
The Effect of Air Flow Rate on Temperature, Yield and Water
Content in the Production of Active Charcoal from Palm Oil Shells
Using Partial Oxidation Method
Firman
1,3
, Siti Hamidah Mohd-Setapar
2,3
, Muh. Irwan
1
and Sitti Sahraeni
1
1
Department of Chemical Engineering, Politeknik Negeri Samarinda, Jalan Dr. Cipto Mangunkusumo,
Kampus Gunung Lipan Samarinda, 75131, Kalimantan Timur Province, Indonesia
2
Malaysia-Japan International Institute of Technology (MJIIT), Universiti Teknologi Malaysia,
Jalan Sultan Yahya Petra, 54100, UTM Kuala Lumpur, Malaysia
3
Razak Faculty of Technology and Informatics, Universiti Teknologi Malaysia, Jalan Sultan Yahya Petra, 54100,
UTM Kuala Lumpur, Malaysia
Keywords: Air Flow Rate, Active Charcoal, Temperature, Palm Kernel Shell, Partial Oxidation, Yield.
Abstract: Palm kernel shell is one of the palm oil processing waste which is quite large, reaching 6.5% of 1 ton of palm
oil. This shell can be used as an ingredient to make activated charcoal. Activated charcoal is widely used as
adsorbent, gas purification, water purification and so on. The palm shell is the hardest part of the components
found in oil palm. Palm kernel shell contain 26.6% cellulose and 27.7% hemicellulose which are good for
making activated charcoal. This study aims to determine the effect of air flow rate on the average temperature,
yield and moisture content in the manufacture of activated charcoal from oil Palm kernel shell by partial
oxidation method. Carbonization and activation were carried out using pyrolysis with the principle of partial
oxidation. The pyrolysis process was carried out with air flow rates of 20, 25, 30, 35 and 40 L/min for 5 hours.
The best results are shown at an air flow rate of 30 L/min with a product yield of 24,03%, a moisture content
of 5.85% and an average temperature of partial oxidation reaching 517.51
o
C.
1 INTRODUCTION
Oil palm is one of the plantation commodities that has
an important role in economic activity in Indonesia
where the area of oil palm plantations increased by
1.88 percent from 2018 to 14.60 million hectares with
an increase in Crude Palm Oil (CPO) production by
12 , 92 percent to 48.42 million tons (BPS, 2020)
Conversion of Fresh Fruit Bunch (FFB) into CPO is
around 25 to 28 percent and conversion of Palm
Kernel Shell (PKS) production from FFB reaches 6.8
to 7.4 percent. This shows that the potential of PKS
in Indonesia reaches 11.8 million tons per year.
several CPO producing countries such as Indonesia,
Malaysia, Colombia and Brazil, the use of PKS is
widely used as boiler fuel for heating fruit as well as
electricity-producing boilers (Nahrul et al., 2020).
The use of PKS to make activated charcoal which has
more selling value is still lacking in Indonesia, one of
which is the potential for PKS to be converted into
activated charcoal. The potential of PKS as Activated
Charcoal really depends on the composition of the
PKS. The composition of oil palm shell itself consists
of 27.7% cellulose, 21.6% hemicellulose, the lignin
content in this plant is 44%, and the results of
proximate analysis are 11% Moisture, 2.1% Ash, 19.7
Fixed Carbon and 67.2% Volatile Matter. .
The ultimate PKS analysis contains 49.7%
Carbon, 5.32% Hydrogen, 0.08% Nitrogen, 44, 86%
oxygen and 0.16% sulfur (Abnisa et al., 2011).
Activated Charcoal Technology has been carried out
by many researchers from various countries. The
technique used in the manufacture of activated
charcoal generally uses a three-stage process
principle, namely carbonization, chemical activation
and physical activation (Huang et al., 2018).
Based on data from the Central Statistics Agency
processed by the Directorate General of Plantation in
2019, Publication of The 2019 Indonesian Oil Palm
Statistics is an annual publication of BPS-Statistics
Indonesia. This publication presents data of area and
production on palm oil by province and by category
of producers, and the export and import of palm oil
Firman, ., Mohd Setapar, S., Irwan, M. and Sahraeni, S.
The Effect of Air Flow Rate on Temperature, Yield and Water Content in the Production of Active Charcoal from Palm Oil Shells Using Partial Oxidation Method.
DOI: 10.5220/0011811600003575
In Proceedings of the 5th International Conference on Applied Science and Technology on Engineering Science (iCAST-ES 2022), pages 423-427
ISBN: 978-989-758-619-4; ISSN: 2975-8246
Copyright © 2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
423

by country of destination and by country of origin.
Indonesia's charcoal exports reached 188,050 tons
with an export value of 145.09 million US dollars
(BPS, 2020). Due to the increasing demand for
Activated Charcoal., there is a strong need to sort out
the manufacturing technology for Activated Charcoal
preparation which must be cost-effective as well as on
par with commercially available Activated Charcoal.
