
Stakeholder’s Perceptions of Value and Risks in Data Governance for
the Secondary Use of Health Data
Hannu Nieminen
1a
, Rima Sermontyte-Baniule
2b
and Nina Helander
3c
1
Faculty of Medicine and Health Technology, Tampere University, Tampere, Finland
2
School of Economics and Business, Kaunas University of Technology, Kaunas, Lithuania
3
Information and Knowledge Management, Tampere University, Tampere, Finland
Keywords: Health Data Governance, Secondary Health Data, Stakeholder Perceptions, Value Creation, Review.
Abstract: The study is a literature study assessing the value expectations and risks perceived by the different
stakeholders related to the governance of secondary use of health data. A key value expectation for all
stakeholders was found to be that data provides public benefits and “common good”, especially through
academic research. Especially for the researchers improvement of health equity in the society was also an
important value expectation. For patients and also for decisionmakers security and privacy related risks were
often mentioned. For all stakeholders the risk of stigma for different groups in the society and for the patient
herself was seen to be important. Constant and clear communication towards all stakeholders about what data
is collected, how it is used, what the expected benefits are and how the risks are managed needs to be a key
element of health data governance solutions. All stakeholders see the importance of involving also the patient
representatives to the governance of health data. Data governance should be developed towards a continuous
and transparent collaborative process, where all stakeholders voice is heard, and they can affect the decisions.
1 INTRODUCTION
Data is often referred as a key resource for creating
value at the level of individuals, organizations and
societies. Value creation is a multifaceted and
complex concept (Sidorchuk 2015, Climent and
Haftor 2021) as multiple elements affect value
perception, such as functionality, aesthetics,
symbolic, financial, social, and emotional aspects
(Karababa and Kjeldgaard, 2014). Value creation can
be defined as the trade-off between benefits captured
and sacrifices made/risks realized (see e.g. Helander
and Kukko,2009).
To ensure effective value creation, good
governance of data is essential. Data governance can
be defined as “the practice of managing data assets
throughout their lifecycle to ensure that they meet
organizational quality and integrity standards”
(Abraham et al. 2019). Data governance is a highly
cross-functional effort to increase the value of data, to
minimize data-related costs and risks and to be able
a
https://orcid.org/0000-0003-1614-2604
b
https://orcid.org/0000-0001-7762-1356
c
https://orcid.org/0000-0003-2201-6444
to utilize data as a strategic asset (Abraham et al.
2019, Carretero et al. 2017, Zhang et al. 2022).
Health data collected by public and private health
care organizations and governments is an extremely
valuable resource that could, in addition to its primary
use in health care, be used in the research aiming to
improve health outcomes, for improving the quality,
safety and cost-effectiveness for health care systems
and, also, for supporting the development of new
products and services. These types of uses are
typically referred to as secondary use of health data.
From the governance perspective secondary use
of health data is in many ways a special case: health
information consists of highly protected personal
health data, maintaining the privacy and security of
many types of health data can be difficult, there are
exceptional needs for data security, data sources are
heterogenous, interoperability between different
countries health care systems is a challenge and the
regulatory framework still remains fragmented (Pinto
et al. 2021, OECD 2015).
Nieminen, H., Sermontyte-Baniule, R. and Helander, N.
Stakeholder’s Perceptions of Value and Risks in Data Governance for the Secondary Use of Health Data.
DOI: 10.5220/0011373900003335
In Proceedings of the 14th International Joint Conference on Knowledge Discovery, Knowledge Engineering and Knowledge Management (IC3K 2022) - Volume 3: KMIS, pages 119-125
ISBN: 978-989-758-614-9; ISSN: 2184-3228
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
119

Different stakeholders (patients, medical
professionals, researchers, health care managers and
decisionmakers) have their own perspectives towards
the value creation. Value expectations of patients /
citizens for the secondary use of data have been
summarized in recent systematic reviews (Aitken et
al. 2016, Skovgaard et al. 2019, Perrin and Mathieu
2021, Kalkman et al. 2022). Patients have been found
to be in general supportive towards the secondary use
of health data, provided that the data is used for
“common good” purposes, and that they trust the
organizations (Aitken et al. 2016, Skovgaard et al.
