
Antibacterial, Antifungal, and Antioxidant Activities and Polyphenol
Content of the Edible Seeds of Archidendron bubalinum
K. Y. Teh, Y. L. Wong and N. W. Sit
*a
Department of Allied Health Sciences, Faculty of Science, Universiti Tunku Abdul Rahman, Bandar Barat, 31900 Kampar,
Perak, Malaysia
Keywords: Antibacterial, Antifungal, Antioxidant, Archidendron bubalinum, Total Phenolic Content
Abstract: This study assessed the kernel extracts of Archidendron bubalinum (family Leguminosae) seeds for
antibacterial, antifungal, and antioxidant activities as well as polyphenol content. The kernels were macerated
using hexane, ethyl acetate, ethanol, and water sequentially and produced four extracts for analysis. All the
extracts showed bactericidal effects against five of the six species of bacteria tested with minimum bactericidal
concentrations of 0.63-2.50 mg/mL, and fungicidal effects against five species of fungi with minimum
fungicidal concentrations of 0.31-2.50 mg/mL. All the extracts also exerted antioxidant activities. The hexane
extract exhibited the lowest oxygen radical absorbance capacity (ORAC) and ferric-reducing antioxidant
power (FRAP) values of 81.63 mmol Trolox equivalent/g of extract and 25.93 mmol Fe
2+
equivalent/g of
extract, respectively. In contrast, ethyl acetate extract exhibited the highest ORAC (300.13 mmol Trolox
equivalent/g of extract) and FRAP (341.36 mmol Fe
2+
equivalent/g of extract) values. Polyphenols were
detected in all extracts with the total phenolic content (TPC) of 3.22-20.19 mg gallic acid equivalent/g of
extract and the total flavonoid content (TFC) of 0.97-4.42 mg quercetin equivalent/g of extract. Association
analysis between ORAC/FRAP and TPC/TFC revealed significant strong positive correlations (r = 0.937-
0.995; all p<0.001). In conclusion, the edible seeds of A. bubalinum possessed antimicrobial and antioxidant
properties and could be promoted as a healthy food.
1 INTRODUCTION
Communicable diseases caused by microorganisms
such as bacteria, fungi, viruses, and parasites are one
of the major contributors to human morbidity and
mortality worldwide. In 2019, more than 26.1 billion
incident cases of communicable diseases were
reported, of which ~93% of the cases involved
respiratory and enteric systems, contributing to 4.2
million death globally (GBD 2019 Diseases and
Injuries Collaborators, 2020). Bacteria have been
recognized as a main etiological agent for human
respiratory and enteric infections. They can be
broadly classified into two groups; Gram-positive
bacteria have a thick peptidoglycan layer anchored
with teichoic acids in the cell wall without an outer
membrane while Gram-negative bacteria possess a
cytoplasmic membrane and a lipopolysaccharides-
containing outer membrane with a thin peptidoglycan
layer in between the membranes (Fisher and
a
https://orcid.org/0000-0002-5949-491X
Mobashery, 2020). Beta-lactams, tetracyclines,
oxazolidinones, macrolides, aminoglycosides,
sulfonamides, and quinolones are among the
antibacterial agents developed for therapeutic use.
However, the misuse and overuse of these agents as
well as the natural evolutionary ability of
microorganisms have driven the increased prevalence
of antibacterial resistance (Chokshi et al., 2019;
Christaki et al., 2020). Resistance to antibacterial
therapy has resulted in patients with more serious
illnesses, prolonged hospitalization, and treatment
failure, as well as increased costs and resource
utilization of the healthcare system (Ahmad and
Khan, 2019). It has been estimated that the total
economic cost of antibacterial resistance caused by
major human pathogens in the United States of
America was $2.9 billion (Shrestha et al., 2018).
Fungi produce a wide spectrum of infections in
humans, ranging from superficial (e.g., skin, hair,
nail, and keratitis), mucosal (e.g., oral and
40
Teh, K., Wong, Y. and Sit, N.
Antibacterial, Antifungal, and Antioxidant Activities and Polyphenol Content of the Edible Seeds of Archidendron bubalinum.
DOI: 10.5220/0011595600003430
In Proceedings of the 8th International Conference on Agricultural and Biological Sciences (ABS 2022), pages 40-49
ISBN: 978-989-758-607-1; ISSN: 2795-5893
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

vulvovaginal candidiasis), allergic (e.g.,
rhinosinusitis and severe asthma with fungal
sensitization), chronic severe (e.g., chronic
pulmonary aspergillosis), to invasive infections (e.g.,
invasive candidiasis, cryptococcosis, and
aspergillosis). People with weakened or
compromised immunity such as cancer patients or
transplant recipients are more susceptible to fungal
infections (Bongomin et al., 2017). Each year, over
300 million people are afflicted with an episode of
fungal infection, resulting in more than 1.6 million
deaths globally (Urban et al., 2021; GAFFI, 2022).
Thus, effective antifungal treatment is important to
reduce the mortality rate. Yet, only polyenes,
flucytosine, azoles, and echinocandins are available
as antifungal agents for clinical use. The emergence
of fungal resistance, drugs’ adverse effects, and
undesirable drug-drug interactions hinder the
outcomes of antifungal treatment (Revie et al., 2018).
In the human body, when the antioxidative
protection system is unable to counteract reactive
species production, oxidative stress ensues (Pisoschi
et al., 2021). Hydroxyl radical, superoxide anion,
singlet oxygen, and peroxynitrite are examples of
these reactive species produced as the byproducts of
aerobic cellular metabolism, or as the response of the
body to exposure to cigarette smoke, ultraviolet
radiation, pesticides, and ozone. Meanwhile,
superoxide dismutase, glutathione peroxidase, metal-
trapping proteins, and vitamins A, C, and E constitute
important elements of the antioxidative protection
system (Rosado-Pérez et al., 2018). The excess
reactive species modify the structures and modulate
the functions of proteins, lipids, and nucleic acids.
Damages to these biomolecules lead to non-
communicable diseases such as cancer,
cardiovascular diseases, diabetes, and
neurodegenerative disorders (Liguori et al., 2018).
Due to the concern of side effects from synthetic
antioxidants (Ito et al., 1985), natural resources such
as plants could offer an alternative source for the
growing demand for exogenous antioxidants.
Edible fruits have long been recognized as an
important source of nutrients such as proteins and
minerals for human health as well as ingredients for
medicines. Different classes of phytochemicals or
secondary metabolites such as alkaloids, terpenoids,
or polyphenols are present in fruits, which endorse
them with various biological properties, including
antimicrobial and antioxidant activities (Forni et al.,
2019). Fruit with a high amount of procyanidins such
as cranberries has been shown to reduce bacterial or
fungal infections of the urinary tract (Vostalova et al.,
2015; Sundararajan et al., 2018). Regular intakes of
flavonoids through diets have been reported to
significantly decrease the risks of cardiovascular
diseases (Wang et al., 2014). In another study, a high
intake of antioxidant-rich fruits and vegetables in
healthy adults lowers the oxidized low-density
lipoprotein level, which is considered a biomarker of
cardiovascular diseases (Bacchetti et al., 2019).
