
pH and SpO
2
Miniaturized Sensors for Fetal Health Monitoring
T. Nguyen
1a
, A. Bessiere
1b
, Q. Rousset
2c
, B. Journet
2d
, S. L’Horset
2e
, H. Takhedmit
1
and G. Lissorgues
1f
1
Université Gustave Eiffel, CNRS, ESYCOM UMR 9007, Noisy-le-Grand, France
2
Lumin, ENS Paris Saclay, CentraleSupelec, Gif-sur-Yvette, France
Keywords: pH Sensor, SpO
2
Sensor, Micro-Electrodes.
Abstract: In this paper we present a prototype which is a first attempt to get continuous fetal health monitoring during
labor. This system is capable of simultaneously measuring pH, SpO
2
and provides a clear
photoplethysmogram in real time. A Titanium nitride pH sensing electrode of 600 μm diameter in size
performed a linear Nernstian sensitivity of 62.8 mV/pH within the pH range of interest from 6 to 8 and a
precision of 0.14 in pH. The reflectance SpO
2
sensor employed two LEDs at 630 nm and 940 nm wavelengths
and is monitored by a MSP432 microcontroller; the result recorded shows close behavior to a commercial
device. This work is under optimization process for a better accuracy and aiming for integration into a specific
miniaturized device with a touch screen as user interface.
1 INTRODUCTION
Monitoring fetal well-being during labor is a common
practice of daily obstetrical activities. Ensuring the
good oxygenation of the fetus is important to prevent
the risk of asphyxiation and its most serious
consequences: peripartum death and distant sequelae,
in particular neurological disorders including
psychomotor disability or cerebral palsy (Carbonne &
Nguyen, 2008). Capillary pH on fetal scalp is
frequently monitored as a second-line examination
beside fetal heart rate in delivery rooms, to reduce
false positives rate for predicting the fetal acidosis.
The technique of collecting capillary samples from
fetal scalp has many limitations in terms of
discontinuity and high rate of failures. Indeed, taking
a discontinuous sample every 30 minutes appears
obsolete in some obstetrical situations.
It was demonstrated that the correlation between
tissue pH and capillary pH at birth is good (Weber,
1980). Several teams have developed continuous
measurement systems for tissue pH at the fetal scalp,
a
https://orcid.org/0000-0002-2355-487X
b
https://orcid.org/0000-0003-0304-4538
c
https://orcid.org/0000-0001-8468-8487
d
https://orcid.org/0000-0001-7278-7170
e
https://orcid.org/0000-0001-7308-0900
f
https://orcid.org/0000-0003-3371-8353
using miniaturized glass electrode (Stamm et al.,
1976), optical fiber and pH indicator colorimetric
(Peterson et al., 1980). However, the methodologies
set out in these publications were stagnant because of
the technical limitations at the time, mainly related to
the fabrication techniques and miniaturization issues
not available in the 1980s.
Potentiometric based pH sensors are the most
favored electrochemical systems due to their simple
design and possibility to be miniaturized (Kurzweil,
2009). A potentiometric based sensor includes one
working electrode (WE) and one reference electrode
(RE). In principle, when the sensor is immersed in the
test solutions, a potential difference between the WE
and the RE (open circuit voltage) is produced and
proportional to the pH variation. The most common
material of RE is Ag/AgCl, due to its stable potential.
Some pH-sensitive materials have been reported
including metal oxides such as Iridium(IV) oxide
(IrO
2
), Ruthenium(IV) oxide (RuO
2
), Tungsten
trioxide (WO
3
) and Titanium dioxide (TiO
2
)etc.,
conductive polymers such as poly-aniline, poly-
pyrrole, etc., and typically, the glass pH electrode
Nguyen, T., Bessiere, A., Rousset, Q., Journet, B., L’Horset, S., Takhedmit, H. and Lissorgues, G.
pH and SpO2 Miniaturized Sensors for Fetal Health Monitoring.
DOI: 10.5220/0010867200003123
In Proceedings of the 15th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2022) - Volume 1: BIODEVICES, pages 155-161
ISBN: 978-989-758-552-4; ISSN: 2184-4305
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
155
with an ideal Nernstian sensitivity. However, these
devices still have some drawbacks in terms of
potential drift and selectivity (metal oxides), stability
and long-term storage (polymers), fragility and
improbability of biomedical application (glass
electrode) (Manjakkal et al., 2020).