2019, Perrin and Mathieu 2021, Kalkman et al. 2022).
Key concerns were related to confidentiality,
individual’s control over their data (Aitken et al.
2016, Perrin and Mathieu 2021, Kalkman et al. 2022)
and misuse of data in a way that puts some groups of
people in bad light (Skovgaard et al. 2019). Also,
citizens often lack knowledge on secondary use of
health data and on how the privacy and security of the
data is protected (Aitken et al. 2016, Perrin and
Mathieu 2021, Kalkman et al. 2022).
While there are recent literature reviews about the
banefits and risks expectation from the patient
perpective, reviews combining the perspectives of
stakeholders are abundant. The ways to create value
for the various stakeholders has recently gained
growing attention in the academic debate on value
creation in general (e.g. Busch et al. 2016), but not yet
much in the context of secondary use of health data.
This research study aims to assess, based on a
literature study, the value expectations and risks
perceived by the different stakeholders related to the
governance of health data for secondary use.
Understanding the value expectations and perceived
risks provides input for building governance
solutions, which meet the expectations of all
stakeholders as well as possible.
In the next section, the research method is
presented, and after this the findings of the literature
review are presented. Finally, the research results and
limitations of the study are discussed and the key
conclusions are summarized.
2 RESEARCH METHOD
To find the right articles related to the topic, the
following search terms were combined to search
articles published during the past 10 years from the
Web of Science database: health data, governance,
(secondary use OR re-use OR data sharing), (value
OR benefit), (risk OR problem), (stakeholder OR
patient OR citizen OR professional OR manager OR
industry OR decisionmaker OR physician).
Articles fulfilling all the following inclusion
criteria were included in the further analysis: 1)
Article utilizes or refers to health data governance for
the secondary use of data, 2) Perceived value,
benefits, risks or problems of secondary use are
studied from the perspective of at least one of the
identified stakeholder groups, 3) Article presents
original results from empirical research.
In addition, searches were done by scanning the
references of included papers. After the analysis, a
total of 23 papers were selected for further study. A
thematic synthesis approach (Thomas and Harden
2008) was adopted. First the key findings describing
the values, benefits, risks and problems perceived by
the different stakeholders were extracted from the
publications. After this, the findings were classified
under descriptive themes emerging from them, and in
the final phase the themes were interpreted in relation
to the research question and analytical themes were
developed.
3 FINDINGS
19 of the selected papers were studying the
patients/citizens, 10 medical researchers and 5
decisionmakers / managers (8 papers studied the
perceptions of several stakeholders).
Different types of mostly qualitative methods
were used in the studies. Most common methods were
structured interviews (4), focus groups (5), surveys
(8) and multi-method studies combining interviews
and focus groups (6). Total aggregated N for studies
using surveys was 15794, varying between 280 and
8004. For other methods the aggregated N was 655,
varying between 18 and 73 in the publications.
3.1 Value / Benefit Expectations
For patients, a total of five themes describing their
expectations for value/benefit from the secondary use
of data were identified: The most common value
expectation (9 papers) was that the data contributes
to public good through academic research (e.g.
Audrey et al. 2016, Grande et al. 2013, Karampela et
al. 2019). Related to this, the second most important
expectation was that data provides public benefit
improving the health of the population and health
equity (7) (e.g. Evans et al. 2020, Tully et al. 2018,
Spencer et al. 2016). Patients strongly except, that
public good for the society is achieved from the data
they share, and that the governance solutions help
KMIS 2022 - 14th International Conference on Knowledge Management and Information Systems
120
them to trust that this expectation is fulfilled.
Improvement of the quality of care or outcomes for
patients was also mentioned in 5 papers (e.g. Adanijo
et al. 2021, Velarde et al. 2021). Other value
expectations from patients included data advancing
innovation (4) (e.g. Colombo et al. 2010, Johansson
et al. 2021) and data improving the quality of
research (3) (e.g. Manhas et al. 2018). Patients often
were suspicious about the use of data for advancing
commercial innovation, but with more information
about the benefits and safeguards they became more
accepting towards it (Tully et al. 2018).