Being one of the tropical countries with mega-
biodiversity, Malaysia has at least 355 species of trees
and 165 species of non-trees bearing edible fruits or
seeds (Milow et al., 2014). More than two-thirds of
them are wild or non-exclusively planted. These fruits
and seeds present a vast opportunity for exploration
as healthy foods.
Archidendron bubalinum (Jack) I.C.Nielsen
(family Leguminosae; synonym Pithecellobium
bubalinum) is a wild evergreen tree native to
Peninsular Malaysia, Thailand, and Indonesia. It has
many vernacular names, for example, ‘kerdas’ or
“keredas” in Malaysia, ‘neing-nok’ in Thailand, and
‘kabau’ or ‘julang-jaling’ in Indonesia (Lim, 2012).
The fruit has a woody pod and contains six to eight
seeds. The seeds are creamy-white, ellipsoid to ovoid,
and laterally flattened, which turn to a shining
reddish-brown color when mature. The seeds have a
strong pungent smell like jering and stink bean but the
odor disappears on cooking (Lim, 2012). The kernels
can be eaten raw after removing the hard husk. The
husk of the seeds has been extensively researched for
various biological activities, including antibacterial,
antidiabetic, anti-uric acid, and antioxidant (Hanafi et
al., 2018; Irawan et al., 2018; Styani et al., 2018;
Riasari et al., 2019). However, the edible kernel has
relatively been less studied. Recently, Riasari and
colleagues (2021) reported that the kernel extract
exerts antihyperglycemic activity in diabetic rats. The
Temuan tribe of aborigines in Peninsular Malaysia
use the seeds for treating diabetes (Ong and Azliza,
2015).
This study evaluated the antibacterial, antifungal,
and antioxidant activities of the kernel extracts of
Archidendron bubalinum seeds. The polyphenol
content of the kernel extracts was also quantified. The
results of this study provide scientific information on
the exploration of this wild edible seed as a healthy
food.
2 METHODOLOGIES
2.1 Sample Processing
Approximately 600 grams of fresh Archidendron
bubalinum seeds were bought from a wet market in
Antibacterial, Antifungal, and Antioxidant Activities and Polyphenol Content of the Edible Seeds of Archidendron bubalinum
41

Bachok, Kelantan, Malaysia on 29
th
May 2017. A few
seeds were kept as a specimen voucher with the code
number UTAR/FSC/17/001.
The husks were removed from the seeds after
cleaning with water. The kernels were then cut into
small pieces with a knife and extracted sequentially
using hexane, ethyl acetate, ethanol, and distilled
water. The maceration was performed at 110 rpm and
ambient temperature for 24 h and the process was
repeated two times. The hexane, ethyl acetate, and
ethanol filtrates were concentrated to dryness at 40°C
using a rotary evaporator whereas the water extract
was freeze-dried. All dry extracts were kept at –20°C
pending bioassay. The percentage of yield for each
extract was calculated based on fresh weight.
2.2 Antibacterial Activity
The antibacterial activity of kernel extracts was
evaluated using a colorimetric broth microdilution
method (Chan et al., 2018) with slight modifications.
The Gram-positive bacteria used were Bacillus
cereus ATCC11778 and Staphylococcus aureus
ATCC6538. While the Gram-negative bacteria tested
were Acinetobacter baumannii ATCC19606,
Escherichia coli ATCC35218, Klebsiella
pneumoniae ATCC13883, and Pseudomonas
aeruginosa ATCC27853. All the bacterial species
were obtained from the American Type Culture
Collection (ATCC) and cultured on Mueller-Hinton
agar (MHA). An extract stock solution was prepared
by dissolving 20 mg of extract in 2 mL of a methanol-
water mixture (2:1, v/v). Subsequent dilutions were
performed using Mueller-Hinton broth (MHB) in a
96-well plate to produce eight concentrations (0.02,
0.04, 0.08, 0.16, 0.31, 0.63, 1.25, and 2.50 mg/mL)
for the assay. The bacterial inoculum was prepared at
1 × 10
8
cfu/mL by adjusting the absorbance value at
625 nm to 0.08-0.10 (equivalent to 0.5 McFarland
turbidity standard) and diluted to 1 × 10
6
cfu/mL with
MHB. After that, 50 µL of prepared bacterial
inoculum was introduced to the wells containing 50
µL of extract and incubated at 37°C for 24 h. Positive
(chloramphenicol), negative (bacterial inoculum),
blank (MHB), and technical (kernel extracts) controls
were incorporated into each plate. The microbial
growth indicator p-iodonitrotetrazolium chloride (0.4
mg/mL; 20 µL) was pipetted to each well after
incubation. After 30 min of incubation at 37°C, the
wells were observed for the formation of a pink or
purple precipitate, and the extract’s minimum
inhibitory concentration (MIC) was ascertained. For
the determination of the extract’s minimum
bactericidal concentration (MBC), 20 µL of the well’s
content that showed inhibitory activity was spread on
MHA, followed by incubation at 37°C for 24 h. MBC
is the lowest concentration of kernel extract that kills
≥99.9% of bacterial inoculum. The experiment was
conducted in triplicate.
2.3 Antifungal Activity
The antifungal activity of kernel extracts was
assessed using a colorimetric broth microdilution
method (Chan et al., 2018) with slight modifications.
Five species of fungi, comprising three yeasts
(Candida albicans ATCC90028, Candida krusei
ATCC6258, and Candida parapsilosis ATCC22019)
and two filamentous fungi (Aspergillus fumigatus
ATCC204305 and Trichophyton rubrum
ATCC28188), were tested. All the fungal species
were obtained from the ATCC. The three yeasts were
cultured on Sabouraud dextrose agar (SDA). The A.
fumigatus and T. rubrum were maintained on potato
dextrose agar (PDA) and oatmeal agar (OA),
respectively. A serial dilution of the extract stock
solution (10 mg/mL) was performed using Roswell
Park Memorial Institute (RPMI)-1640 medium to
produce a concentration range of 0.02-2.50 mg/mL
for evaluation. For the fungal inoculum preparation,
the absorbances for Candida spp. and A. fumigatus
were adjusted to 0.12-0.15 and 0.09-0.11,
respectively, at 530 nm. The inoculums were then
diluted to 1.5 × 10
3
cfu/mL with RPMI-1640 medium.