To the best of our knowledge, Titanium nitride
(TiN) is the only metal nitride reported for
potentiometric pH sensors, and is considered as an
alternative pH-sensing material. Thin TiN films have
been reported to reach 0.01 pH precision which is
critical for the fetal scalp tissue pH measurements
(Paul Shylendra et al., 2020). Therefore, the first part
of our work focuses on developing a TiN
potentiometric pH micro-sensor.
In this study, we attempt to design a prototype for
the first time to continuously monitor fetal tissue pH
during labor, combined with fetal oxygen saturation
(SpO
2
) as control. A microelectrode of 600 µm
diameter was fabricated using Titanium nitride (TiN)
as the potentiometric sensing material for pH
variations. An optical sensor made of two LEDs and
one photodiode was used for SpO
2
monitoring, based
on the absorption spectra of the oxygenated and
deoxygenated hemoglobins. The signal waveform
also known as photoplethysmogram (PPG) can be
useful to determine pulse wave characteristics, such
as dicrotic notch, systolic and diastolic phases. The
data acquired from these sensors including pH, SpO
2
,
heart rate and PPG can be saved in a SD card and be
displayed on a LCD touchscreen.
2 DESIGN AND FABRICATION
2.1 pH Sensor Fabrication and
Characterization Procedure
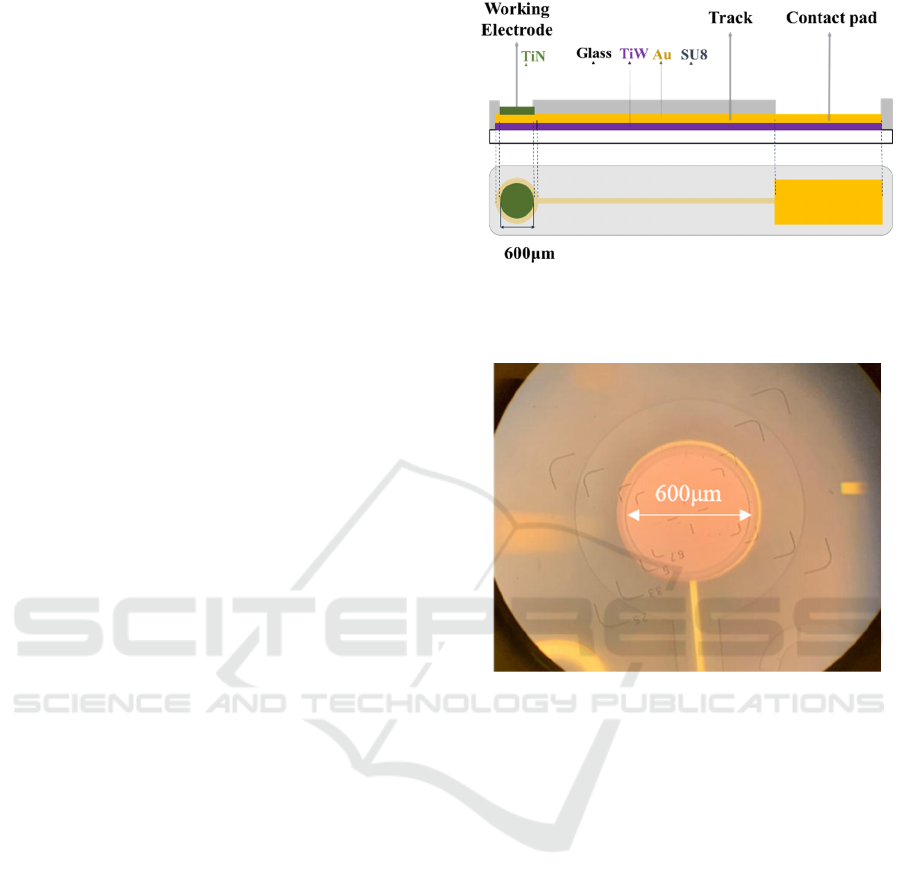
Electrodes of 600 μm diameter in size were fabricated
following this process: (1) a gold/titanium tungsten
(Au/TiW) layer of 520 nm in thickness was deposited
on a glass substrate (Alcatel sputtering), (2) a positive
photoresist was spin coated on top of the gold layer
for ultraviolet photolithography, development and
etching, (3) the photoresist was removed, (4) the
process was repeated with the second which is then
TiN of 200 nm thick (Plassys sputtering) and (5)
completed with the third layer of 1.7 μm thick
photoresist SU8 used as an insulator with the
openings on the electrode and contact pad areas.
