
Wavelet based Method of Mapping the Brain Activity Waves
Travelling over the Cerebral Cortex
Bozhokin Sergey
a
, Suslova Irina
b
and Tarakanov Daniil
Peter the Great Polytechnic University, Polytechnicheskaya str. 29, Saint-Petersburg, Russia
Keywords: Continuous Wavelet Transform, Spectral Integrals, Cortical Travelling Waves.
Abstract: The brain electroencephalogram is treated as a set of electrical activity bursts in various spectral ranges.
Spectral integrals calculated by the wavelet transform method are used to study time-frequency properties of
such bursts. The mathematical theory has been developed to describe quantitatively the change in the shape
of EEG bursts while their propagating along the cerebral cortex. The proposed model of neural activity uses
nonlinear approximation of EEG record as a sum of several Gaussian peaks moving along different trajectories
with different speeds. Such model together with the continuous wavelet transform provides an opportunity to
receive analytical solutions. The proposed method allows us to draw the maps showing the trajectories of
EEG bursts moving along the cerebral cortex. It also becomes possible to study the change in the shape of
bursts in the process of their motion. The method was applied to study EEG records of a healthy subject at
rest with his eyes closed.
1 INTRODUCTION
At the macro level, the electrical activity of numerous
neural ensembles is recorded as an
electroencephalogram signal (EEG) from many brain
channels in different spectral ranges ,
where -rhythm (0,5–4 Hz), -rhythm (4–7,5 Hz),
-rhythm (7,5–14 Hz), -rhythm (14–30 Hz)
(Nunez and Srinivasan 2006; Gnezditskii 2004;
Ivanitsky et al. 2009; Tong and Thakor 2009; Zenkov
2013). This is the most common non-invasive
research method. It is known that EEG is an
inherently unsteady process. Even at rest, in the
absence of any external stimuli, we observe numerous
temporary bursts due to spontaneous fluctuations in
the level of electrical activity caused by
synchronization and desynchronization processes
associated with individual characteristics of mental
activity during registration (Hramov et al. 2015).
EEG structure represents various forms of
oscillatory patterns related to the electrical activity of
neural ensembles and reflecting the functional states
of the brain (Borisyuk and Kazanovich 2006; Quiles
et al. 2011; Chizhov and Craham 2008; Tafreshi et al.
a
https://orcid.org/0000-0001-5653-6574
b
https://orcid.org/0000-0002-4497-1867
2019). It was shown (Kaplan and Borisov 2003) that
the amplitude, temporal, and spatial characteristics of
neural activity segments indicate the rate of
formation, the lifetime, and the rate of decay of neural
ensembles. In this work, it is noted that the duration
of the quasi-stationary segments of alpha activity is
approximately equal to 𝜏300 - 350 ms depending
on the channel. This value exceeds approximately
three times the characteristic period of alpha
oscillations 𝑇
1/𝑓
100 ms, where 𝑓
10Hz.
As a rule, to analyse the variation in spectral
properties of the signal, the Short Time Fourier
Transform (STFT) is applied. We have good
resolution of temporal behaviour of the signal in the
case when the window duration 𝑊 satisfies the
condition 1/𝑓
𝑊 𝜏. However, in the case
when the condition 𝜏3𝑇
is satisfied, the
application of the STFT method leads to incorrect
determination of spectral properties.
One of the most difficult tasks in EEG processing
is to determine the localization of spatial and temporal
sources of neural activity from the signals recorded on
the outer surface of the skull. Such a problem relates to
the inverse problems of mathematical physics. Even in
the case of the most simplified model of neural activity
sources as electric dipoles located in a homogeneous
,, ,
Sergey, B., Irina, S. and Daniil, T.
Wavelet based Method of Mapping the Brain Activity Waves Travelling over the Cerebral Cortex.
DOI: 10.5220/0010888000003123
In Proceedings of the 15th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2022) - Volume 4: BIOSIGNALS, pages 63-73
ISBN: 978-989-758-552-4; ISSN: 2184-4305
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
63

sphere, the problem does not have a unique solution.
The real topology of the cortex has a complex structure
with a lot of convolutions and furrows. In addition, we
should notice that the spatial form of brain anatomical
structures is individual for each person. In solving
these problems, it is also necessary to take into account
the anisotropy of brain conductivity in various
directions (Lopes de Silva 1991; Pfurtscheller et al.
1990; Verkhlyutov et al. 2019; Ozaki et al. 2012).
The experiments show the successive shifts of
electrical activity maxima over different trajectories.
This movement can be interpreted as wave propagation
in certain direction along the surface of the brain. The
work (Manjarrez et al., 2007) calculated the trajectories
of waves in -rhythm range, originating mainly in
the frontal or occipital region. The trajectories of these
waves always cross the central zones of the brain. The
characteristic velocity of such waves is 2.1 ± 0.29 ms.
Currently, it is believed that EEG wave generators are
a group of combined nerve cells (columns or dipoles)
that transmit their excitation to neighboring neural
centers (Ng et al. 2014).
The Fast Fourier transform (STFT) with dividing
the entire EEG record into separate epochs lasting 4 s
is used to solve the problem of the propagation of
disturbances over the surface of the brain in the articles
(Massimini et al. 2004; Riedner et al. 2007). By using
cross-correlation analysis (Kulaichev 2016), the work
(Belov et al. 2014) studies the influence of
interhemispheric asymmetry and the patient’s
psychological type on the characteristics of the
“traveling EEG wave”. The method of segmentation of
EEG signals with the subsequent use of indicators of
coherence and synchronism (Phase-locking value) was
used to quantify the performance of traveling EEG
waves in (Trofimov et al. 2015; Bahramisharif et al.
2013). In the article (Getmanenko et. al. 2006),
temporary mismatches in the electrical activity of the
cerebral cortex are calculated from the shift in the
maximum of cross-correlation function. However, it
was shown (Kulaichev 2016) that the coherence value
of the two signals depends very much on the averaging
procedure, on the choice of the window size, on the
window function, on the magnitude of the window
pitch shift. Consequently, the coherence value cannot
be considered as a sufficiently accurate quantitative
measure of the correlation of two signals 𝑍
𝑡
and
𝑍
𝑡
, where 𝐼 and 𝐾 are the numbers of EEG
channels.
The velocities of travelling waves (TW)
propagation in various spectral ranges are calculated in
(Patten et al. 2012). In this work, it was shown that
-waves (the speed of 6.5 m/s) propagate faster than
-waves having the speed of 4 m/s. According to the
theory of diffuse signal transmission through nerve
tissue (Lopes da Silva 1991; Pfurtscheller and Lopes
da Silva 1999), the signal pathway consists of many
fibers with different conduction speeds. TW are
associated with switching the activity of different brain
centers. With each such switching, the outburst of
neural activity being compact at the beginning
stretches in time and decreases in amplitude due to the
dispersion of the medium.