While for T. rubrum, the cell number was enumerated
using a hemocytometer and adjusted to 2 × 10
3
cfu/mL (CLSI, 2008a; 2008b). The diluted fungal
inoculum (50 µL) was then pipetted into the extract
solution and incubated at 35°C and 48 h for Candida
spp., 35°C and 72 h for A. fumigatus, and 30°C and
96 h for T. rubrum. Two antibiotics, griseofulvin for
T. rubrum and amphotericin B for others, were used
as positive controls. Blank (medium), negative
(fungal inoculum), and technical (kernel extracts)
controls were incorporated into each plate. The p-
iodonitrotetrazolium chloride (20 µL) was pipetted to
each well one day before completion of the
incubation period and the MIC of the extract was
determined after incubation. After that, the spread
plate method using SDA/PDA was used to determine
the minimum fungicidal concentration (MFC) of the
active extracts.
ABS 2022 - The International Conference on Agricultural and Biological Sciences
42

2.4 Antioxidant Activity
2.4.1 2, 2-Diphenyl-1-picrylhydrazyl
(DPPH) Radical Scavenging Assay
The DPPH radical scavenging assay was performed
using the method of Pavithra and Vadivukkarasi
(2015) with slight modifications. The kernel extract
(4 mg/mL) and vitamin C (0.4 mg/mL), which served
as a positive control, were diluted two-fold serially
with the methanol-water mixture in a 96-well plate to
produce final concentration ranges of 1000-8 µg/mL
and 100-0.8 µg/mL, respectively. One hundred µL of
0.2 mM DPPH radical solution was pipetted to 100
µL of kernel extract/vitamin C solution, and the plate
was maintained in the dark at ambient temperature for
30 min. DPPH radical solution and extract solution
without the addition of DPPH were used as blank and
sample blank, respectively. The absorbance value
was recorded at 517 nm. The DPPH radical
scavenging activity was expressed in percentage and
plotted against the concentration of kernel extract.
The half-maximum inhibitory concentration (IC
50
) of
the extract was then determined graphically.
2.4.2 Oxygen Radical Absorbance Capacity
(ORAC) Assay
The peroxyl radical scavenging activity of the kernel
extracts was evaluated using the ORAC assay (Heng
et al., 2020). Six concentrations (3.13, 6.25, 12.5, 25,
50, and 100 µg/mL) were prepared for each extract.
Fifty µL of extract and one hundred fifty µL of 0.08
µM fluorescein were pipetted into a black 96-well
plate. Sodium phosphate buffer (75 mM, pH 7.0) was
used as a blank while Trolox solutions at 0.78, 1.56,
3.13, 6.25, and 12.5 µM were used to generate a
calibration curve. Following the addition of 2,2-
azobis (2-methylpropionamidine) dihydrochloride,
the fluorescence intensity of the fluorescein at 485 nm
(λ
ex
) and 520 nm (λ
em
) was monitored at 37°C every
1.5 min for 1 h. The area under the fluorescence
intensity versus time curve (AUC) was then
calculated. The ORAC value was interpolated from
the plot of the net AUC value versus the concentration
of Trolox. The equation and regression coefficient for
the plot were y = 1.7577x + 1.998 and 0.9932,
respectively. The ORAC value was expressed as
mmol Trolox equivalent/g of extract. The assay was
conducted in three independent experiments.
2.4.3 Ferric-Reducing Antioxidant Power
(FRAP) Assay
The FRAP assay was conducted using the method of
Benzie and Strain (1996) with slight modifications.
The standard curve was constructed using 0.2, 0.4,
0.6, 0.8, 1.0, 1.2, 1.4, and 1.6 mM of ferrous sulfate.
Vitamin C at 1 mg/mL served as a positive control
whereas the methanol-water mixture was deployed as
a negative control. A 270 µL of FRAP reagent was
pipetted to 30 µL of extract/standard/controls and
incubated at 37°C. After 4 min of incubation, the
absorbance was read at 593 nm. The FRAP reagent
contained 300 mM acetate buffer (pH 3.6), 20 mM
FeCl
3
solution, and 10 mM 2,4,6-tri(2-pyridyl)-s-
triazine solution at a ratio of 10:1:1 (v/v/v). The
equation and regression coefficient for the standard
curve were y = 2.7655x + 0.0953 and 0.9990,
respectively. The FRAP value for each extract was
interpolated from the standard curve and expressed as
mmol Fe
2+
equivalent/g of extract.
2.5 Polyphenol Content
2.5.1 Total Phenolic Content (TPC) Assay
The Folin-Ciocalteu method was used to determine
the TPC of each kernel extract in triplicate (Herald et
al., 2012). Eight gallic acid solutions (2.5, 5, 10, 20,
40, 80, 160, and 320 µg/mL) were used to generate a
calibration curve. A 10 mg/mL of extract (25 µL),
deionized water (75 µL), and 50% Folin-Ciocalteu
reagent (25 µL) were mixed in a 96-well plate and
shaken at 70 rpm for 6 min. For the sample blank, the
50% Folin-Ciocalteu reagent was replaced with
deionized water. The methanol-water mixture was
deployed as blank. Following the addition of 100 µL
of 700 mM sodium carbonate, the plate was
maintained in the dark at ambient temperature for 90
min, prior to the absorbance measurement at 765 nm.
The actual absorbance value of sample was obtained
after subtracting its absorbance value from the
absorbance value of the sample blank. The equation
and regression coefficient for the gallic acid
calibration curve were y = 0.0065x – 0.0031 and
0.9977, respectively. The TPC of each kernel extract
was expressed as mg gallic acid equivalent/g of
extract.
2.5.2 Total Flavonoid Content (TFC) Assay
The aluminium chloride method was used to
determine the TFC of each kernel extract in triplicate
(Herald et al., 2012). A 725 mM of sodium nitrite (10
µL), 10 mg/mL of extract (25 µL), deionized water
Antibacterial, Antifungal, and Antioxidant Activities and Polyphenol Content of the Edible Seeds of Archidendron bubalinum
43

(100 µL), and 750 mM of aluminium chloride (15 µL)
were mixed in a 96-well plate and shaken at 70 rpm
for 6 min. Quercetin (7.8, 15.6, 31.3, 62.5, 125, and
250 µg/mL) was used for constructing a calibration
curve whereas the methanol-water mixture was used
as a blank. The aluminium chloride in the mixture
was substituted with deionized water for the sample
blank. Each well was then added with 50 µL of 1 M
sodium hydroxide, followed by 50 µL of deionized
water. The plate was maintained in the dark at
ambient temperature for 60 min before the
absorbance was read at 420 nm. The equation and
regression coefficient for the quercetin calibration
curve were y = 0.0014x + 0.0068 and 0.9979,
respectively. The TFC of each extract was expressed
as mg quercetin equivalent/g of extract.