Figure 1 illustrates the design of the electrode with
the corresponding microscope image on Figure 2.
Figure 1: pH sensing electrode design with three layers
TiW/Au, TiN and SU8 on a glass substrate, top/cut view,
bottom/top view.
Figure 2: Image of the fabricated pH sensing electrode
under microscope.
The fabricated electrodes were equilibrated in
deionized water overnight before characterization to
obtain stable potential.
The potentiostat SP-200 from Biologic Science
Instrument was used to carry out electrochemical
measurements. An Ag/AgCl electrode was used as
the RE. Open circuit voltage (OCV) or open circuit
potential was measured in phosphate-buffered saline
(PBS) test solutions with pH ranging from 6 to 8 at
25ºC, with 0.2 pH step to determine sensitivity,
stability, response time, hysteresis and
reproducibility of the pH sensor. The WE and the RE
were submerged sequentially in the test solutions
disregarding cross-contamination. A commercial
glass electrode from Atlas Scientific was used to
confirm the pH level of the solution under testing.
BIODEVICES 2022 - 15th International Conference on Biomedical Electronics and Devices
156

2.2 SpO
2
Sensor Scheme and PCB
Design
Figure 3: Scheme of the optical reflectance SpO
2
sensor.
The circuit consists of two LEDs of 630 nm (Red) and 940
nm (IR) wavelength, one photodiode (D0), switching
signals from microcontroller MSP432 (MC1 and MC2
inputs), transistors MMBT3904 (T1 and T2) and two
operational amplifiers OPA376 (U0 and U1).
Figure 4: a) SpO
2
sensing board composed of a Red LED
(630 nm), an IR LED (940 nm), a photodiode, a
transimpedance amplifier and a second amplifier stage. b)
The flexible printed circuit board (PCB) made in
Polyimide, without components, for next step integration.
Figure 3 shows the scheme of the SpO
2
sensor. The
circuit consists of two LEDs of 630 nm (Red, LS
L29K) and 940 nm (IR, KP-2012F3C) wavelengths.
The light transmitted through the skin is detected by
a PIN photodiode (BP 104 S). On the sensing board a
transimpedance amplifier stage and a second
operational amplifier stage, both based on operational
amplifier OPA376, are used to amplify the signal and
to drive the cable connected to the microcontroller
board.
This sensor is fabricated on a classical FR4
Printed Circuit Boards (PCB) and will be later
implemented on a Polyimide flexible substrate
(Figure 4). MSP432 is used to control the LEDs, to
achieve the data sampling by a 14 bits Analog to
Digital Converters (ADC) and to perform some signal
processing. A band pass filter with 5 Hz high cutoff
frequency and 1 Hz low cutoff frequency is
implemented to obtain clear PPG signals.
The LEDs are activated sequentially from pulses
delivered by the microcontroller at a frequency of 200
Hz, the pulse width is 220 µs. Sampling is done in the
middle of a pulse. The LEDs information is acquired
and separated in the microcontroller leading to two
digitized signals for each one. The corresponding
peak-to-peak amplitudes (AC for Alternating
Current) and mean values (DC for Direct Current) are
determined to calculate a ratio (R) value as given by
equation 1. As until now we did not make the
complete calibration, the SpO
2
is determined using
the R value and an empirical formula (equation 2),
based on application note AN1525 from Zhang Feng,
Microchip Technology.
