%
SVMEMD 111 76.66%
(Xu et al., 2017) FDR + DNN 236 80.64%
(Jadhav et al., 2014) Random supspace PART tree _ 91.11%
CFS: Correlation based feature selection, PCA: Principal component analysis, HE: Heterogeneous Ensemble,
RF: Rotation Forest, SMO: Sequential Minimal Optimization, AdaBoost: Adaptive boosting, BO: Bayesian
Optimization, RS: Random search, DNN: Deep neural networks, FDR: Fisher discriminant ratio
5 CONCLUSION
The aim of this study was to investigate the
performance of ensemble feature ranking techniques
compared to single ones for heart disease prediction
To this, the relevant features of six heart disease
datasets were selected using five single and two
ensemble ranking techniques constructed using mean
and median combination methods. The subsets
selected with ensemble rankers as well as single ones
were evaluated KNN, SVM, MLP and DT classifiers.
The results of the empirical experiments showed that
linear correlation seem to be the best performing
single univariate filter while symmetrical uncertainty
is the worst performing one. Moreover, the results
obtained with ensemble feature ranking techniques
are very promising.
We believe that our results can still be improved
by building ensembles of different sizes, using feature
ranks instead of feature scores, using other
combination methods, and optimizing the hyper-
parameters of the constructed classifiers. These
aspects will be taken into consideration in future
work. Moreover, other missing data handling
strategies and multi-class classification will be
investigated.
HEALTHINF 2022 - 15th International Conference on Health Informatics
374

REFERENCES
Alizadehsani, R., Habibi, J., Hosseini, M. J., Mashayekhi,
H., Boghrati, R., Ghandeharioun, A., Bahadorian, B., &
Sani, Z. A. (2013). A data mining approach for
diagnosis of coronary artery disease. Computer
Methods and Programs in Biomedicine.
https://doi.org/10.1016/j.cmpb.2013.03.004
Bashir, S., Qamar, U., & Hassan, F. (2015). Bagmoov: A
novel ensemble for heart disease prediction bootstrap
aggregation with multi-objective optimized voting.
Australasian Physical and Engineering Sciences in
Medicine. https://doi.org/10.1007/s13246-015-0337-6
Bashir, S., Qamar, U., & Javed, M. Y. (2015). An ensemble
based decision support framework for intelligent heart
disease diagnosis. International Conference on
Information Society, i-Society 2014. https://doi.org/
10.1109/i-Society.2014.7009056
Benhar, H., Idri, A., & Fernández-Alemán, J. L. (2019). A
Systematic Mapping Study of Data Preparation in Heart
Disease Knowledge Discovery. Journal of Medical
Systems, 43(1), 17. https://doi.org/10.1007/s10916-
018-1134-z
Benhar, H., Idri, A., & L Fernández-Alemán, J. (2020).
Data preprocessing for heart disease classification: A
systematic literature review. In Computer Methods and
Programs in Biomedicine. https://doi.org/10.1016/
j.cmpb.2020.105635
Cüvitoǧlu, A., & Işik, Z. (2018). Classification of CAD
dataset by using principal component analysis and
machine learning approaches. 2018 5th International
Conference on Electrical and Electronics Engineering,
ICEEE 2018. https://doi.org/10.1109/ICEEE2.2018.83
91358
Gárate-Escamila, A. K., Hajjam El Hassani, A., & Andrès,
E. (2020). Classification models for heart disease
prediction using feature selection and PCA. Informatics
in Medicine Unlocked. https://doi.org/10.1016/
j.imu.2020.100330
Gardner, M. ., & Dorling, S. (1998). Artificial neural
networks (the multilayer perceptron)—a review of
applications in the atmospheric sciences. Atmospheric
Environment, 32(14–15), 2627–2636. https://doi.org/
10.1016/S1352-2310(97)00447-0
Gooch, J. W. (2011). Pearson Product-Moment Correlation
Coefficient. In Encyclopedia of Measurement and
Statistics. Sage Publications, Inc.
https://doi.org/10.4135/9781412952644.n338
H., K., H., J., & J., G. (2016). Diagnosing Coronary Heart
Disease using Ensemble Machine Learning.
International Journal of Advanced Computer Science
and Applications. https://doi.org/10.14569/
ijacsa.2016.071004
Hall, M. a., & Smith, L. a. (1998). Practical feature subset
selection for machine learning. Computer Science.