2.6 Data Analysis
The results obtained from the ORAC, FRAP, TPC,
and TFC assays were examined for statistical
significance using one-way analysis of variance
(ANOVA). Subsequently, the significant difference
between the kernel extracts within an assay was
determined using Duncan’s multiple range test.
Correlation analysis between ORAC or FRAP and
TPC or TFC was examined using the Pearson
correlation test (Chan, 2003). The significance level
was set at p<0.05. All statistical analyses were
performed using the IBM SPSS Statistics for
Windows Version 23.0 software.
3 RESULTS
3.1 Yield of Extraction
Four extracts were obtained from the extraction of the
kernel of A. bubalinum seeds. The percentages of the
yield of hexane, ethyl acetate, ethanol, and water
extracts were 0.01%, 0.05%, 5.33%, and 3.58%
(w/w), respectively. The total yield of extraction was
8.97% (w/w).
3.2 Antibacterial Activity
The bacteriostatic and bactericidal effects of an
extract are designated by its MIC and MBC values,
respectively. All the kernel extracts showed a
bacteriostatic effect against the six species of
bacteria. The MIC values ranged from 0.31 to 2.50
mg/mL (Table 1). However, the extracts required
higher concentrations to exert their bactericidal
effects. Moreover, none of the extracts exerted the
bactericidal effect against E. coli (Table 1),
suggesting an extract concentration higher than 2.50
mg/mL might be needed to kill this species. All the
bacterial species were susceptible to chloramphenicol
(positive control) with MIC values of 4 µg/mL
against the Gram-positive bacteria and 2-64 µg/mL
against the Gram-negative bacteria.
3.3 Antifungal Activity
All the kernel extracts possessed antifungal activity
against the three species of yeasts and two species of
filamentous fungi (Table 2). Their MIC values were
the same as the MFC values, indicating the extracts
possessed direct fungicidal effects on the fungi
evaluated. The fungicidal effects were slightly
stronger on the yeasts (MFC: 0.31-1.25 mg/mL) than
that of the filamentous fungi (MFC: 0.63-2.50
mg/mL). All the fungi were susceptible to the positive
controls, amphotericin B and griseofulvin, with MIC
values of 1-2 and 0.50 µg/mL, respectively.
Table 1: Antibacterial activities of the kernel extracts of Archidendron bubalinum seeds.
Extract
Gram-
p
ositive bacteria Gram-negative bacteria
Bacillus
cereus
ATCC11778
Staphylococ
cus aureus
ATCC6538
Acinetobacte
r baumannii
ATCC19606
Escherichia
coli
ATCC35218
Klebsiella
pneumoniae
ATCC13883
Pseudomonas
aeruginosa
ATCC27853
Minimum inhibitory concentration (mg/mL)
Hexane 1.25 1.25 0.31 0.63 0.63 0.63
Eth
y
l acetate 1.25 1.25 0.31 0.63 1.25 0.63
Ethanol 2.50 1.25 0.31 1.25 1.25 0.63
Wate
r
2.50 2.50 0.31 1.25 1.25 0.63
Minimum bactericidal concentration (mg/mL)
Hexane 1.25 2.50 2.50 >2.50 0.63 2.50
Eth
y
l acetate 1.25 2.50 2.50 >2.50 1.25 2.50
Ethanol 2.50 2.50 2.50 >2.50 1.25 2.50
Wate
r
2.50 2.50 2.50 >2.50 2.50 2.50
Notes: The concentrations are shown in mean values of triplicate.
ABS 2022 - The International Conference on Agricultural and Biological Sciences
44
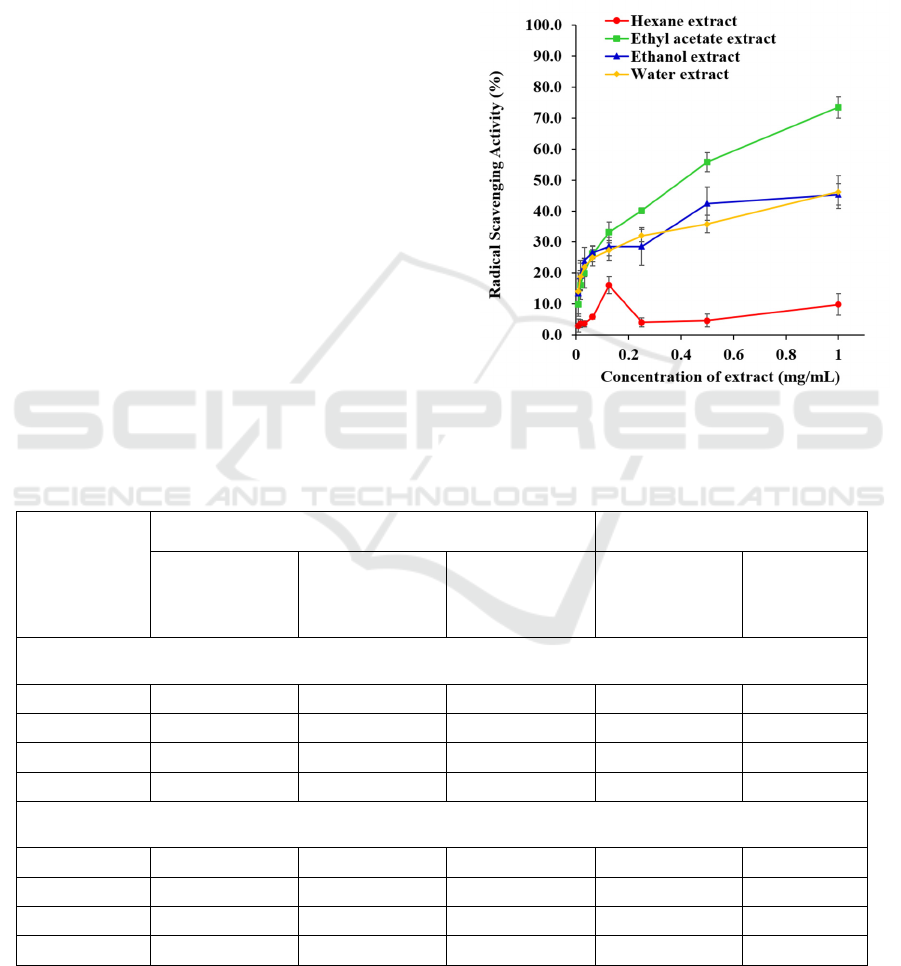
3.4 Antioxidant Activity
Two radical scavenging activity assays (DPPH and
ORAC) and an ion reducing activity assay (FRAP)
were deployed to assess the antioxidant activities of
the kernel extracts. Out of the four extracts, only ethyl
acetate extract was able to reduce >50% of DPPH
radicals when the concentration exceeded 0.5 mg/mL
(Figure 1), and the IC
50
value for this extract was 0.41
± 0.04 mg/mL, much higher than the positive control,
vitamin C (IC
50
= 2.51 ± 0.12 µg/mL). Besides that,
the highest ORAC value was exhibited by the ethyl
acetate extract (300.13 ± 13.15 mmol Trolox
equivalent/g of extract), followed by the ethanol
extract. Meanwhile, the ORAC values for the hexane
and water extracts were the lowest and similar
(p>0.05). As shown in the FRAP assay (Table 3), the
kernel extracts of A. bubalinum seeds also possessed
the ability to reduce the ion Fe
3+
to Fe
2+
. The ethyl
acetate extract showed the highest FRAP value
(341.36 ± 20.23 mmol Fe
2+
equivalent/g of extract),
followed by the ethanol, water, and hexane extracts.