For researchers the public benefit of improving
the health of the population and health equity was the
most often mentioned value expectation (4/10
papers). Health data was seen as an essential public
resource to protect and produce population health
(Evans et al. 2020, Mbuthia et al. 2019) and a key tool
to help reach better health equity e.g. for low-to-
middle income settings (e.g. Jao et al. 2015). Other
benefits included data improving care and saving
resources (3 papers, e.g. Neves et al. 2019),
improvement of societal decision-making and
regulation (Hate et al. 2015) and improving the
quality of research (Adanijo et al. 2021). For
decisionmakers the most often mentioned value
expectations were improvement of care and saving of
resources (3/5, e.g. Mazor et al. 2017), data
improving the quality of research (2) and public good
benefits (2).
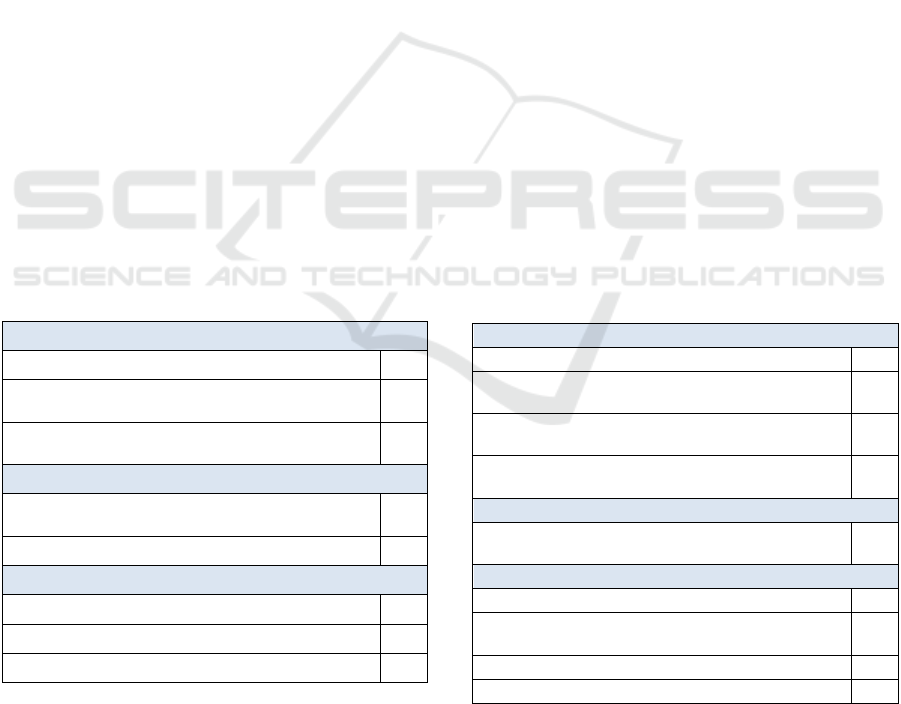
Table 1: Most common value/benefit expectations.
Patients (19 papers)
Public good through academic research
9
Public benefit improving the health of the
population and health equity
7
Improvement of the quality of care or outcomes
f
or
p
atients
5
Researchers (10 papers)
Public benefit of improving the health of the
population and health equity
4
Data improving care and saving resources
3
Decisionmakers (5 papers)
Improvement of care and saving of resources
3
Data improving the quality of research
2
Public good benefits
2
Some of the studied papers did not specifically
address the value expectations, but were more
focusing on risks, foreseen problems and
recommendations for data governance solutions.
3.2 Foreseen Risks and Problems
Several themes were identified for the foreseen risks
and problems in the governance of health data.
Patients most often mentioned privacy and
confidentiality related risks (10 papers). These
included especially risks of being able to identify a
person even from anonymized data (e.g. Cheah et al.
2018) and data and privacy breaches and
infringements (e.g. Seltzer et al. 2019). Mistrust in the
organizations governing data so that patients cannot
affect how and by whom the data is used was the
second most commonly mentioned risk (8 papers, e.g.