R=
AC
red
/DC
red
AC
IR
/DC
IR
(1)
SpO
2
=119-32.5R (2)
3 RESULTS AND DISCUSSION
3.1 TiN Electrode Characterization for
pH Sensing Application
The sensitivity of the TiN electrodes fabricated with
the above described clean room process as a
potentiometric sensor was determined by measuring
the OCV values at different pH levels of the test
solutions during 100 s. The recorded data from 50 s
to 100 s were averaged to calculate the sensitivity of
TiN electrode as shown in Figure 5. The slope of the
curve is 62.8 mV/pH corresponding to the sensitivity
a)
b
)
Photodiode
Red LED
IR LED
pH and SpO2 Miniaturized Sensors for Fetal Health Monitoring
157
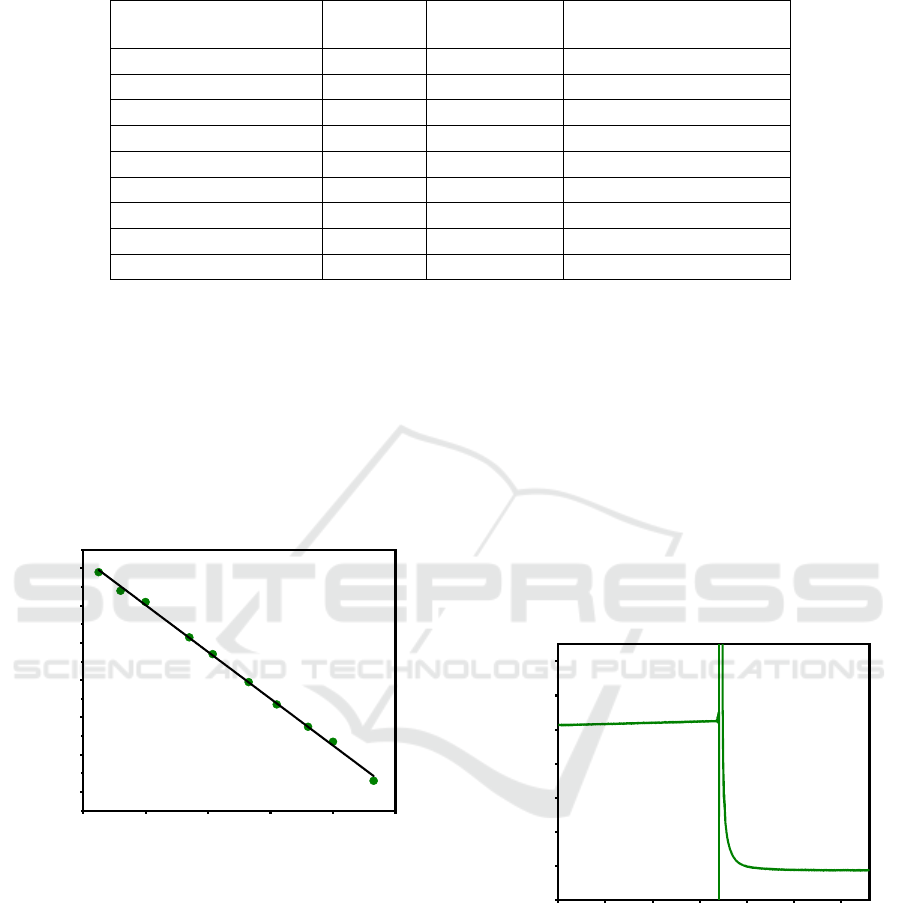
Table 1: Potentiometric pH sensor performance of different materials.
Material Sensitivity
(mV/pH)
Response time
(s)
Reference
TiN NP 46.48 5.2 (Liu et al., 2016)
TiN NTA 55.33 4.4 (Liu et al., 2016)
TiN thin film 57.5 - (Paul Shylendra et al., 2020
IrO
2
69.9 ± 2.2 0.5 s (Chung et al., 2014)
IrO
2
51 0.9 to 2 s (Huang et al., 2011)
Polyaniline 58 ± 0.3 20 (Guinovart et al., 2014)
Polyaniline 62.4 12.8 (Park et al., 2019)
Polyaniline/Zeolite blend 310 ± 40 - (Malkaj et al., 2006)
Polypyrrole/Zeolite blend 1300 ± 100 - (Malkaj et al., 2006)
of the TiN electrode. The correlation coefficient was
calculated to be 0.998 and the standard reduction
potential E is 402.6 mV, corresponding to the
intercept of the curve.
This sensitivity is higher than the reported results
of sputtered TiN film with the same thickness of TiN
layer (57.5 mV/pH, R
2
= 0.9999), (Paul Shylendra et
al., 2020), TiN nanotube array (TiN NTA, 55.33
mV/pH, R
2
= 0.995), and TiN nano powder (TiN NP,
46.48 mV/pH, R
2
= 0.992), (Liu et al., 2016).