Currently, the dynamics of the cerebral cortex of
clinical patients is often analyzed by using
intracranial electrocorticogram record (ECoG)
(Zhang et al. 2018; Belov et al. 2016). The review
(Muller et al. 2018) presents the conceptual basis of
the traveling wave phenomenon as a response
generated by intra-cortical contours to external
stimuli. The analysis of traveling waves as a non-
stationary process caused by the internal or external
stimulus allows us to obtain information not only
about the spatial localization of the stimulus, but also
about the time it occurred (Muller et al. 2018; Patten
et al. 2012). When studying the memory mechanisms
(Zhang et al. 2018), the traveling waves were
identified at different frequencies in a wide frequency
range (from 2 to 15 Hz) and with various electrode
configurations, in most cases, traveling waves
propagate from the posterior to the anterior regions of
the brain (Voytek et al. 2010; Zhang et al. 2018). The
main mathematical tools for studying traveling waves
are neural network methods (Villacorta et al. 2013;
Terman et al. 2001). The wavelet transform method
(Patten et al. 2012; Alexander et al. 2013; Zhang et al.
2018) is also widely used in the quantitative
description of the dynamics of neural ensembles.
This work proposes a new mathematical model of
neural activity based on the nonlinear approximation
of each EEG burst in the form of the sum of moving
Gaussian peaks. The maxima of electrical activity
bursts calculated in all spectral ranges 𝜇
𝛿,𝜃,𝛼,𝛽
take place at different points in time. The
movements of the activity maxima calculated for a
given channel show the trajectories of EEG waves
propagating through the cerebral cortex. Using the
proposed special model and wavelet based
mathematical tools, the trajectories and speeds of
neural activity bursts along the cerebral cortex can be
found. The studies have been carried out by
calculating the correlation in time of the electrical
activity bursts in various EEG channels (Bozhokin
and Suslova 2015) based on the continuous wavelet
transform method (CWT –continuous wavelet
transform) and on the analysis the time variation of
spectral integrals.
BIOSIGNALS 2022 - 15th International Conference on Bio-inspired Systems and Signal Processing
64

The advantage of using the wavelet methods in
this article is the ability to correctly describe the
behavior in time of the EEG activity bursts in any
spectral range 𝜇
𝛿,𝜃,𝛼,𝛽
. This approach gives
the opportunity to study EEG bursts in all spectral
ranges and their evolution in time. The new model of
EEG bursts together with the new techniques related
to CWT allows us finding both the trajectories of the
motion and the change in the shape of EEG bursts
while their travelling along the cerebral cortex. In
addition, the proposed model gives analytical
solution, which can be used to check the numerical
procedures. Thus, we can consider the proposed
methods as giving some additional information and
capabilities in the study of brain waves propagation.
2 MATERIALS AND METHODS
In this work, we processed the spontaneous EEG of a
healthy subject at rest with his eyes closed (Anodina-
Andrievskaya et al. 2011). Background EEG activity is
a desynchronized activity of neural ensembles of the
cerebral cortex. In addition to the background activity,
the EEG signal contains various oscillatory patterns,
which are continuously appearing and disappearing
bursts of rhythms characterizing the coherent electrical
activity of neural ensembles. When recording the EEG,
the standard channels were used according to the 10-
20% scheme, where the index J= 1,2, ... 21 takes the
values J = {Fp1, Fpz, Fp2; F7, F3, Fz, F4, F8; T3, C3,
Cz, C4, T4; T5, P3, Pz, P4, T6; O1, Oz, O2}. The shifts
of the maxima of electrical activity on the cerebral
cortex are approximately equal to 5-10 ms, therefore,
the signal sampling frequency should be at least = 500
Hz. The duration of the EEG recording is
approximately equal to 𝑇 = 30 s.
2.1 Continuous Wavelet Transform
(CWT) and Spectral Integrals
The modified form 𝑉
𝜈,𝑡
of the continuous
wavelet transform (СWT) for an EEG signal 𝑍
𝑡
from EEG channel J, depending on the frequency 𝜈
and time 𝑡, as well as the explicit form of the Morlet
mother wavelet function, which we will use in this
paper, are given in (Bozhokin and Suvorov 2008;
Bozhokin et al. 2017). Fig.1 shows the absolute value
𝑉
𝜈,𝑡
for the occipital channel 𝐽𝑂
. The
spectral integrals 𝐸
𝐽,𝑡
, which represent the local
density of signal energy spectrum integrated over the
given frequency range 𝜇 , are determined in
(Bozhokin and Suslova 2015).
We define a burst of EEG activity in 𝜇-frequency
range as the appearance and disappearance of a group
of waves different from the background EEG in
frequency, shape and amplitude. This can continue
for a certain period of time. The maximum of the
electrical activity of such a burst is localized at a
certain point in time 𝑡
(the center of the burst).
Figure 1: CWT modulus 𝑉
𝜈,𝑡
depending on
frequency ν and time t for the EEG channel 𝐽𝑂
.
The burst has its beginning and end, and we can
calculate the time-behavior of the local
frequency 𝐹
(t), which corresponds to the maximal
value of 𝑉
𝜈,𝑡
at fixed moment of time in the
given frequency range 𝜇. Fig.1 shows that the real
EEG signal from the given brain channel can be
treated as a set of EEG activity bursts taking place at
different time moments in different spectral ranges.
Fig.2 shows spectral integrals 𝐸
𝐽,𝑡
in 𝛼-range
for three brain channels 𝐽𝐹
;𝐶
;𝑂
. Based
entirely on Fig.2, we conclude that in 𝛼-range, brain
activity is a sequence of bursts (in this case, sleep
spindles), and the intensity of 𝛼-bursts in the occipital
channel is much higher than in the frontal. Fig.2
demonstrates the strong non-stationarity of the EEG,
expressed in the fact that the amplitude and spectral
properties of such a signal strongly depend on time.
By examining the performance of spectral
integrals 𝐸
𝐽,𝑡
, and by setting the cut off level
relative to the maximum level, we can represent the
EEG in the entire observation interval for each
channel 𝐽 as a set of bursts with certain duration.
Figure 2: Spectral integrals 𝐸
𝐽,𝑡
depending on 𝑡,𝑠 for 𝑂
-
brain channel (thin line), 𝐶
(dot line), and 𝐹
(bold line).