Han, J., Kamber, M., & Pei, J. (2012). Data Mining:
Concepts and Techniques. In Data Mining: Concepts
and Techniques. https://doi.org/10.1016/C2009-0-
61819-5
Hosni, M., Carrillo de Gea, J. M., Idri, A., El Bajta, M.,
Fernández Alemán, J. L., García-Mateos, G., &
Abnane, I. (2020). A systematic mapping study for
ensemble classification methods in cardiovascular
disease. Artificial Intelligence Review. https://doi.org/
10.1007/s10462-020-09914-6
Hosni, M., Carrillo de Gea, J. M., Idri, A., El Bajta, M.,
Fernández Alemán, J. L., García-Mateos, G., &
Abnane, I. (2021). A systematic mapping study for
ensemble classification methods in cardiovascular
disease. Artificial Intelligence Review. https://doi.org/
10.1007/s10462-020-09914-6
Jadhav, S., Nalbalwar, S., & Ghatol, A. (2014). Feature
elimination based random subspace ensembles learning
for ECG arrhythmia diagnosis. Soft Computing.
https://doi.org/10.1007/s00500-013-1079-6
Jin, X., Xu, A., Bie, R., & Guo, P. (2006). Machine learning
techniques and chi-square feature selection for cancer
classification using SAGE gene expression profiles.
Lecture Notes in Computer Science (Including
Subseries Lecture Notes in Artificial Intelligence and
Lecture Notes in Bioinformatics). https://doi.org/
10.1007/11691730_11
Kadam, V. J., & Jadhav, S. M. (2020). Performance
analysis of hyperparameter optimization methods for
ensemble learning with small and medium sized
medical datasets. Journal of Discrete Mathematical
Sciences and Cryptography. https://doi.org/10.1080/
09720529.2020.1721871
Kadi, I., Idri, A., & Fernandez-Aleman, J. L. (2017).
Systematic mapping study of data mining–based
empirical studies in cardiology. Health Informatics
Journal, 1. https://doi.org/10.1177/1460458217717636
Lo, Y. T., Fujita, H., & Pai, T. W. (2016). Prediction of
coronary artery disease based on ensemble learning
approaches and co-expressed observations. Journal of
Mechanics in Medicine and Biology. https://doi.org/
10.1142/S0219519416400108
Ozcift, A., & Gulten, A. (2011). Classifier ensemble
construction with rotation forest to improve medical
diagnosis performance of machine learning algorithms.
Computer Methods and Programs in Biomedicine.
https://doi.org/10.1016/j.cmpb.2011.03.018
Pilnenskiy, N., & Smetannikov, I. (2020). Feature selection
algorithms as one of the python data analytical tools.
Future Internet. https://doi.org/10.3390/fi12030054
Qin, C.-J., Guan, Q., & Wang, X.-P. (2017). Application Of
Ensemble Algorithm Integrating Multiple Criteria
Feature Selection In Coronary Heart Disease Detection.
Biomedical Engineering: Applications, Basis and
Communications, 29(06). https://doi.org/10.4015/
S1016237217500430
Quinlan, J. R. (1986). Induction of Decision Trees.
Machine Learning. https://doi.org/10.1023/A:102264
3204877
Saikhu, A., Arifin, A. Z., & Fatichah, C. (2019). Correlation
and symmetrical uncertainty-based feature selection for
multivariate time series classification. International
Journal of Intelligent Engineering and Systems.
https://doi.org/10.22266/IJIES2019.0630.14
Ensemble Feature Selection for Heart Disease Classification
375

Schlemmer, A., Zwirnmann, H., Zabel, M., Parlitz, U., &
Luther, S. (2014). Evaluation of machine learning
methods for the long-term prediction of cardiac
diseases. 2014 8th Conference of the European Study
Group on Cardiovascular Oscillations, ESGCO 2014.
https://doi.org/10.1109/ESGCO.2014.6847567
Scott, A. J., & Knott, M. (1974). A Cluster Analysis
Method for Grouping Means in the Analysis of
Variance. Biometrics. https://doi.org/10.2307/2529204
Seijo-Pardo, B., Porto-Díaz, I., Bolón-Canedo, V., &
Alonso-Betanzos, A. (2017). Ensemble feature
selection: Homogeneous and heterogeneous
approaches. Knowledge-Based Systems, 118, 124–139.
https://doi.org/10.1016/j.knosys.2016.11.017
Urbanowicz, R. J., Meeker, M., La Cava, W., Olson, R. S.,
& Moore, J. H. (2018). Relief-based feature selection:
Introduction and review. In Journal of Biomedical
Informatics. https://doi.org/10.1016/j.jbi.2018.07.014
Vapnik, V. N. (2000). The Nature of Statistical Learning
Theory. Springer New York. https://doi.org/10.1007/
978-1-4757-3264-1
Witten, I. H., Frank, E., & Hall, M. A. (2011). Data Mining:
Practical Machine Learning Tools and Techniques.
Elsevier. https://doi.org/10.1016/C2009-0-19715-5
Xu, S. S., Mak, M. W., & Cheung, C. C. (2017). Deep
neural networks versus support vector machines for
ECG arrhythmia classification. 2017 IEEE
International Conference on Multimedia and Expo
Workshops, ICMEW 2017. https://doi.org/10.1109/
ICMEW.2017.8026250
Zhou, Z. H. (2012). Ensemble methods: Foundations and
algorithms. In Ensemble Methods: Foundations and
Algorithms. https://doi.org/10.1201/b12207
HEALTHINF 2022 - 15th International Conference on Health Informatics
376