6,0 6,4 6,8 7,2 7,6 8,0
-100
-80
-60
-40
-20
0
20
Potential (mV)
pH
Slope = -62.8 ± 0.1
Intercept = 402.6 ± 7.1
R
2
= 0.9976
Figure 5: OCV curve and sensitivity of our fabricated TiN
electrode.
Data from Table 1 show that we should achieve a
better sensitivity than most of the other materials.
Especially compared to IrO
2
, which has been
developed for in-vivo and in-vitro applications, our
TiN electrode has shown a comparable sensitivity. It
was however reported a greater sensitivity in
conductive polymer - based sensors such as
polyaniline and polypyrrole blends (310 ± 40 mV/pH
and 1300 ± 100 mV/pH, respectively) (Korostynska
et al., 2008). Yet, their application is still limited due
its tendency to become unstable over time.
The response time of the TiN electrode was
measured by immersing the WE and the RE
continuously in PBS solutions with pH levels from
6.10 to pH 7.74. The time for the signal to reach 90 %
of equilibrium value is 10 s (Figure 6), which is
comparable and even better than some reported
polymer-based potentiometric pH sensors (Table 1).
This response time is slower than that of TiN NTA
(4.4 s), TiN NP (5.2 s) (Liu et al., 2016) and IrO
2
(0.5
s to 2s) (Manjakkal et al., 2020). This can be
explained by the low porosity of the surface of our
TiN layer (ability to trap more ions in a certain area),
which is expected to be improved in the next
fabrication process after a specific surface treatment.
0 50 100 150 200 250 300
-100
-80
-60
-40
-20
0
20
40
pH 7.74
Potential (mV)
pH
pH 6.10
Figure 6: The response time of TiN in PBS. The parasite
signal in between two pH levels is only due to manipulation
during the experiment.
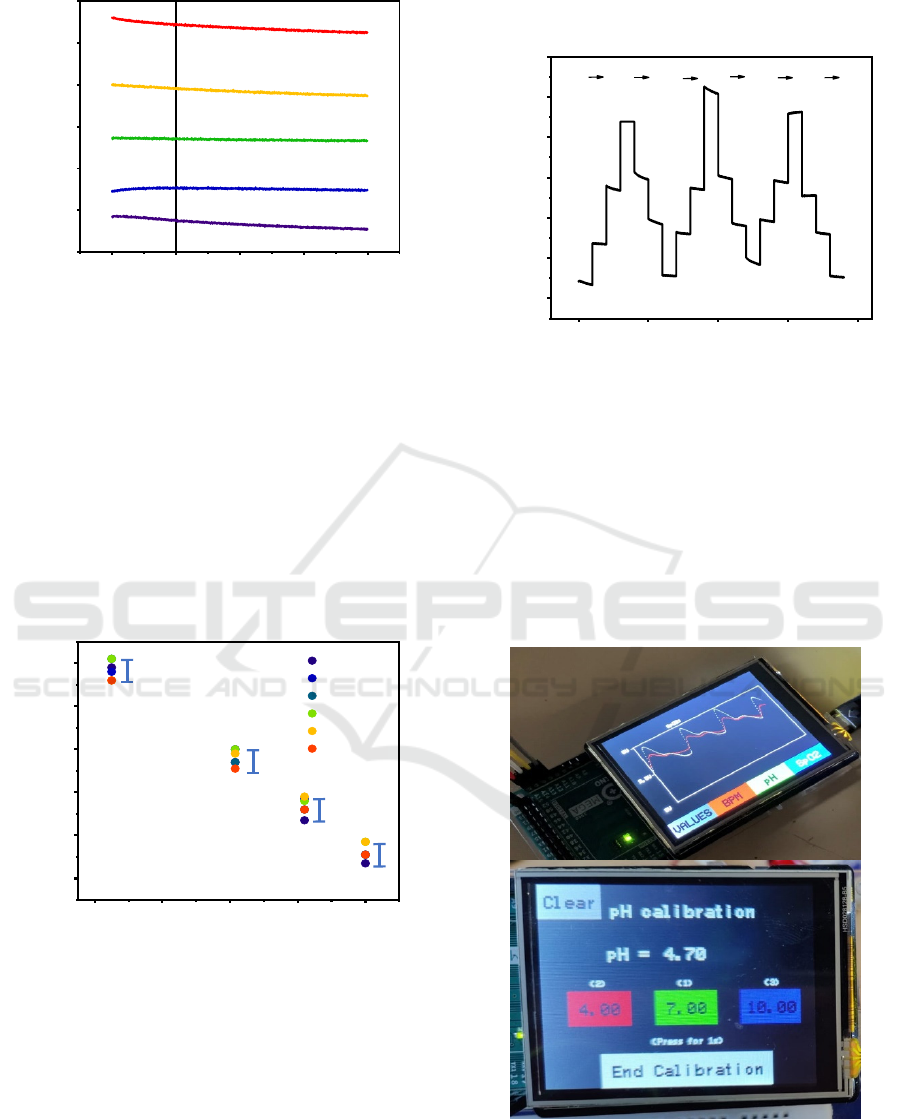
Figure 7 shows the stability of the open circuit
potential of the same electrode in different pH levels
during 200 s. A potential drift of less than 3 mV was
observed during the first 50 s but stabilized after that.