Wavelet based Method of Mapping the Brain Activity Waves Travelling over the Cerebral Cortex
65
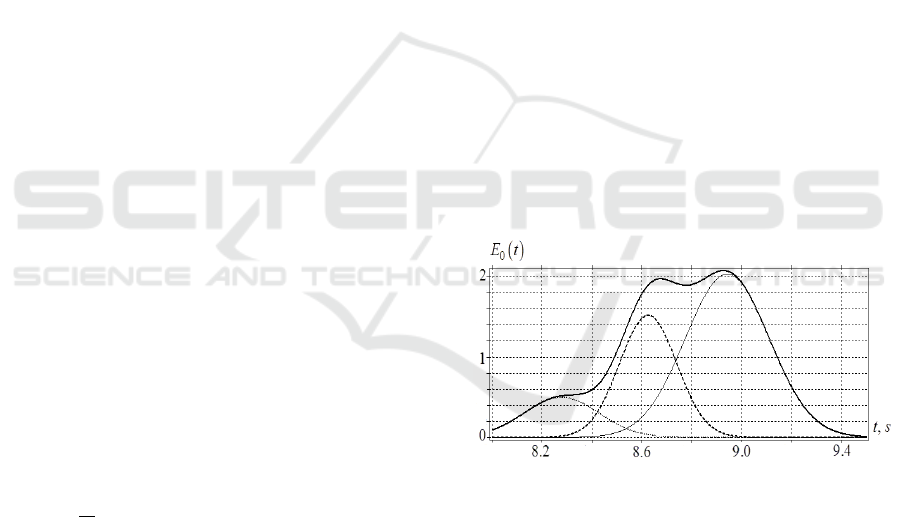
The maximum of each burst is also localized in
time and frequency. The wavelet images of the EEG
signals from the other brain channels are similar in
general, but different in patterns. The bursts differ in
the form, and their maximums also vary in values and
times of occurrence (Fig.1). The quantitative
parameters characterizing each burst, and their
classification are given in (Bozhokin and Suslova
2014; Bozhokin and Suslova 2015).
2.2 Mathematical Model of a Complex
EEG Burst
Let us develop a mathematical theory, which will
allow us to describe quantitatively the change in the
shape of EEG bursts while their moving over the
cerebral cortex. In the spectral range 𝜇𝛼,𝛽,𝛾,𝛿,
the EEG burst detected in the given brain channel 𝐽 is
characterized by spectral integral 𝐸
𝐽,𝑡
. First, to
develop a mathematical model 𝐸
𝐽,𝑡
of the burst,
we consider a single Gaussian peak
𝐺
𝑔
⃗
,𝑡
𝑎
𝑒𝑥𝑝
𝑡𝑏
/2𝑐
(1)
depending on time 𝑡 , and the vector 𝑔⃗
𝑎
;𝑏
;𝑐
with the parameters: 𝑎
− the amplitude,
𝑏
− the time localization center, 𝑐
− the peak’s width.
Then, we represent mathematical model 𝐸
𝐽,𝑡
as a
sum of 𝑛 Gaussian peaks 𝐺
𝑔⃗
,𝑡
:
𝐸
𝐽
,𝑡
𝐺
𝐽
,𝑔
⃗
,𝑡
,
(2)
where 𝑔⃗
is the vector corresponding to 𝑠-peak. As
the simplest example, we take the sum of three
Gaussian peaks (with nine parameters 𝑔⃗
;𝑔⃗
;𝑔⃗
) to
simulate 𝛼-burst ( 𝜇𝛼). The parameters of the
Gaussian peaks for a fixed burst are selected from the
condition
𝛥
1
𝑁
𝐸
𝐽
,𝑡
𝐸
𝐽
,𝑡
→𝑚𝑖𝑛
(3)
where 𝐸
𝐽,𝑡
is the burst observed
experimentally; 𝐸
𝐽,𝑡 is the theoretical model (2);
𝛥 is the standard error of approximation. In (3) the
value of 𝛥 represents the standard error of the
approximation. The summation in (3) is carried out
over all time instants 𝑡
for the selected burst
localized in the time interval 𝑡
;𝑡
in the alpha
range. To find the minimum (3), we applied the
modified Newton method with accuracy control. The
program was tested on the example of determining
the parameters of Gaussian peaks, when the program
input 𝐸
𝑡
consists of three ideal Gaussian peaks
with the parameters 𝑔⃗
𝑎
;𝑏
;𝑐
(1) close to the
real situation: 𝑔⃗
= (0.49938; 8.2785; 0.15001); 𝑔⃗
=
(1.5194; 8.6258; 0.11486); 𝑔⃗
= (2.0357; 8.9427;
0.16810). The result of the program is shown in Fig.3.
Analysing this dependence, it is important to note: the
true peaks of the Gaussian curves and the peaks of the
signal 𝐸
𝑡
can occupy different positions in time.
This conclusion will be important in the study of real
records of EEG activity bursts. In addition, a slight
change in the values of 𝑔⃗
𝑎
;𝑏
;𝑐
can
significantly change the topology of the overall
picture 𝐸
𝑡
. The approximation of the system of
nonlinear equations 𝐸
𝑡
using the nonlinear
approximation program (3) reproduces, with
accuracy to the fifth decimal place, the parameters
𝑔⃗
;𝑔⃗
;𝑔⃗
of the test signal 𝐸
𝑡
. The mean square
error between 𝐸
𝑡
and 𝐸
𝑡
is 𝛥 8.3 ⋅ 10
,
therefore, the curves in Fig.3 merge.
To increase the accuracy of approximation (3), the
experimentally observed values 𝐸
𝐽,𝑡
were
interpolated using the theory of splines. This led to
the fact that the signal sampling step 𝛥𝑡 = 2 ms was
reduced by 20 times. The calculations showed that
most real EEG bursts are satisfactorily described by
three-Gaussian approximation ( 𝑛3), and the
standard deviation between the experimental and
theoretical models is Δ 0.03.
Figure 3: Three Gaussian peaks with the parameters
𝑔⃗
;𝑔⃗
;𝑔⃗
are shown by dots, dashes, and thin lines,
respectively. The bold line corresponds to the sum of three
ideal Gaussian peaks with the parameters𝑔⃗
;𝑔⃗
;𝑔⃗
. The
curve 𝐸
𝑡
and its approximation 𝐸
𝑡
merge entirely into
the bold line.