McCormick and Hopkins 2021, Shah et al.2019). Due
to lack of transparency and awareness of the ways
how the data will be used, data could be misused by
companies, government or other actors for “bad
intentions” (Evans et al. 2020). Third most common
theme (6 papers) was the risk of data leading to
stigmatizing or discriminating some groups in the
society, for example gays, ethnic groups or people
living in a certain area (e.g. . Audrey et al. 2016,
Cheah et al. 2018). Not achieving public good due to
the data used for profit-making purposes was seen as
a risk in 5 papers, e.g. Mazor et al. 2017. Also,
negative effect on care for example due to the long
lifespan of data “permanently marking” the
individual (Evans et al. 2020), patients not
understanding what they are consenting to and later
inconvenience for the patient were seen as risks.
Table 2: Most common foreseen risks and problems.
Patients (19 papers)
Privacy and confidentiality
10
Mistrust in organizations: patients cannot affect
how and b
y
whom the data is used
8
Data stigmatizing or discriminating some groups
in the societ
y
6
Not achieving public good due to the data used for
profit-making purposes
5
Researchers (10 papers)
Data stigmatizing or discriminating some groups
in the societ
y
5
Decisionmakers (5 papers)
Security and privacy problems
4
Lack of transparency and awareness in the way
how data is used
4
Problems in data quality
3
Data stigmatizing or discriminating some groups
3
For medical researchers the variation in the
foreseen risks was high. 5 papers mentioned the risk
of data leading to stigmatizing groups in society as a
risk (e.g. Jao et al. 2015). Other risks included
Stakeholder’s Perceptions of Value and Risks in Data Governance for the Secondary Use of Health Data
121

excessive costs for the governance (3, e.g. Ballantyne
et al. 2020), additional burden for the patient (4),
patients not understanding what they are consenting
to (3), privacy and confidentiality (3), mistrust in the
organization governing the data (3), lack of
transparency in the ways how data is used (2), data
affecting care in a negative way (3), prioritizing profit
(3) and problems in research quality (2).
Decisionmakers identified as risks and problems
security and privacy problems (4), lack of
transparency and awareness in the way how data is
used (4), problems in data quality affecting research
results (3) for example inherent biases in collecting
data misguiding decisions (Evans et al. 2020) and
data leading to stigmatizing groups in the society (3).
3.3 Recommendations for Data
Governance
Based on the study findings, many papers provided
recommendations and requirements for the data
governance. From patients the most common
recommendation was the need to provide clear and
understandable information about how the data is
shared and what the key benefits are (6 papers, e.g.
Audrey et al. 2016, Kim et al. 2015). Existing
safeguarding procedures need to be highlighted
(Adanijo et al. 2021) and a review process to oversee
the use of data needs to be at place (Johansson et al.
2021). All stakeholders, including patient
representatives, should be involved in the governance
of data (Adanijo et al. 2021, Hate et al. 2015) and
patients should have choice on what data is shared
and have a possibility to opt out (3 papers). Ensuring
trust in the organizations governing the data (4) and
safety and security (4) is essential. Sanctions should
be at place in case of data misuse (Colombo et al.
2019). In order to maintain trust good communication
is important (Hate et al. 2015).
Researchers agree with the patients that all
stakeholders should be involved in the governance of
data (4, e.g. Manhas et al. 2018). Community
engagement in the data governance committees is
seen as an essential element of ethical practice (e.g.
Jao et al. 2015). Ensuring patient consent is a key
requirement (3, e.g. Hate et al 2015, Stevenson 2015).
Effective processes are needed for the governance of
requests for data re-use in later projects, and a broad
form of consent would make this easier (Jao et al.
2015). Other recommendations include need to
provide clear and understandable information how
data is shared and what the benefits are and ensuring
security and safety through strict safeguards.
Decisionmakers provide very similar
recommendations as researchers, emphasizing the
involvement of all stakeholders in the governance,
ensuring clear and understandable information for all
stakeholders, ensuring safety and security, ensuring
patient consent and building trust in all entities
participating in the data governance process.