3.5 Polyphenol Content
All four kernel extracts of A. bubalinum seeds
contained polyphenols. The TPC of the extracts
ranged from the lowest 3.22 ± 0.35 mg gallic acid
equivalent/g of extract for the hexane extract to the
highest 20.19 ± 0.23 mg gallic acid equivalent/g of
extract for the ethyl acetate extract (Table 3). While
for TFC, the ethyl acetate extract also recorded the
highest amount with a value of 4.42 ± 0.08 mg
quercetin equivalent/g of extract. Although the
ethanol extract had the lowest TFC, it was not
significantly different (p>0.05) from that of the
hexane and water extracts (Table 3).
Figure 1: DPPH radical scavenging activity of the kernel
extracts of Archidendron bubalinum seeds. Each value is
shown in mean ± standard deviation of triplicate.
Table 2: Antifungal activities of the kernel extracts of Archidendron bubalinum seeds.
Extract
Yeasts Filamentous Fungi
Candida
albicans
ATCC90028
Candida
krusei
ATCC6258
Candida
parapsilosis
ATCC22019
Aspergillus
fumigatus
ATCC204305
Trichophyton
rubrum
ATCC28188
Minimum inhibitory concentration (mg/mL)
Hexane 0.63 0.63 0.31 0.63 1.25
Ethyl acetate 1.25 0.63 0.63 1.25 2.50
Ethanol 1.25 1.25 0.63 1.25 2.50
Water 1.25 1.25 0.63 2.50 2.50
Minimum fungicidal concentration (mg/mL)
Hexane 0.63 0.63 0.31 0.63 1.25
Ethyl acetate 1.25 0.63 0.63 1.25 2.50
Ethanol 1.25 1.25 0.63 1.25 2.50
Water 1.25 1.25 0.63 2.50 2.50
Notes: The concentrations are shown in mean values of triplicate.
Antibacterial, Antifungal, and Antioxidant Activities and Polyphenol Content of the Edible Seeds of Archidendron bubalinum
45
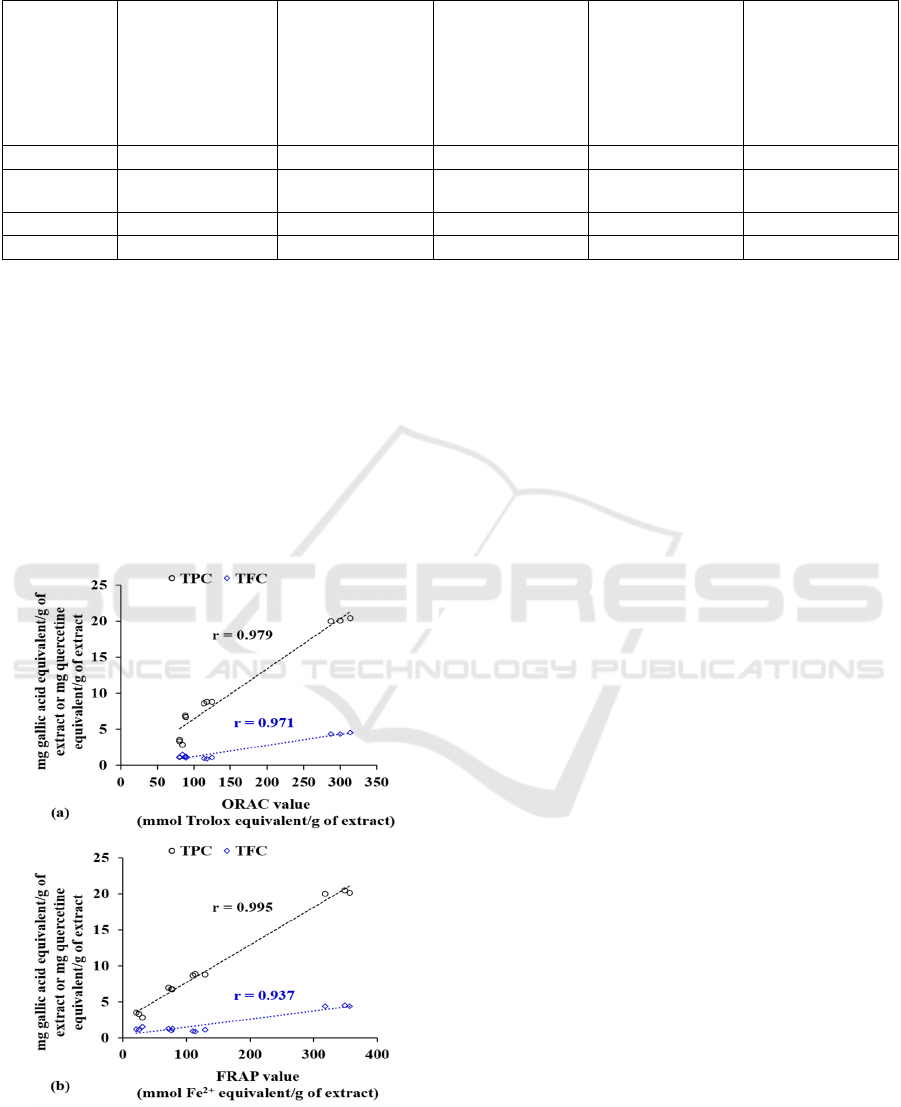
Table 3: Antioxidant activities and polyphenol content of the kernel extracts of Archidendron bubalinum seeds.
Extract
DPPH radical
scavenging
activity, half-
maximum
inhibitory
concentration
(
m
g
/mL
)
Oxygen radical
absorbance
capacity (mmol
Trolox
equivalent/g of
extract)
Ferric-reducing
antioxidant power
(mmol Fe
2+
equivalent/g of
extract)
Total phenolic
content (mg gallic
acid equivalent/g
of extract)
Total flavonoid
content (mg
quercetin
equivalent/g of
extract)
Hexane Nil 81.63 ± 2.08
a
25.93 ± 4.70
a
3.22 ± 0.35
a
1.25 ± 0.23
a
Ethyl
acetate
0.41 ± 0.04 300.13 ± 13.15
c
341.36 ± 20.23
d
20.19 ± 0.23
d
4.42 ± 0.08
b
Ethanol Nil 118.90 ± 5.41
b
118.13 ± 10.18
c
8.73 ± 0.10
c
0.97 ± 0.11
a
Water Nil 88.62 ± 0.26
a
75.22 ± 3.47
b
6.79 ± 0.11
b
1.16 ± 0.12
a
Notes: Each value is shown in mean ± standard deviation of triplicate. Values with different alphabetical superscripts
denote significant differences (p<0.05) among the extracts by one-way ANOVA test.