2.3 Results
2.3.1 Comparison of EEG Simulation
Results and Experimental Data
Let us construct the mathematical model of EEG
burst with the duration [8-9.15 s] in 𝛼 -spectral range,
and follow the change in the shape of this burst while
BIOSIGNALS 2022 - 15th International Conference on Bio-inspired Systems and Signal Processing
66
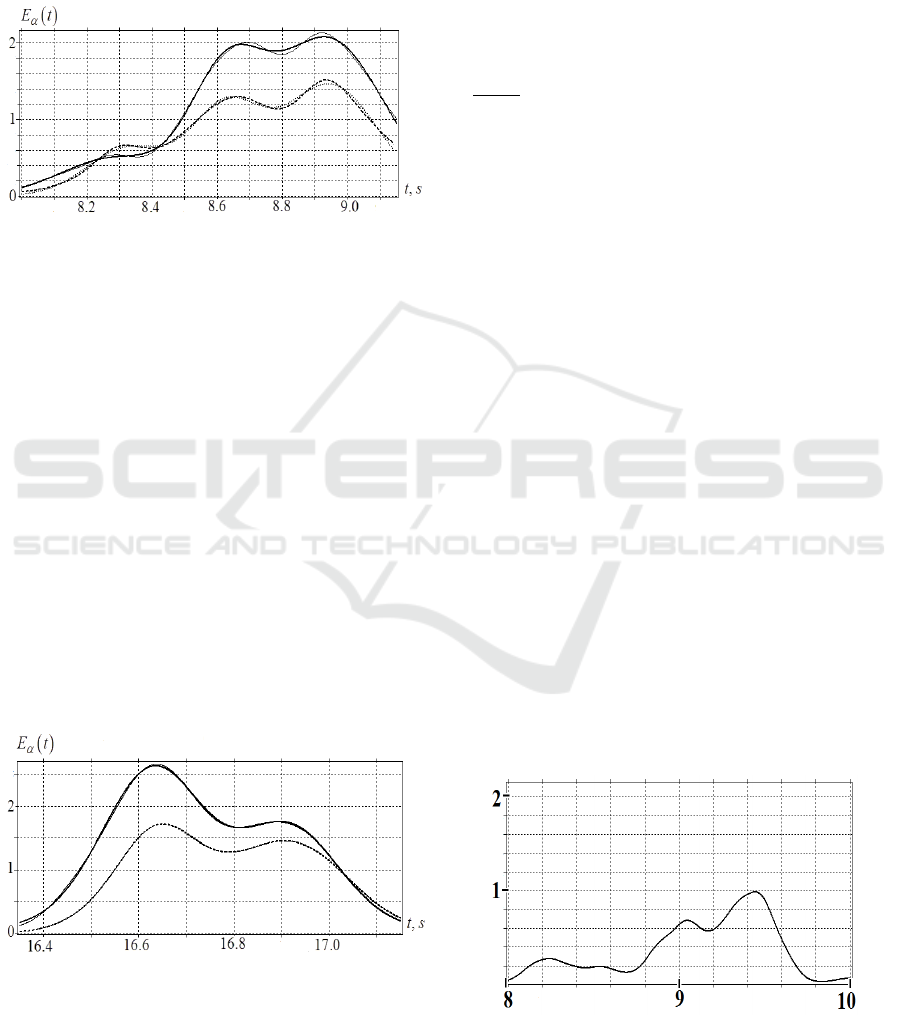
moving between two occipital electrodes 𝐽𝑂
→
𝐽𝑂
.
For the burst in the interval [8-9.15 s], the
difference (3) between the model 𝐸
𝐽,𝑡
and
experimental 𝐸
𝐽,𝑡
is 𝛥
𝑂
0.024 , and
𝛥
𝑂
0.027.
Figure 4: Comparison of the spectral
integrals 𝐸
𝐽,𝑡
obtained for the experimental record of
EEG burst in the interval [8–9.15 s] with their mathematical
models 𝐸
𝐽,𝑡: thin line −𝐸
𝑂
;𝑡
; bold line −𝐸
𝑂
;𝑡
;
dash line −𝐸
𝑂
;𝑡
; dot line −𝐸
𝑂
;𝑡
.
When the burst propagates in the direction𝑂
→
𝑂
, the behavior of three Gaussian peaks, which form
the model burst in the range [8–9.15 s], is different
(Fig.4). The amplitude of the left peak increases:
𝑎
𝑂
/𝑎
𝑂
= 1.26, the time shift between the
peaks 𝑂
→𝑂
is positive 𝛥𝑡 𝑏
𝑂
𝑏
𝑂
=
0.0424 s. The amplitude of the central peak decreases
𝑎
𝑂
/𝑎
𝑂
= 0.74. The time shift of the central
peak is negligible 𝛥𝑡 𝑏
𝑂
𝑏
𝑂
= –0.004.
The amplitude of the right peak decreases 𝑎
𝑂
/
𝑎
𝑂
= 0.712, and its maximum lags behind the
maximum of the central peak 𝛥𝑡 𝑏
𝑂
𝑏
𝑂
= 0.0037. The characteristic widths of all three peaks
с
;𝑐
;𝑐
during their propagation 𝑂
→𝑂
vary
slightly.
Figure 5: Comparison of the spectral integrals
𝐸
𝐽,𝑡
obtained for the experimental record of EEG burst
in the interval [16.2-17.2 s] with their mathematical models
𝐸
𝐽,𝑡: thin line −𝐸
𝑂
;𝑡
; bold line −𝐸
𝑂
;𝑡
; dash
line −𝐸
𝑂
;𝑡
; dot line −𝐸
𝑂
;𝑡
.
Fig.5 represents the curves 𝐸
𝐽,𝑡
and 𝐸
𝐽,𝑡
for 𝑂
and 𝑂
channels, which correspond to the
burst in the time interval [16.3-17.2 s] (compare with
Fig.4). For such a burst, the agreement between
experiment and theory is better: 𝛥
𝑂
0.003 ,
𝛥
𝑂
0.02, as compared to that in the interval [8–
9.15 s].
For the burst in the interval [16.2-17.2 s]
propagating in the direction 𝑂
→𝑂
(Fig.5), the
amplitude of the left main peak decreases:
0.258. The time shift between two main
peaks is positive: 𝛥𝑡 𝑏
𝑂
𝑏
𝑂
=0.007s.
Provided the distance between the nearest (𝑂
→𝑂
)
electrodes 𝐿5cm, the propagation velocity of this
alfa-rhythm peak is 𝑉
𝑂
→𝑂
𝐿/𝛥𝑡, where
𝑉
7.4 ms. Note that the amplitude of the rightmost
peak of this burst increases slightly: 𝑎
𝑂
/
𝑎
𝑂
=1.06. The time shift between the third peaks
during the transition 𝑂
→𝑂
is also positive: 𝛥𝑡
𝑏
𝑂
𝑏
𝑂
= 0.002 s, and its width becomes
larger 𝑐
𝑂
/𝑐
𝑂
= 1.21. Such an expansion of
the right peak of the burst and a slight increase in its
amplitude can be observed in Fig.5 at time 𝑡 17.1s.