As a general observation, the reviewed papers did
not provide detailed level descriptions on the health
data governance models. Thus, there still remains in
the literature a gap of the discussion on successful
health data governance model in practice.
Table 3: Most common data governance recommendations.
Patients (19 papers)
Clear and understandable information about how
the data is shared and what the key benefits are
6
Ensuring trust in the organizations governing the
data
4
Ensuring safety and security
5
Patients should involved in the governance, and
s
hould have a choice on what data is shared
3
Researchers (10 papers)
All stakeholders should be involved in the
g
overnance of data
4
Ensuring patient consent
3
Decisionmakers (5 papers)
All stakeholders should be involved in the
g
overnance of data
2
Ensuring clear and understandable information
f
or all stakeholders,
2
4 DISCUSSION
Understanding of the stakeholder’s expectations for
the values and risks for the secondary use of health
data is important to ensure the development of high-
quality solutions for health data governance, which
can be accepted both by the citizens/patients and by
the medical professionals.
Recent literature reviews have indicated that for
the patients a key value expectation is that the
secondary use of health data should ensure public
benefits and “common good”, and that trust in the
organizations utilizing the data is essential for them
(Aitken et al. 2016, Skovgaard et al. 2019, Perrin and
Mathieu 2021, Kalkman et al. 2022). Our review
results indicate, that this key value expectation is also
shared by the other stakeholders. For the researchers
improvement of health equity was also seen as an
important value expectation. Big data is seen as a
valuable resource to improve and develop the society
towards being more equal. These types of altruistic
KMIS 2022 - 14th International Conference on Knowledge Management and Information Systems
122

value goals are mentioned more often than values
related to care improvement or innovation, and
especially patients seem to be somewhat suspicious
towards industry’s use of data for innovation
purposes. Most probably being more transparent in
explaining the benefits of innovation, ways how data
would be used and the safeguards at place would
make the stakeholders more positive towards these
types of uses.
Summarising the results of value expectations of
patients, researchers and decisionmakers we have
identified that despite common value expectations
there exist unique value expectations, specifically for
patients and researchers. Analyzed papers report such
value expectation by patients as ability of data to
advance innovations. Interestingly, it is not so much
reported by researchers or decisionmakers which
perhaps are more involved into research and
innovation processes than patients. Other interesting
insight is the fact, that researchers report such value /
benefit expectation as improvement of societal
decision-making and regulation which is not reported
by decisionmakers themselves as value / benefit.
Contrary, decision makers see the benefit of
secondary data more as helping to improve the quality
of research. In other words, researchers and decision
makers do not assign certain value as applicable to
them even though it is closely related.
Regarding the perceived risks, for patients and
also for decisionmakers security and privacy related
risks were most often mentioned. This corresponds
well with the earlier reviews (Aitken et al. 2016,
Perrin and Mathieu 2021, Kalkman et al. 2022).
Researchers, however, did not see these risks as so
important. Possible reason for this is that
professionals know in more detail the ways how
privacy is protected. Interestingly, for all stakeholder
groups the risk of stigma for different groups in the
society was seen to be important. This would come
from the misuse of data or from the inherent biases
and limitations in the collected data.
Full transparency towards all stakeholders on the
ways what data is collected and used and on the
expected benefits would be essentially important for
building the trust. Specifically, literature analysis
highlights, that a worry of not achieving public good
due to the data used for profit-making commercial
purposes is expressed by patients, but not reported by
researchers or decisionmakers. Constant and clear
communication towards all stakeholders needs to be
a key element for the future data governance models.
When data is collected, it is required that patient
signs an informed consent. This consent typically is
only for needs of one study, and it describes in detail
what data is collected, how it is protected and what it
is used for. Broad forms of consent are also in use,
where patient gives the right for using data also for
secondary studies. While consent forms provide
information for the patients about the future use of
data, this “one governance contact with the patient”
principle is not enough. Advanced systems, where the
patient can monitor the requests for data use and opt
out or adapt her preferences are also being developed
(e.g. Williams et al. 2015).