3.6 Correlations between Antioxidant
Activities and Polyphenol Content
The correlation analyses unveiled significant strong
positive correlations between ORAC value and TPC
(r = 0.979; p<0.001) or TFC (r = 0.971; p<0.001)
(Figure 2). Similarly, strong positive correlations
were noted between FRAP value and TPC (r = 0.995;
p<0.001) or TFC (r = 0.937; p<0.001).
Figure 2: Association analysis between total phenolic
content (TPC) or total flavonoid content (TFC) and (a)
oxygen radical absorbance capacity (ORAC) and (b) ferric-
reducing antioxidant power (FRAP) of the kernel extracts
of Archidendron bubalinum seeds using the Pearson
correlation test.
4 DISCUSSIONS
The results of this study indicated that the edible
seeds of A. bubalinum possessed antibacterial,
antifungal, and antioxidant activities. The ability of
all the kernel extracts to exert antibacterial and
antifungal activities implied that different classes of
phytochemicals were present in the kernels and
contributed to the activities. During the sequential
solvent extraction, phytochemicals in the kernels are
segregated according to the polarity of the solvent
used. Non-polar solvents such as hexane and
chloroform commonly remove alkaloids, fatty acids,
sterols, terpenoids, etc. from plants. Phytochemicals
such as anthraquinones, flavones, polyphenols,
saponins, tannins, and terpenoids are obtained by
ethyl acetate and ethanol, which are more polar
solvents. The most polar solvent water could yield
secondary metabolites like polypeptides and lectins
(Heng et al., 2020).
The polyphenols quantified in all the kernel
extracts may account, at least in part, for the
antibacterial and antifungal activities. Polyphenols
constitute one of the biggest groups of
phytochemicals with approximately 8000 different
structures. They are classified into flavonoids,
stilbenes, phenolic acids, coumarins, anthraquinones,
tannins, and xanthones (Forni et al., 2019).
Polyphenols from plants have been reported to have
inhibitory or killing effects against bacteria and fungi
(Kumar et al., 2021; Manso et al., 2022). Polyphenols
exert antimicrobial activities via various mechanisms,
such as damaging fungal cell wall or bacterial
lipopolysaccharide layer, formation of pores in cell
ABS 2022 - The International Conference on Agricultural and Biological Sciences
46

membrane leading to leakage of cytoplasmic content,
inhibition of metabolic enzymes, ergosterol
biosynthesis, or efflux pumps, repression of genes,
disruption of ionic imbalance, induction of cell death,
and inhibition of biofilm formation (Seleem et al.,
2017; Simonetti et al., 2020; Kumar et al., 2021). The
kernel extracts exhibited a direct killing effect on the
fungi evaluated, suggesting the antifungal
components of A. bubalinum seeds may mainly
disrupt the integrity of the cell wall and cause
cytolysis of the fungal cells.
Riasari et al. (2019) reported the DPPH radical
scavenging activity of the ethanol extracts of the
seeds of A. bubalinum from Lampung and South
Sumatra (Indonesia) with half-maximum inhibitory
concentrations of 163 and 446 µg/mL, respectively.
In contrast, the ethanol kernel extract of A. bubalinum
seeds used in this study exerted weak DPPH radical
scavenging activity (<50% inhibition at 1 mg/mL).
The differences could be attributed to agro-
geographical reasons and/or extraction techniques
used. Ghasemzadeh et al. (2018) studied the stink
beans collected from different regions of Peninsular
Malaysia and found that the sample collected from
Perak had the highest DPPH radical scavenging and
FRAP activities than the ones collected from Negeri
Sembilan and Johor. Another study found that 18
metabolites from black bean and 11 metabolites from
soybean were different significantly when their
metabolite profiles were compared using two
different extraction techniques (Maria John et al.,
2018).
All four kernel extracts of A. bubalinum seeds
exhibited antioxidant activities via the ORAC and
FRAP assays. These antioxidant activities were likely
contributed by polyphenols including flavonoids in
the kernels due to the significant results from the
correlation analyses. Significant strong positive
correlations (r > 0.80) between ORAC values and
polyphenol content (TPC and TFC) have also been
documented for adlay seeds (Xu et al., 2017), seeds
and fruit of Phoenix dactylifera (Djaoudene et al.,
2021), and fruit of Eleiodoxa conferta (Go et al.,
2021). Similarly, strong positive correlations between
FRAP and TPC as well as between FRAP and TFC
have been reported for stink beans (Ghasemzadeh et
al., 2018), kiwifruit (Wang et al., 2018), and peels and
seeds of pomegranates (Sabraoui et al., 2020). The
existence of at least one phenyl ring in the molecular
structure allows polyphenols to have free radical
scavenging, singlet oxygen quenching, and metal ion
reducing or chelating properties (Ullah et al., 2020).
As the ethyl acetate extract was the most active
among all kernel extracts, further analysis using
gas/liquid chromatography-mass spectrometry could
shed some light on the identity of antioxidants in the
extract.
5 CONCLUSIONS
This study suggests that the edible seeds of A.
bubalinum could be promoted as healthy food due to
its health-promoting activities. All four kernel
extracts (hexane, ethyl acetate, ethanol, and water)
from the seeds had bactericidal activities against
Gram-positive and Gram-negative bacteria and
fungicidal activities against yeasts and filamentous
fungi. The antimicrobial activities of the seeds are
likely to be contributed by different classes of
phytochemicals. All the kernel extracts also
possessed antioxidant activities, as revealed by the
ORAC and FRAP assays. Among the extracts, ethyl
acetate extract showed the strongest antioxidant
activity by having the highest ORAC and FRAP
values. The antioxidant activities were attributable
mainly to the polyphenols present in the kernels, due
to the significant strong positive correlations between
antioxidant activities and polyphenol content. Further
work is essential to identify the bioactive components
and elucidate the mechanisms of action. More
research is needed if A. bubalinum is to be cultivated
sustainably as a crop in Malaysia, as currently the
fruit is mainly collected from forests for sales in wet
markets.
ACKNOWLEDGEMENTS
The authors thank Dr. Sugumaran Manickam
(Faculty of Science, University of Malaya, Malaysia)
for the identification of plant species and Lee Vwen
Tan for sourcing the plant material. The work is
supported by the UTAR Research Publication
Scheme (6251/S02).