2.3.2 The Trajectories of the EEG Bursts in
α -range and the Map of Cerebral
Cortex Electrical Activity
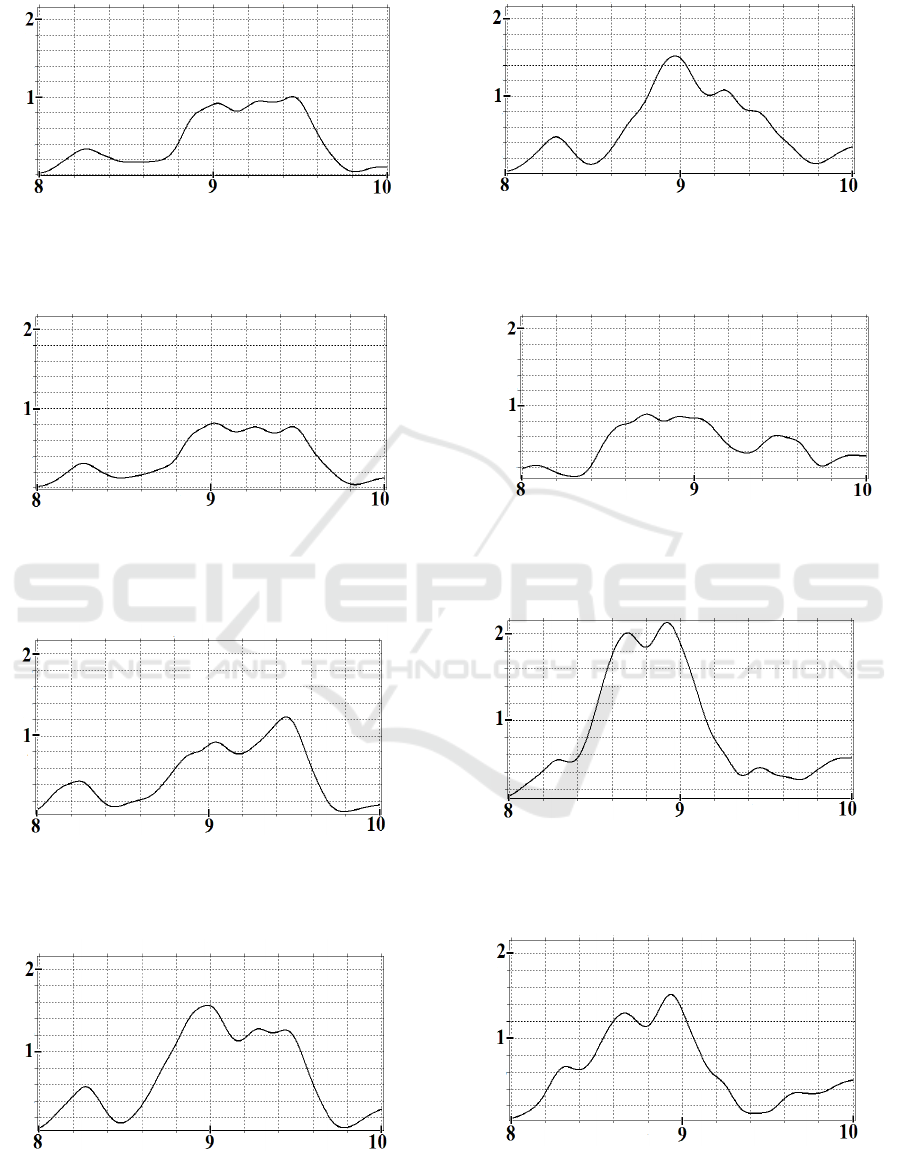
Let us consider the methodology for calculating the
propagation rates of bursts moving along the cerebral
cortex on the example of EEG bursts in α-range.) We
study two bursts in α-range but in different time
intervals: one evolves in the time interval [8–9.15 s]
(Fig.4), the second in [16.3–17.2 s] (Fig.5). .For each
brain channel, the coordinates of the maximum value
of the spectral integral in the given time interval are
indicated in brackets.
𝐸
𝐽;𝑡
t, s
Figure 6a: Spectral integral in the channel C
3
(9.444;0.998)
in the interval [8–9.15 s].
Wavelet based Method of Mapping the Brain Activity Waves Travelling over the Cerebral Cortex
67
𝐸
𝐽;𝑡
t,s
Figure 6b: Spectral integral in the channel C
z
(9.456;1.003)
in the interval [8–9.15 s].
𝐸
𝐽;𝑡
t, s
Figure 6c: Spectral integral in the channel C
4
(9.018;0.815)
in the interval [8–9.15 s].
𝐸
𝐽;𝑡
t, s
Figure 6d: Spectral integral in the channel P
3
(9.454;1.226)
in the interval [8–9.15 s].
𝐸
𝐽;𝑡
t, s
Figure 6e: Spectral integral in the channel Pz (8.982;1.56)
in the interval [8–9.15 s].
𝐸
𝐽;𝑡
t, s
Figure 6f: Spectral integral in the channel P
4
(8.970;1.518)
in the interval [8–9.15 s].
𝐸
𝐽;𝑡
t, s
Figure 6g: Spectral integral in the channel 0
1
(8.724;0.888)
in the interval [8–9.15 s].
𝐸
𝐽;𝑡
t, s
Figure 6h: Spectral integral in the channel 0
z
(8.920;2.13)
in the interval [8–9.15 s].
𝐸
𝐽;𝑡
t, s
Figure 6i: Spectral integral in the channel 0
2
(8.934;1.514)
in the interval [8–9.15 s].
BIOSIGNALS 2022 - 15th International Conference on Bio-inspired Systems and Signal Processing
68
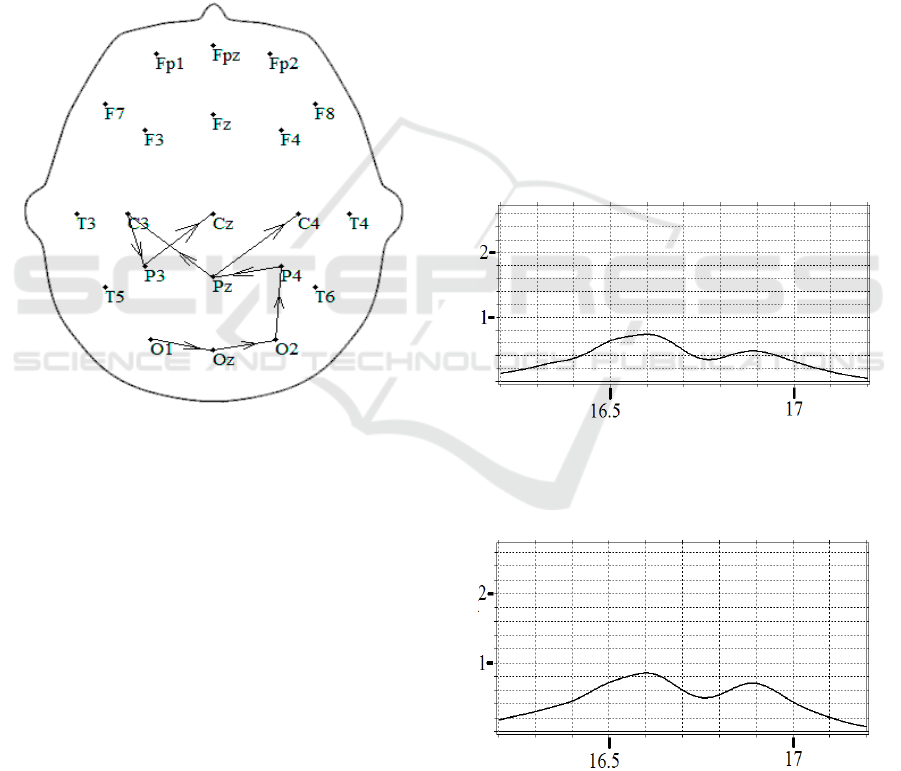
Fig.6 shows the behavior of spectral integrals
𝐸
𝐽;𝑡
in all central channels 𝐽
𝐶
,𝐶
,𝐶
;𝑃
,𝑃
,𝑃
;𝑂
,𝑂
,𝑂
on a single scale in
the interval [8–9.15 s] The development of the burst
during time interval [8–9.15 s] starts in the occipital
channel 𝑂
. Then it reaches a maximum in 𝑂
, and,
gradually decreasing in size, reaches the central
channels 𝑃
and 𝑃
. After that, the burst arrives into
the channel 𝐶
, decreasing in amplitude by 2.6 times
compared with the maximum in 𝑂
. In addition, the
maximum of the burst from the channel 𝑃
moves
along the trajectory 𝐶
→𝑃
→𝐶
(Fig.7). So, the
disturbance spreads from occipital to central and
parietal regions with its activity decreasing in time.