Based on our review, stakeholders see the
importance of involving also the patient
representatives to the governance of health data. Data
sharing could be overseen by a committee involving
e.g. (Hate et al 2015) decisionmakers, internal
researchers, patient representatives, representatives
from the communities where data is collected and
ethicists. As Joa et al. (2015) states: “governance
processes need to include openness, solidarity,
fairness, and truth-telling”. Building this kind of
stakeholder involvement and continuous and
transparent communication process is a key challenge
for the future health data governance solutions and
thus, important avenue for further research.
The results of this literature study will be utilized
and further verified in our ongoing empirical study on
stakeholders perceptions on value, benefits and risks
of secondary use of health data. Study is focusing on
long-term home care of chronic diseases, and data is
being collected in 5 countries (Finland, Sweden,
France, Lithuania and Spain) as part of the DiHECO
(Digital Healthcare Ecosystem research and
innovation capability building) project.
Limitations of the Study
Even though this literature based research at hand was
able to give some guidelines for further research, the
research also faced limitations. For example, the
number of articles describing the perceptions of
researchers / medical professionals (10) and
decisionmakers (5) was smaller than the number of
articles analyzing the patients perspective (19),
leading to potential emphasis on patients’ views.
5 CONCLUSIONS
Based on our literature study, a key value expectation
for the secondary use of health for all stakeholders is
that data provides public benefits and “common
good”, especially through academic research.
Especially for the researchers improvement of health
equity in the society is also seen as an important value
expectation. For patients and also for decisionmakers
Stakeholder’s Perceptions of Value and Risks in Data Governance for the Secondary Use of Health Data
123

different security and privacy related risks were the
most often mentioned risks, while researchers did not
mention these risks so often. For all stakeholder
groups the risk of stigma for different groups in the
society and for the patient herself was seen to be
important.
Constant and clear communication towards all
stakeholders about what data is collected, how it is
used, what the expected benefits are and how the risks
are managed need to be a key element of health data
governance solutions. Communications is the
essential enabler for building the needed trust
between the stakeholders. All stakeholders see the
importance of involving also the patient
representatives to the governance of health data. Data
governance should be developed towards a
continuous and transparent collaborative process,
where all stakeholders voice is heard, and they can
affect the decisions. Building this kind of stakeholder
involvement and continuous and transparent
communication process is a key challenge for the
future health data governance solutions.
ACKNOWLEDGEMENTS
This work is a part of the DiHECO project, which has
received funding from the European Union’s Horizon
2020 research and innovation programme under grant
agreement No. 952012.
REFERENCES
Abraham, R., Schneider, J., von Brocke, J. (2019) Data
governance: A conceptual framework, structured
review, and research agenda. International Journal of
Information Management, Volume 49, December 2019,
Pages 424-438.
Adanijo, A., McWilliams, C., Wykes, T., Jilka, S. (2021)
Investigating mental health service user opinions on
clinical data sharing; Qualitative focus group study.
JMIR Mental Health, vol. 8, iss. 9
Aitken, M., St Jorre, J., Pagliari, C., Jepson, R.,
Cunningham-Burley, S. (2016) Public responses to the
sharing linkage of health data for research purposes: a
systematic review and thematic synthesis of qualitative
studies. BMC Medical Ethics (2016) 17:73.
Audrey, S., Brown, L., Campbell, R., Boyd, A., Macleod,
J. (2016) Young people’s views about consenting to
data linkage: findings from the PEARL qualitative
study. BMC Medical Research Methodology 16:34.
Ballantyne, A., Moore, A., Bartholomew, K., Aagaard, N.
(2020) Points of contention: Qualitative research
identifying where researchers and research ethics
committees disagree about consent waivers for
secondary research with tissue and data. PLoS ONE
15(8): e0235618.
Busch, T., Hamprecht, J. & Waddock, S. (2018) Value(s)
for Whom? Creating Value(s) for Stakeholders.
Organization & Environment Vol. 31, 210-222.
Carretero, A.G., Gualo, F., Caballero, L., Piattini, M.