REFERENCES
Ahmad, M. and Khan, A. U. (2019). Global economic
impact of antibiotic resistance: A review. Journal of
Global Antimicrobial Resistance, 19:313-316.
Bacchetti, T., Turco, I., Urbano, A., Morresi, C., and
Ferretti, G. (2019). Relationship of fruit and vegetable
intake to dietary antioxidant capacity and markers of
oxidative stress: A sex-related study. Nutrition, 61:164-
172.
Antibacterial, Antifungal, and Antioxidant Activities and Polyphenol Content of the Edible Seeds of Archidendron bubalinum
47

Benzie, I. F. F. and Strain, J. J. (1996). The ferric reducing
ability of plasma (FRAP) as a measure of “antioxidant
power”: The FRAP assay. Analytical Biochemistry,
239:70-76.
Bongomin, F., Gago, S., Oladele, R. O., and Denning, D.
W. (2017). Global and multi-national prevalence of
fungal diseases–estimate precision. Journal of Fungi,
3(4):57.
Chan, Y. H. (2003). Biostatistics 104: Correlational
analysis. Singapore Medical Journal, 44(12):614-619.
Chan, Y. S., Ong, C. W., Chuah, B. L., Khoo, K. S., Chye,
F. Y., and Sit, N. W. (2018). Antimicrobial, antiviral
and cytotoxic activities of selected marine organisms
collected from the coastal areas of Malaysia. Journal of
Marine Science and Technology, 26(1):128-136.
Chokshi, A., Sifri, Z., Cennimo, D., and Horng, H. (2019).
Global contributors to antibiotic resistance. Journal of
Global Infectious Diseases, 11(1):36-42.
Christaki, E., Marcou, M., and Tofarides, A. (2020).
Antimicrobial resistance in bacteria: Mechanisms,
evolution, and persistence. Journal of Molecular
Evolution, 88(1):26-40.
Clinical and Laboratory Standards Institute (CLSI).
(2008a). Reference Method for Broth Dilution
Antifungal Susceptibility Testing of Yeasts; Approved
Standard, CLSI Document M27-A3, 3rd ed. Clinical
and Laboratory Standards Institute.
Clinical and Laboratory Standards Institute (CLSI).
(2008b). Reference Method for Broth Dilution
Antifungal Susceptibility Testing of Filamentous Fungi;
Approved Standard, CLSI Document M38-A2, 2nd ed.
Clinical and Laboratory Standards Institute.
Djaoudene, O., Mansinhos, I., Gonçalves, S., Jara-Palacios,
M. J., Bachir Bey, M., and Romano, A. (2021).
Phenolic profile, antioxidant activity and enzyme
inhibitory capacities of fruit and seed extracts from
different Algerian cultivars of date (Phoenix dactylifera
L.) were affected by in vitro simulated gastrointestinal
digestion. South African Journal of Botany, 137:133-
148.
Fisher, J. F. and Mobashery, S. (2020). Constructing and
deconstructing the bacterial cell wall. Protein Science,
29(3):629-646.
Forni, C., Facchiano, F., Bartoli, M., Pieretti, S., Facchiano,
A., D'Arcangelo, D., Norelli, S., Valle, G., Nisini, R.,
Beninati, S., Tabolacci, C., and Jadeja, R. N. (2019).
Beneficial role of phytochemicals on oxidative stress
and age-related diseases. BioMed Research
International, 2019:8748253.
GBD 2019 Diseases and Injuries Collaborators. (2020).
Global burden of 369 diseases and injuries in 204
countries and territories, 1990–2019: A systematic
analysis for the Global Burden of Disease Study 2019.
The Lancet, 396(10258):1204-1222.
Ghasemzadeh, A., Jaafar, H. Z. E., Bukhori, M. F. M.,
Rahmat, M. H., and Rahmat, A. (2018). Assessment
and comparison of phytochemical constituents and
biological activities of bitter bean (Parkia speciosa
Hassk.) collected from different locations in Malaysia.
Chemistry Central Journal
, 12(1):12.
Global Action Fund for Fungal Infections (GAFFI). (2022).
Fungal disease frequency. Retrieved from
https://gaffi.org/why/fungal-disease-frequency/.
Go, H. C., Low, J. A., Khoo, K. S., and Sit, N. W. (2021).
Nutritional composition, biological activities, and
cytotoxicity of the underutilized fruit of Eleiodoxa
conferta. Journal of Food Measurement and
Characterization, 15(5):3962-3972.
Hanafi, H., Irawan, C., Rochaeni, H., Sulistiawaty, L.,
Roziafanto, A. N., and Supriyono. (2018).
Phytochemical screening, LC-MS studies and
antidiabetic potential of methanol extracts of seed shells
of Archidendron bubalinum (Jack) I.C. Nielson (Julang
Jaling) from Lampung, Indonesia. Pharmacognosy
Journal, 10(6): S77-S82.
Heng, Y. W., Ban, J. J., Khoo, K. S., and Sit, N. W. (2020).
Biological activities and phytochemical content of the
rhizome hairs of Cibotium barometz (Cibotiaceae).
Industrial Crops and Products, 153:112612.
Herald, T. J., Gadgil, P., and Tilley, M. (2012). High-
throughput micro plate assays for screening flavonoid
content and DPPH-scavenging activity in sorghum bran
and flour. Journal of the Science of Food and
Agriculture, 92(11):2326-2331.
Irawan, C., Foliatini, Hanafi, Sulistiawaty, L., and
Sukiman, M. (2018). Volatile compound analysis using
GC-MS, phytochemical screening and antioxidant
activities of the husk of “Julang-jaling” (Archidendron
bubalinum (Jack) I.C Nielsen) from Lampung,
Indonesia. Pharmacognosy Journal, 10(1):92-98.
Ito, N., Fukushima, S., and Tsuda, H. (1985).
Carcinogenicity and modification of the carcinogenic
response by BHA, BHT, and other antioxidants.
Critical Reviews in Toxicology, 15(2):109-150.
Kumar, H., Bhardwaj, K., Cruz-Martins, N., Nepovimova,
E., Oleksak, P., Dhanjal, D. S., Bhardwaj, S., Singh, R.,
Chopra, C., Verma, R., Chauhan, P. P., Kumar, D., and
Kuča, K. (2021). Applications of fruit polyphenols and
their functionalized nanoparticles against foodborne
bacteria: A mini review. Molecules, 26(11):3447.
Liguori, I., Russo, G., Curcio, F., Bulli, G., Aran, L., Della-
Morte, D., Gargiulo, G., Testa, G., Cacciatore, F.,
Bonaduce, D., and Abete, P. (2018). Oxidative stress,
aging, and diseases. Clinical Interventions in Aging,
13:757-772.