Figure 7: The trajectory of the burst activity maximum in
the time interval [8-9.15 s].
This can be considered as a certain wave process
associated with the excitation of local neural
ensembles caused by some stimuli.
It is important to note that for the peripheral
channels 𝐽𝐹
;𝐹
;𝑇
;𝑇
;𝑇
;𝑇
the values of
𝐸
𝐽,𝑡
in α-range fall by more than 𝑒 2,72 times
as compared to the maximum value of the burst
observed in the central occipital. The numerical
values 𝐸
𝐽,𝑡
are given for each 𝐽, where 𝑡
is the time moment in seconds at which the maximum
value of the spectral integral for the given channel is
reached. Figures 6 show that the intensities and
shapes of bursts are different for each channel.
Moreover, the maximums of the bursts also vary in
amplitude and time localization, so we may talk about
bursts moving at a certain speed along their own path,
that is, about a wave propagating in a dispersive
medium. The study of Fig.6 makes it possible to find
the trajectories of the maxima of the bursts along the
cerebral cortex. This can be done, if we trace the
maxima of the corresponding spectral integrals in
different channels and connect them.
A different trajectory characterizes the burst in
[16.3-17.2 s] (Fig.8-9). The burst of small amplitude
(0.844) occurs in the central channel 𝐶
. This burst
also reaches its maximum value (2.664) in 𝑂
. A
careful analysis of the shape of the bursts in Fig.4,
Fig.5 shows that an individual burst often has a
complex shape and consists of several peaks. In this
case, the trajectories of the burst maximum do not
take into account the changes in the intensity maxima
redistribution of inside the burst itself. It turns out that
in the case of approximating the total burst 𝐸
𝐽,𝑡
by the mathematical model (2), the velocities,
amplitudes, and widths of each Gaussian peak
appeared to be individual.
The results of calculation of spectral integrals
𝐸
𝐽;𝑡
for different EEG channels in the time
interval [16.3-17.2 s] are shown in Fig.8.
𝐸
𝐽;𝑡
t, s
Figure 8a: Spectral integral in the channel C
3
(16.600;0,726) in the interval [16.3-17.2 s].
𝐸
𝐽;𝑡
t, s
Figure 8b: Spectral integral in the channel C
z
(16.602;0,844) in the interval [16.3-17.2 s].
Wavelet based Method of Mapping the Brain Activity Waves Travelling over the Cerebral Cortex
69
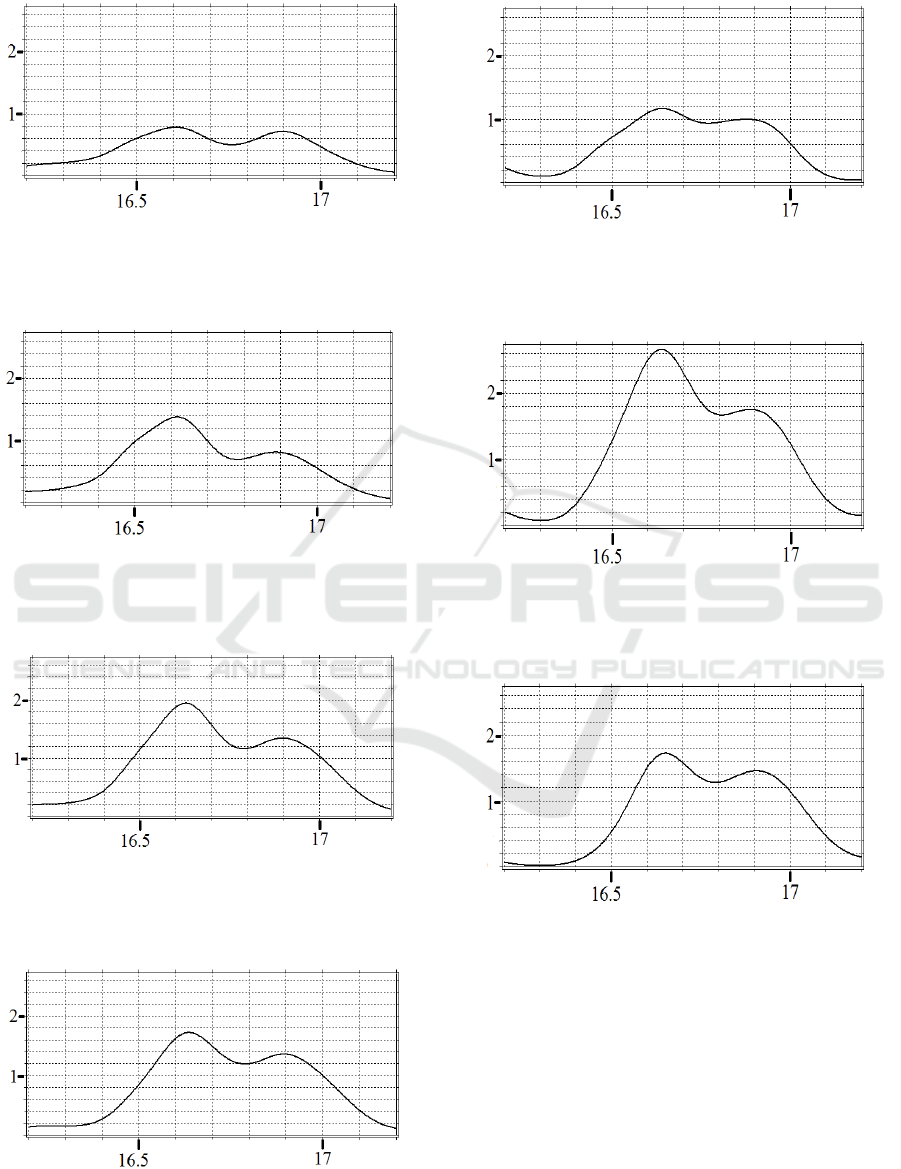
𝐸
𝐽;𝑡
t, s
Figure 8c: Spectral integral in the channel C
4
(16.606;0,78)
in the interval [16.3-17.2 s].
𝐸
𝐽;𝑡
t, s
Figure 8d: Spectral integral in the channel P
3
(16.616;1,378) in the interval [16.3-17.2 s].
𝐸
𝐽;𝑡
t, s
Figure 8e: Spectral integral in the channel P
z
(16.628;1,947)
in the interval [16.3-17.2 s].