(2017). MAMD 2.0: Environment for data quality
processes implementation based on ISO-8000-6X and
ISO/IEC 33000. Computer Standards and Interfaces,
54(3), 139-151.
Cheah, P., Jatupompimol, N., Hanboonkunupakam, B.,
Khirikoekkong, N., Jittamala, P., Pukrittayakamee, S.,
Day, N., Parker, M., Bull, S. (2018) Challenges arising
when seeking broad consent for health research data
sharing: a qualitative study of perspectives in Thailand.
BMC Medical Ethics, 19_68.
Climent, R.C., Haftor, D.M. (2021) Value creation through
the evolution of business model themes. Journal of
Business Research, Vol. 122, January 2021, pp. 353-361.
Colombo, C., Roberto, A., Krieza-Jeric, K., Parmelli, E.,
Banzi, R. (2019) Sharing individual participant data
from clinical studies: a cross-sectional online survey
among Italian patient and citizen groups. BMJ Open
2019;9:e024863.
Evans, E., Delorme, E., Cyr, K., Goldstein, D. (2020) A
qualitative study of big data and the opioid epidemic:
recommendations for data governance. BMC Med
Ethics, 21:101.
Grande, D., Mitra, N., Shah, A., Wan, F., Asch, D. (2013)
Public preferences about secondary uses of electronic
health information. JAMA Inter Med. 173(19), 1798-
1806
Hate, K., Meherally, S., More, N, Jayaraman, A., Bull, S.,
Parker, M., Osrin, D. (2015) Sweat, skepticism, and
uncharted territory: A qualitative study of opinions on
data sharing among public health researchers and
research participants in Mumbai, India. Journal of
Empirical Research on Human Research Ethics, Vol.
10(3), 239-250
Helander, N., & Kukko, M. (2009) A value-creating
network analysis from software business. International
Journal of Management and Marketing Research, 2(1),
73-88.
Jao, I., Kombe, F., Mwalukore, S., Bull, S., Parker, M.,
Kamuya, D., Molyneux, S., Marsh, V. (2015) Involving
research stakeholders in developing policy on sharing
public health research data in Kenya: Views on fair
process for informed consent, access oversight and
community engagement. J. of Empirical Research on
Human Research Ethics, Vol 19(3), 264-277.
Johansson, J., Bentzen, H., Shah, N., Haraldsdottir, E.,
Jonsdottir, A., Kaye, J., Mascalzoni, D., Veldwijk, J.
(2021) Preference of the public for sharing health data:
Discrete choice experiment. JMIR Med Inform, vol9,
iss. 7, e29614.
Kalkman, S., van Delden, J., Banerjee, A., Tyl, B.,
Mosterts, M., van Thiel, G. (2022) Patients’ and public
views and attitudes towards the sharing of health data
for research: a narrative review of the empirical
evidence. J Med Ethics 2022; 48:3-13.
KMIS 2022 - 14th International Conference on Knowledge Management and Information Systems
124

Karababa, E. and Kjeldgaard, D. (2014) Value in
marketing: Toward sociocultural perspectives.
Marketing Theory, 14(1), pp.119–127.
Karampela, M., Ouhbi, S., Isomursu, M. (2019) Connected
health user willingness to share personal health data:
Questionnaire study. J Med Internet Res; 21(11)
Kim, K., Joseph, J., Ohno-Machado, L. (2015) Comparison
of consumers’ views on electronic data sharing for
healthcare and research. J Am Med Inform Assoc;
22:821-830.
Manhas, K. P., Dodd, S. X., Page, S., Letourneau, N., Adair,
C. E., Cui, X., & Tough, S. C. (2018). Sharing
longitudinal, non-biological birth cohort data: a cross-
sectional analysis of parent consent preferences. BMC
medical informatics and decision making, 18(1), 1-11.
Mazor, K. M., Richards, A., Gallagher, M., Arterburn, D.
E., Raebel, M. A., Nowell, W. B., ... & Toh, S. (2017).
Stakeholders’ views on data sharing in multicenter
studies. Journal of comparative effectiveness research,
6(6), 537-547.