This drift might have been caused by the hydrogen
ion trapping and diffusion phenomena into the lattice
of the material.
BIODEVICES 2022 - 15th International Conference on Biomedical Electronics and Devices
158
0 50 100 150 200
-80
-70
-60
-50
-40
-30
-20
pH 7.60
pH 7.44
pH 7.24
pH 7.06
Potential (mV)
Time (s)
pH 6.83
Figure 7: The stability of one TiN electrode at different pH
levels of PBS solutions during 200 s.
A hysteresis of 10 mV was observed with the pH
range from 6 to 7.6 during 6 cycles, as shown in
Figure 8. The hysteresis could be caused by the cross-
contamination and possibly by condition differences
in the testing process, such as the stability of pH test
solutions, temperature and connection problems.
Since we are looking at a small variation of pH in a
narrow clinical-related pH range, the experiment still
needs to be improved to evaluate accurately the
performance of the electrode including operation at
37°C.
Figure 8: OCV of one TiN electrode during 6 cycles.
Reproducibility during 3 loops of measurements
on the same electrode is presented in Figure 9. The
variation of the pH value was calculated to be 0.14
(by hysteresis over sensitivity), which represented the
precision of our electrode. It is not yet sufficient for
the fetal tissue pH monitoring application but already
interesting for other applications. The optimization is
undergoing by varying the TiN fabrication
parameters, changing its surface properties
(roughness) to improve the performance of the TiN
electrodes.
0 500 1000 1500 2000
-80
-60
-40
-20
0
20
40
7.60
7.24
6.83
6.10
6.83
7.24
Potential (mV)
Time (s)
7.60
Figure 9: The reproducibility of one TiN electrode during 3
loops measurements in 4 pH levels: 6.10, 6.83, 7,24, 7.60.
3.2 SpO
2
Measurement as Control for
Fetal Monitoring
We present here the characterisation of our SpO
2
circuit described in Figure 3. We need to develop our
own chip for later integration of both pH and SpO
2
sensors in a unique miniaturized device adapted to the
fetal head positioning.
Figure 10: A touchscreen (2.8") to display in real time the
signals (choice between pH or SPO
2
), integrated to a SD
card to save data as simple user interface.