𝐸
𝐽;𝑡
t, s
Figure 8f: Spectral integral in the channel P
4
(16.636;1,722)
in the interval [16.3-17.2 s].
𝐸
𝐽;𝑡
t, s
Figure 8g: Spectral integral in the channel 0
1
(16.640;1,164)
in the interval [16.3-17.2 s].
𝐸
𝐽;𝑡
t, s
Figure 8h: Spectral integral in the channel 0
z
(16.638;2,664)
in the interval [16.3-17.2 s].
𝐸
𝐽;𝑡
t, s
Figure 8i: Spectral integral in the channel 0
4
(16.650;1,721)
in the interval [16.3-17.2 s].
Just like the Figures 6, the Figures 8 show
significant change in intensities and shapes of bursts
in different EEG channels. The maximums of the
bursts also vary in amplitude and time of occurrence,
which indicates the movement of an excitement along
the brain cortex.
BIOSIGNALS 2022 - 15th International Conference on Bio-inspired Systems and Signal Processing
70

Figure 9: The trajectory of the burst activity maximum in
the time interval [16.3-17.2 s].
2.4 Discussion and Conclusion
In this paper, we propose the mathematical model of
EEG bursts propagating over the cerebral cortex as a
combination of Gaussian peaks with their centers
moving along different trajectories at different
speeds. Each Gaussian peak in the burst is
characterized by its amplitude, maximum in time and
width. In accordance with the nonlinear
approximation model, we approximate each burst by
the sum of three Gaussian peaks with vectors 𝑔⃗
𝑎
;𝑏
;𝑐
, where 𝑖 1,2,3. Calculating the
parameters of each burst makes it possible to
determine changes in the amplitude, shape, direction
of motion, and velocity of the whole burst.
For mapping the movement of bursts over the
cerebral cortex, we used continuous wavelet
transform (CWT) with the Morlet mother wavelet
function, and the temporary analysis of spectral
integrals. By the method of CWT, the spectral
integrals of non-stationary EEG signal have
been calculated for each brain channel 𝐽 in all
frequency ranges 𝜇𝛼,𝛽,𝛾,𝛿. The advantage of
CWT applied in this work over the traditional
window Fourier transform is that the latter requires
the choice of window size 𝑊, which is the additional
problem. For CWT the window size is adjusted
automatically depending on the frequency 𝜈. When
studying signals at low frequencies, the window
becomes wide. In the high-frequency part of the
spectrum (𝛼and 𝛽 rhythms), the window becomes
narrow. In the case when the EEG signal is a
superposition of several non-stationary signals with a
wide spread in characteristic durations and
frequencies, the choice of a single value 𝑊 optimal
for all spectral components 𝜇 may not be possible at
all.
The spectral integrals used in this work
represent the value of the local density of the signal
spectrum integrated over a certain frequency range 𝜇.
An EEG record is presented as some non-stationary
signal − a sequence of bursts, each of which is
characterized by its spectral composition, beginning
and end, and also by the time of electrical activity
maximum. By using the EEG analysis in 𝛼 -range as
an example, it is shown that there are various
scenarios for the appearance and propagation of
bursts in the cerebral cortex. Comparing the
propagation of two bursts, we detected that in one 𝛼 -
burst, an increase in perturbation occurs from the
parietal region of the brain to the occipital. In another
burst, the disturbances spread from the occipital
region. Such a burst fades in the parietal and central
brain channels. Based on the calculation of 𝐸
𝑡
, the
map of activity in those areas of the brain, where the
bursts reach the maximum values, is defined.
In the article (Anodina-Andrievskaya EM et al
2011) on the correlations of various EEG channels at
solving cognitive problems, it was shown that the
correlation map is individual for each person. We
may assume that the map of velocities and directions
of motion (Fig.7, 9) is individual for each person too.
The intensity of the activity bursts and their
localization in time and space recorded in EEG and
ECoEG reflects the spatio-temporal picture of local
neural ensembles reorganization.
The method of tracking the path of the disturbance
propagation over the cerebral cortex can have many
applications. It can be applied in the quantitative
analysis and classification of transients that
characterize the properties of the central nervous
system of a person at the macro level. The method of
restoring the movement of perturbation along the
surface of the brain was used to determine the
dynamics of assimilation and forgetting the rhythm of
photo-stimulation for non-stationary EEG under the
influence of flash. Thus, the mathematical techniques
proposed in the article are the tool for processing
numerous experimental data related to both the
diagnosis of diseases and the study of cognitive
processes in the human brain.
ACKNOWLEDGEMENT
State Task for Basic Research (topic code FSEG-
2020-0024).
Et
Et
Wavelet based Method of Mapping the Brain Activity Waves Travelling over the Cerebral Cortex
71

REFERENCES
Alexander DM, Jurica P, Trengove C, Nikolaev AR,
Gepshtein S, Zvyagintsev M, Mathiak K, Schulze-
Bonhage A, Ruescher JA, Ball T, Leeuwen C (2013)
Traveling waves and trial averaging: The nature of
single-trial and averaged brain responses in large-scale
cortical signals. NeuroImage, 73, 95–112
Anodina-Andrievskaya EM, Bozhokin SV, Polonsky Yu Z,
Suvorov NB, Marusina MY (2011) Perspective
approaches to analysis of informativity of physiological
signals and medical images of human intelligence.
Journal of instrumental Engineering, 54(7), 27-35
Bahramisharif A, Gerven MAJ, Aarnoutse EJ, Mercier MR,
Schwartz TH, Foxe JF, Ramsey NF, Jensen OJ (2013)
Propagating Neocortical Gamma Bursts Are Coordinated by
Traveling Alpha Waves. Journal of Neuroscience, 33(48),
18849-18854
Belov DR, Kolodyazhnyi SF, Smit NYu (2004) Expression
of Hemispheric Asymmetry and Psychological Type in
the EEG Traveling Wave. Human Physiology, 30(1),
5-19Belov DR, Volnova AV, Ahmediev D(2016)
Travelling wave of ECoG epileptic activity in local
cortical seizure modeling in awake rats. Journal of
Higher Nervous Activity, 66(6), 751-762
Bozhokin SV (2010) Wavelet analysis of learning and
forgetting of photostimulation rhythms for a
nonstationary electroencephalogram. Technical Phys,
55(9), 1248-1256
Bozhokin SV, Suvorov NB (2008) Wavelet analysis of
transients of an electroencephalogram at
photostimulation. Biomed. Radioelectron, N3, 21-25
Bozhokin SV, Suslova IB (2014) Analysis of non-
stationary HRV as a frequency modulated signal by
double continuous wavelet transformation method.