Mbuthia, D., Molyneux, S., Njue, M., Mwalukore, S., &
Marsh, V. (2019). Kenyan health stakeholder views on
individual consent, general notification and governance
processes for the re-use of hospital inpatient data to
support learning on healthcare systems. BMC medical
ethics, 20(1), 1-16.
McCormick, J. B., & Hopkins, M. A. (2021). Exploring
public concerns for sharing and governance of personal
health information: a focus group study. JAMIA open,
4(4), ooab098.
Neves, A. L., Poovendran, D., Freise, L., Ghafur, S., Flott,
K., Darzi, A., & Mayer, E. K. (2019) Health care
professionals’ perspectives on the secondary use of
health records to improve quality and safety of care in
England: qualitative study. Journal of medical Internet
research, 21(9), e14135.
OECD (2015), Health Data Governance: Privacy,
Monitoring and Research, OECD Health Policy
Studies, OECD Publishing, Paris. http://dx.doi.org/
10.1787/9789264244566-en
Perrin, Z., Mathieu, L. (2021) Citizen’s perception of and
engagement with health data secondary use and sharing
in Europe – a literature review. Project report,
TEHDAS – Towards European Health Data Space, 25
November 2021. Accessed 28.3.2022. from
www.tehdas.eu.
Pinto, C., van Gool C., Cascini, F. et al. (2021) Why health
is a special case for data governance. Project report,
TEHDAS – Towards European Health Data Space, 23
June 2021. Accessed 21.3.2022. from www.tehdas.eu.
Seltzer, E., Goldshear, J., Guntuku, S. C., Grande, D., Asch,
D. A., Klinger, E. V., & Merchant, R. M. (2019)
Patients’ willingness to share digital health and non-
health data for research: a cross-sectional study. BMC
medical informatics and decision making, 19(1), 1-8.
Shah, N., Coathup, V., Teare, H., Forgie, I., Giordano, G.
N., Hansen, T. H., ... & Kaye, J. (2019) Motivations for
data sharing—views of research participants from four
European countries: a DIRECT study. European
Journal of Human Genetics, 27(5), 721-729.
Sidorchuk, R. (2015) The concept of “value” in the theory
of marketing. Asian Social Science, 11(9), pp.320–325.
Skovgaard, L., Wadmann, S., Hoeyer, K. (2019) A review
of attitudes towards the reuse of health data among
people in the European Union: The primacy of purpose
and the common good. Health Policy 123 (2019), 564-
571.
Spencer, K., Sanders, C., Whitley, E. A., Lund, D., Kaye,
J., & Dixon, W. G. (2016) Patient perspectives on
sharing anonymized personal health data using a digital
system for dynamic consent and research feedback: a
qualitative study. Journal of medical Internet research,
18(4), e5011.
Stevenson, F. (2015) The use of electronic patient records
for medical research: conflicts and contradictions.
BMC health services research, 15(1), 1-8.
Thomas J, Harden A. (2008) Methods for the thematic
synthesis of qualitative research in systematic reviews.
BMC Med Res Methodol. 8(45):1–10.
Tully, M., Hassan, L., Oswald, M., Ainsworth, J. (2018)
Commercial use of health data – A public “trial” by
citizens’ jury. Learn Health Sys 3: e10200.
Velarde, M. C. R., Tsantoulis, P., Burton-Jeangros, C.,
Aceti, M., Chappuis, P., & Hurst-Majno, S. (2021)
Citizens' views on sharing their health data: the role of
competence, reliability and pursuing the common good.
BMC Medical Ethics, 22(1), 1-12.
Williams, H., Spencer, K., Sanders, C., Lund, D., Whitley,
E., Kaye, J., Dixon, W. (2015) Dynamic consent: A
possible solution to improve patient confidence and
trust in how electronic patient records are used in
medical research. JMIR Medical Informatics, 3(1).
Zhang, Q., Sun, X., Zhang, M. (2022) Data matters: A
strategic action framework for data governance.
Information and Management, Vol. 59, Issue 4, June
2002.
Stakeholder’s Perceptions of Value and Risks in Data Governance for the Secondary Use of Health Data
125