Lim, T. K. (2012). Edible Medicinal and Non-Medicinal
Plants: Volume 2, Fruits. Springer Science+Business
Media B.V.
Maria John, K. M., Harnly, J., and Luthria, D. (2018).
Influence of direct and sequential extraction
methodology on metabolic profiling. Journal of
Chromatography B: Analytical Technologies in the
Biomedical and Life Sciences, 1073:34-42.
Manso, T., Lores, M., and de Miguel, T. (2022).
Antimicrobial activity of polyphenols and natural
polyphenolic extracts on clinical isolates. Antibiotics,
11(1):46.
Milow, P., Malek, S. B., Edo, J., and Ong, H. C. (2014).
Malaysian species of plants with edible fruits or seeds
ABS 2022 - The International Conference on Agricultural and Biological Sciences
48

and their valuation. International Journal of Fruit
Science, 14(1):1-27.
Ong, H. C. and Azliza, M. A. (2015). Medicinal plants for
diabetes by the Orang Asli in Selangor, Malaysia.
Studies on Ethno-Medicine, 9(1):77-84.
Pavithra, K. and Vadivukkarasi, S. (2015). Evaluation of
free radical scavenging activity of various extracts of
leaves from Kedrostis foetidissima (Jacq.) Cogn. Food
Science and Human Wellness, 4(1):42-46.
Pisoschi, A. M., Pop, A., Iordache, F., Stanca, L., Predoi,
G., and Serban, A. I. (2021). Oxidative stress mitigation
by antioxidants–An overview on their chemistry and
influences on health status. European Journal of
Medicinal Chemistry, 209:112891.
Riasari, H., Fauzi, N. I., Anggadiredja, K., Hartati, R., and
Sukrasno. (2021). In vivo antidiabetic screening of
kabau (Archidendron bubalinum (Jack) I.C Nielsen)
seeds. International Journal of Applied Pharmaceutics,
13(S4):228-234.
Riasari, H., Fitriansyah, S. N., Hartati, R., Anggadiredja,
K., and Sukrasno. (2019). Comparison of extraction
methods, antioxidant activities, total phenol in seeds
and seed shells of Kabau (Archidendron bubalinum
(Jack) I.C. Nielsen) from Lampung and South Sumatra.
Pharmacognosy Journal, 11(6):1278-1284.
Revie, N. M., Iyer, K. R., Robbins, N., and Cowen, L. E.
(2018). Antifungal drug resistance: Evolution,
mechanisms and impact. Current Opinion in
Microbiology, 45:70-76.
Rosado-Pérez, J., Aguiñiga-Sánchez, I., Arista-Ugalde, T.
L., Santiago-Osorio, E., and Mendoza-Núñez, V. M.
(2018). The biological significance of oxidative stress,
effects of fruits as natural edible antioxidants. Current
Pharmaceutical Design, 24(40):4807-4824.
Sabraoui, T., Khider, T., Nasser, B., Eddoha, R., Moujahid,
A., Benbachir, M., and Essamadi, A. (2020).
Determination of punicalagins content, metal chelating,
and antioxidant properties of edible pomegranate
(Punica granatum L) peels and seeds grown in
Morocco. International Journal of Food Science,
2020:8885889.
Seleem, D., Pardi, V., and Murata, R. M. (2017). Review of
flavonoids: A diverse group of natural compounds with
anti-Candida albicans activity in vitro. Archives of
Oral Biology, 76:76-83.
Shrestha, P., Cooper, B. S., Coast, J., Oppong, R., Do Thi
Thuy, N., Phodha, T., Celhay, O., Guerin, P. J.,
Wertheim, H., and Lubell, Y. (2018). Enumerating the
economic cost of antimicrobial resistance per antibiotic
consumed to inform the evaluation of interventions
affecting their use. Antimicrobial Resistance and
Infection Control, 7(1):98.
Simonetti, G., Brasili, E., and Pasqua, G. (2020).
Antifungal activity of phenolic and polyphenolic
compounds from different matrices of Vitis vinifera L.
against human pathogens. Molecules
, 25(16):3748.
Styani, E., Hanafi, Sulistiswaty, L., Irawan, C., and Imalia.
(2018). Liquid chromatograph-mass spectrophotometer
and anti-uric acid potential studies of ethyl acetate
extract of Archidendron bubalinum (Jack) I.C. Nielsen
fruit seed shell. In Proceedings of the international
conference on science and technology, pages 293-297.
Atlantis Press.
Sundararajan, A., Rane, H. S., Ramaraj, T., Sena, J.,
Howell, A. B., Bernardo, S. M., Schilkey, F. D., and
Lee, S. A. (2018). Cranberry-derived
proanthocyanidins induce a differential transcriptomic
response within Candida albicans urinary biofilms.
PLoS One, 13(8): e0201969.
Ullah, A., Munir, S., Badshah, S. L., Khan, N., Ghani, L.,
Poulson, B. G., Emwas, A. H., and Jaremko, M. (2020).
Important flavonoids and their role as a therapeutic
agent. Molecules, 25(22):5243.
Urban, K., Chu, S., Scheufele, C., Giesey, R. L., Mehrmal,
S., Uppal, P., and Delost, G. R. (2021). The global,
regional, and national burden of fungal skin diseases in
195 countries and territories: A cross-sectional analysis
from the Global Burden of Disease Study 2017. JAAD
International, 2:22-27.
Vostalova, J., Vidlar, A., Simanek, V., Galandakova, A.,
Kosina, P., Vacek, J., Vrbkova, J., Zimmermann, B. F.,
Ulrichova, J., and Student, V. (2015). Are high
proanthocyanidins key to cranberry efficacy in the
prevention of recurrent urinary tract infection?
Phytotherapy Research, 29(10):1559-1567.
Wang, X., Ouyang, Y. Y., Liu, J., and Zhao, G. (2014).
Flavonoid intake and risk of CVD: A systematic review
and meta-analysis of prospective cohort studies. British
Journal of Nutrition, 111(1):1-11.
Wang, Y., Zhao, C. L., Li, J. Y., Liang, Y. J., Yang, R. Q.,
Liu, J. Y., Ma, Z., and Wu, L. (2018). Evaluation of
biochemical components and antioxidant capacity of
different kiwifruit (Actinidia spp.) genotypes grown in
China. Biotechnology and Biotechnological
Equipment, 32(3):558-565.
Xu, L., Wang, P., Ali, B., Yang, N., Chen, Y., Wu, F., and
Xu, X. (2017). Changes of the phenolic compounds and
antioxidant activities in germinated adlay seeds.
Journal of the Science of Food and Agriculture,
97(12):4227-4234.
Antibacterial, Antifungal, and Antioxidant Activities and Polyphenol Content of the Edible Seeds of Archidendron bubalinum
49