Biomedical Signal Processing and Control, 10, 34-40
Bozhokin SV, Suslova IB (2015) Wavelet-based analysis of
spectral rearrangements of EEG patterns and of non-
stationary correlations. Physica A: Statistical
Mechanics and its Applications, 421(1), 151–160
Bozhokin SV, Zharko SV, Larionov NV, Litvinov AN,
Sokolov IM (2017) Wavelet Correlation of
Nonstationary Signals. Technical Physics, 62(6), 837-
845. https://doi.org/ 10.1134/S1063784217060068
Borisyuk R, Kazanovich Y (2006) Oscillations and waves
in the models of interactive neural populations.
Biosystems, 86, 53-62
Buschman TJ, Denovellis EL, Diogo C, Bullcock D, Miller
EK (2012) Synchronous oscillatory neural ensembles
for rules in the prefrontal cortex. Neuron, 76(1), 838-
846
Getmanenko OV, Belov DR, Kanunikov IE, Smit NY,
Sibarov DA (2006) Reflection of cortical activation
pattern in the human EEG phase structure. Russian
Journal of Physiology, 92(8), 930-948
Chizhov AV, Graham LJ (2008) Efficient evaluation of
neuron populations receiving colored-noise current
based on a refractory density method. Phys.Rev. E, 77,
011910
Gnezditskii VV (2004) A Reverse EEG Problem and
Clinical Electroencephalography. MEDpress-inform,
Moscow
Ivanitsky АМ, Ivanitsky GA, Nikolaev AR, Sysoeva OV
(2009) Encyclopedia of Neuroscience.
Electroencephalography, Springer
Hramov AE, Koronovskii AA, Makarov VA, Pavlov AN,
Sitnikova E (2015) Wavelets in neuroscience. Springer
Series in Synergetics, Springer-Verlag, Berlin,
Heidelberg
Kaplan A, Borisov SV (2003) Dynamic properties of
segmental characteristics of EEG alpha activity in rest
conditions and during cognitive tasks. Zhurnal Vysshei
Nervnoi Deiatelnosti, 53(1), 22-32
Kulaichev AP (2011) The informativeness of coherence
analysis in EEG studies. Neuroscience and behavioral
physiology, 41(3), 321-328
Lopes da Silva F (1991) Neural mechanisms underlying
brain waves: from neural membranes to networks.
Electroenceph. Clin. Neurophysiol, 79, 81–93
Manjarrez E, Vázquez M, Flores A (2007) Computing the
center of mass for traveling alpha waves in the human
brain. Brain Research, 1145, 239 – 247
Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G
(2004) The Sleep Slow Oscillation as a Traveling
Wave. Neurosci., 24(31), 6862– 6870
Muller L, Chavane F, Reynolds J, Sejnowski TJ (2018)
Cortical travelling waves: mechanisms and
computational principles. Nature Reviews
Neuroscience, 19(5), 255–268. https:// doi.org/
10.1038/nrn.2018.20
Mysin IE, Kitchigina VF, Kazanovich YJ (2015)
Modeling synchronous theta activity in the medial
septum: Key role of local communications between
different cell populations. Journal of Computational
Neuroscience, 39, 1-16. https://doi.org/10.1007/
s10827-015-0564-6
Ng V, Barker GJ, Hendler T (2003) Psychiatric
Neuroimaging, Series 1: Life and Behavioural Science,
V.348 of NATO Science Series
Nikolaev AR, Ivanitskii GA, Ivanitskii AM (2001)
Studies of Cortical Interactions over Short Periods of
Time during the Search for Verbal Associations.
Physiology, Neuroscience and Behavioral
Physiology, 31(2), 119-132
Nunez PL (2000) Towads a quantitative description of
large-scale neocortical dynamic function and EEG.
Behavioral and brain sciences, 23, 371–437
Nunez PL, Srinivasan R (2006) Electric Fields of the Brain:
The Neurophysics of EEG, second ed., Oxford
University Press
Ozaki TJ, Sato N, Kitajo K, Someya Y, Anami K, Mizuhara
H, Ogawa S, Yamaguchi Y (2012) Traveling EEG slow
oscillation along the dorsal attention network initiates
spontaneous perceptual switching. Cognitive
Neurodynamics, 6, 185–198
Patten TM, Rennie CJ, Robinson PA, Gong P (2012)
Human Cortical Traveling Waves: Dynamical
Properties and Correlations with Responses. PLoS
ONE, 7(6), e38392
BIOSIGNALS 2022 - 15th International Conference on Bio-inspired Systems and Signal Processing
72

Pfurtscheller G, Lopes da Silva FH (1999) Event-related
EEG/MEG synchronization and desynchronization:
basic principles. Clinical Neurophysiology, 110, 1842-
1857
Quiles MG, Wang D, Zhaoc L, Romeroc RAF, Huang DS
(2011) Selecting salient objects in real scenes: An
oscillatory correlation model. Neural Networks, 24, 54–
64
Riedner BA, Vyazovskiy VV, Huber R, Massimini M,
Esser S, Murphy M, Tononi G (2007) Sleep
homeostasis and Cortical Synchronization. III. A high-
density EEG Study of Sleep Slow Waves in Humans.
Sleep, 30(12), 1643-1657
Tafreshi TF, Daliri MR, Ghodousi M (2019) Functional and
effective connectivity-based features of EEG signals
for object recognition. Cognitive Neurodynamics,
13(6), 555-566
Trofimov AG, Kolodkin IV, Ushakov LV, Velichkovsckii
BM (2015) Agglomerative method for isolating
microstates EEG related to the characteristics of the
traveling wave. Neuroinformatic-2015, MIFI, Moscow,
Part 1. P. 66-77
Terman DH, Ermentrout GB, Yew AC (2001) Propagating
activity patterns in thalamic neuronal networks. SIAM
Journal on Applied Mathematics, 61, 1578–1604
Tong S, Thakor NN (2009) Quantitative EEG Analysis
Methods and Clinical Applications. Artech House.
Boston. London
Verkhlyutov VM, Balaev VV, Ushakov VL, Velichkovsky
BM (2019) A Novel Methodology for Simulation of
EEG Traveling Waves on the Folding Surface of the
Human Cerebral Cortex. Studies in Computational
Intelligence, 799, 51-63. https://doi.org/10.1007/978-3-
030-01328-8_4
Villacorta-Atienza JA, Makarov VA (2013) Wave-
Processing of Long-Scale Information by Neuronal
Chains. PLoS ONE 8(2), e57440.
https://doi.org/10.1371/journal.pone.0057440
Voytek B, Canolty R, Shestyuk A, Crone N, Parvizi J,
Knight R (2010) Shifts in gamma phase–amplitude
coupling frequency from theta to alpha over posterior
cortex during visual tasks. Frontiers in Human
Neuroscience, 4, 191
Zenkov LR (2013) Clinical Electroencephalography,
MEDpress-inform
Zhang H, Watrous AJ, Patel A, Jacobs J (2018) Theta and
alpha oscillations are traveling waves in the human
neocortex. Neuron, 98(6), 1269-1281. https://doi.org/
10.1016/j.neuron.2018.05.019
Wavelet based Method of Mapping the Brain Activity Waves Travelling over the Cerebral Cortex
73