6,0 6,4 6,8 7,2 7,6
-80
-60
-40
-20
0
20
Cycle 1
Cycle 2
Cycle 3
Cycle 4
Cycle 6
Cycle 6
Potential (mV)
pH
10mV
9mV
11mV
10mV
pH and SpO2 Miniaturized Sensors for Fetal Health Monitoring
159
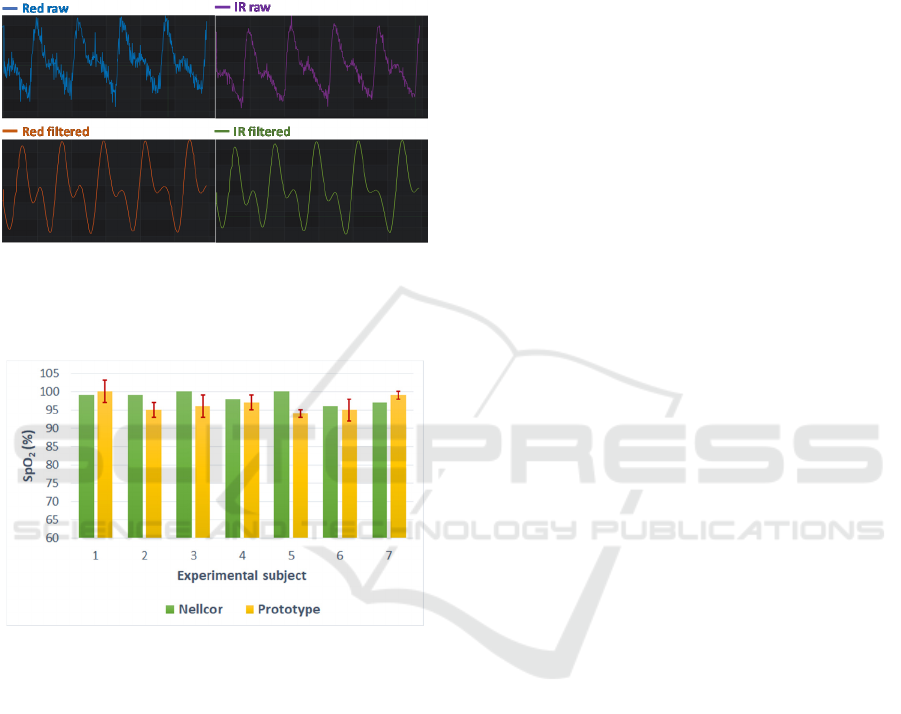
Figure 10 shows a simple user interface based on
a touch screen display and Figure 11 shows a
comparison between the raw data collected from the
sensing board and the filtered data for display
purposes and easy readings. The noise, including 50
Hz, is significantly reduced to obtain clear
waveforms.
Figure 11: Comparison between raw signals sent from the
sensing board and the filtered signals by a band-pass filter
with 5 Hz high cutoff frequency and 1 Hz low cutoff
frequency.
Figure 12: Comparison between the SpO
2
measured by
Nellcor device (green) and our prototype (yellow) on the
middle finger of seven healthy individuals. The error bars
are in absolute values.
A commercial Nellcor pulse oximeter was used
for a comparison with the SpO
2
prototype. We have
done measurements with both tools simultaneously
on the middle finger of seven healthy individuals. In
each measurement by the prototype, we waited at
least one minute before recording data to be sure that
the signal was stabilized. We accessed the results by
comparing the average values in 2 minute intervals.
The errors of measured signal by the prototype varied
from 1 to 6% in absolute values, as seen on Figure 12.
The average relative error of the prototype compared
to the Nellcor device is -1,9%. The difference
between the two mean values comes from the use of
equation 2 for a non-calibrated device. Some
influencing factors have been observed, which are the
pressure of the finger to the sensing board, the skin
color, the perspiration and the difference in thickness
of epidermis. This should be overcome by improving
the mechanical design and packaging of the sensor.
However, the behaviour of the prototype is quite close
to the reference system from Nellcor.
4 CONCLUSIONS
A prototype capable of measuring simultaneously pH
and SpO
2
has been developed showing promising
initial performance. At this stage, the sensors are
separated but an integrated package is considered for
the next step using a flexible electronic circuit for
SpO
2
and flexible substrate fabrication for the pH
sensor.
A 600 µm diameter pH sensing electrode that was
constructed from Titanium nitride (TiN), performed a
linear Nernstian sensitivity of 62.8 mV/pH within the
pH range from 6 to 8 and a precision of 0.14. This pH
sensing electrode is under optimization targeting fetal
tissue pH monitoring application.
The optical reflectance SpO
2
sensor was designed,
a microcontroller MSP432 being employed to control
the light emission and data acquisition at 200 Hz
frequency. Our SpO
2
sensor produced a quite close
behavior to commercial devices and a clear PPG
signal available through a customized simple
interface. This work is under the optimization stage
and is expected to be embedded with all above-
mentioned sensors into a flexible solution for later in
vivo testing.
Indeed, as stated in the introduction, capillary pH
on fetal scalp is frequently monitored as a second-line
examination beside fetal heart rate in delivery rooms,
to reduce false positives rate for predicting the fetal
acidosis. Our project is to integrate on a unique device
both SpO
2
and pH sensors with a continuous
monitoring.
In the final form of the device, the flexible pH
electrodes could be considered to be attached into a
specific needle, which is adaptable for clinical skin
insertion. This pH sensing needle will be fixed with
the SpO
2
sensing board using a flexible polyimide
substrate in a customized design package.
The important challenges to be considered for this
work are: 1) to find the best mechanical design to be
easily used by the medical staffs and prevent any
damage for the foetus and the mother; 2) to guarantee
the sensitivity in real use-case and 3) to establish a
protocol for sterilization and storage of the pH
sensing electrodes and the SpO
2
sensor.
BIODEVICES 2022 - 15th International Conference on Biomedical Electronics and Devices
160

ACKNOWLEDGEMENTS
We express our gratitude to L. Rousseau, C.
Wilfinger and U.T. Sarah for their support on TiN
fabrication in ESIEE PARIS clean room and Prof. E.
Lecarpentier at intercommunal hospital center in
Créteil (CHIC) for his advice on medical application
issues.
REFERENCES
Carbonne, B., & Nguyen, A. (2008). Surveillance fœtale
par mesure du pH et des lactates au scalp au cours du
travail. Journal de Gynécologie Obstétrique et Biologie
de la Reproduction, 37(1), S65–S71.
Chung, H.-J., Sulkin, M. S., Kim, J.-S., Goudeseune, C.,
Chao, H.-Y., Song, J. W., Yang, S. Y., Hsu, Y.-Y.,
Ghaffari, R., Efimov, I. R., & Rogers, J. A. (2014).
Stretchable, Multiplexed pH Sensors With
Demonstrations on Rabbit and Human Hearts
Undergoing Ischemia. Advanced Healthcare Materials,
3(1), 59–68.
Guinovart, T., Valdés-Ramírez, G., Windmiller, J. R.,
Andrade, F. J., & Wang, J. (2014). Bandage-Based
Wearable Potentiometric Sensor for Monitoring Wound
pH. Electroanalysis, 26(6), 1345–1353.
Huang, W.-D., Cao, H., Deb, S., Chiao, M., & Chiao, J. C.
(2011). A flexible pH sensor based on the iridium oxide
sensing film. Sensors and Actuators A: Physical,
169(1), 1–11.
Korostynska, O., Arshak, K., Gill, E., & Arshak, A. (2008).
Review Paper: Materials and Techniques for In Vivo pH
Monitoring. IEEE Sensors Journal, 8(1), 20–28.
Kurzweil, P. (2009). Metal Oxides and Ion-Exchanging
Surfaces as pH Sensors in Liquids: State-of-the-Art and
Outlook. Sensors, 9(6), 4955–4985.
Liu, M., Ma, Y., Su, L., Chou, K.-C., & Hou, X. (2016). A
titanium nitride nanotube array for potentiometric
sensing of pH. The Analyst, 141(5), 1693–1699.
Malkaj, P., Dalas, E., Vitoratos, E., & Sakkopoulos, S.
(2006). PH electrodes constructed from
polyaniline/zeolite and polypyrrole/zeolite conductive
blends. Journal of Applied Polymer Science, 101(3),
1853–1856.
Manjakkal, L., Dervin, S., & Dahiya, R. (2020). Flexible
potentiometric pH sensors for wearable systems. RSC
Advances, 10(15), 8594–8617.
Park, H. J., Yoon, J. H., Lee, K. G., & Choi, B. G. (2019).
Potentiometric performance of flexible pH sensor based
on polyaniline nanofiber arrays. Nano Convergence,
6(1), 9.
Paul Shylendra, S., Lonsdale, W., Wajrak, M., Nur-E-
Alam, M., & Alameh, K. (2020). Titanium Nitride Thin
Film Based Low-Redox-Interference Potentiometric
pH Sensing Electrodes. Sensors, 21(1), 42.
Peterson, J. I., Goldstein, S. R., Fitzgerald, R. V., &
Buckhold, D. K. (1980). Fiber optic pH probe for
physiological use. Analytical Chemistry, 52(6), 864–
869.
Stamm, O., Latscha, U., Janecek, P., & Campana, A.
(1976). Development of a special electrode for
continuous subcutaneous pH measurement in the infant
scalp. American Journal of Obstetrics and Gynecology,
124(2), 193–195.
Weber, T. (1980). Continuous Fetal Scalp Tissue pH
Monitoring During Labor: An Analysis of 152
Consecutive Cases. Acta Obstetricia et Gynecologica
Scandinavica, 59(3), 217–223.
pH and SpO2 Miniaturized Sensors for Fetal Health Monitoring
161