Although various feedstocks have been explored for
the preparation of Activated Charcoal in previous
studies, scientists are still trying to explore new
materials depending on their availability and
suitability for Activated Charcoal production.
However, the utilization of Plantation waste as a raw
material for making activated charcoal has increased
rapidly in recent years. the use of making activated
charcoal by the partial oxidation method is still poorly
practiced.
Charcoal is a light carbon black residue produced
by heating wood (or other animal and plant materials)
with minimal oxygen to remove all water and other
volatile materials. In most cases, this pyrolysis
process, called charcoal burning, often results in the
formation of a charcoal furnace, where heat is
supplied by burning part of the starting material itself,
with a limited supply of oxygen. Materials can also
be heated in a closed medium. Activated charcoal has
the same initial process of making activated carbon.
Activated carbon (AC) is a non-graphite, non-
graphitizable carbon that has a very irregular
microstructure. It is famous for its high adsorption
capacity due to its high surface area and porosity.
Generally activated carbon can be made from various
raw materials including agricultural and forestry
residues. Generally most of the precursors used for
the manufacture of activated carbon are rich in carbon
(Prahas et al., 2008). AC production is achieved
usually through two methods, physical activation
method and chemical activation method (Bansal et
al., 1988)
Physical activation methods involve
carbonization of the feedstock followed by activation
at high temperatures (between 800 and 1100
o
C) in
the presence of an oxidizing gas such as carbon
dioxide or steam, while the chemical activation
method is mixing chemicals with precursors and then
followed by pyrolysis at moderate temperatures in the
absence of air High activated carbon uptake is closely
related to pore characteristics such as surface area,
pore volume, and pore size distribution. All activated
carbon has a porous structure, containing up to 15%
mineral matter in the form of ash content (Bansal et
al., 1988). The AC structure is formed during the
carbonization process and is continued during
activation, when the space between the forming
crystals is cleared of tar and other carbonated
materials. The structure of the hole and the size of the
hole are very dependent on the nature of the raw
material and the activation process. The activating
process removes the disorganized carbon by exposing
the crystallites to the action of the activating agent
leading to the construction of the diamond structure.
Activated carbon pore systems are of various types
and the individual pores may differ greatly in size and
shape. The drying, pyrolysis, and reduction processes
are heat-absorbing (endothermic), while the oxidation
process is heat-releasing (exothermic). On drying, the
moisture content of the solid fuel is evaporated by
heat absorbed from the oxidation process. In
pyrolysis, the separation of volatile matters (water
vapor, organic liquids, and non-condensed gases)
from charcoal or fuel carbon solids also uses heat
absorbed from the oxidation process. Combustion
oxidizes the carbon and hydrogen content of the fuel
by an exothermic reaction, whereas gasification
reduces the combustion product to gas by an
endothermic reaction. Further explanation regarding
these processes is given in the following description.
One of the important aspects of bioenergy to generate
heat, power and biofuels and products in the form of
activated charcoal for useful applications is biomass
gasification. As technology and materials advanced,
the development of gasification technology has
increased significantly for applications compared to
conventional power sources. This article presents an
overview of technical advances, developments in
biomass gasification technology and obstacles faced
by various stakeholders in the widespread
dissemination of technology for the needs of
individual communities and the business world to
support downstream to upstream activities (Shuit et
al., 2009).
2 METHODOLOGY
Palm oil shells from PT. Kebun Mandiri Sejahahtera
there are still impurities in the form of fibers and palm
seeds. Before carrying out the main process, it is
necessary to clean the shell by separating the
impurities. After it is considered clean, the drying
stage is carried out using sunlight. After that, five
kilograms of each process are weighed and then
stored and ready for use. The method used in this
research is to measure the results of the analysis of
activated charcoal based on SNI 06-3730-1995
standards with the main parameters of Moisture
content (%). The quality of activated charcoal greatly
iCAST-ES 2022 - International Conference on Applied Science and Technology on Engineering Science
424
affects the water content. The equation used to
calculate the water content is
Moisture content (%) = (((M2-M3))/
((M2-M1)))x100%
(1)
Information:
M1 = Weight of empty petri dish (grams)
M2 = Weight of petri dish containing activated
carbon before oven (grams)
M3 = Weight of petri dish containing activated
carbon after oven (grams)
3 RESULT AND DISCUSSION
The purpose of this study was to determine the effect
of air flow rate on activated charcoal using oil palm
shells with a pyrolysis process using the principle of
partial oxidation on a pilot plant scale with a raw
material capacity of 5 kg/batch, and to determine the
quality of charcoal produced by pyrolysis of oil palm
shells. The pyrolysis process stage is burned for 5
hours. The results of the study obtained the following
data:
Table 1: Research result.
Air
Flow
Rate
(L/min)
Temperature
Average (
o
C)
Yield
(%)
Moisture
Content
(%)
20
432.69
29.8
6.94
25
474.12
29.01
6.40
30
517.51
24.03
5.85
35
565.34
20.2
5.82
40
585.98
19.01
5.80
SNI06-3730-1995
Max 15
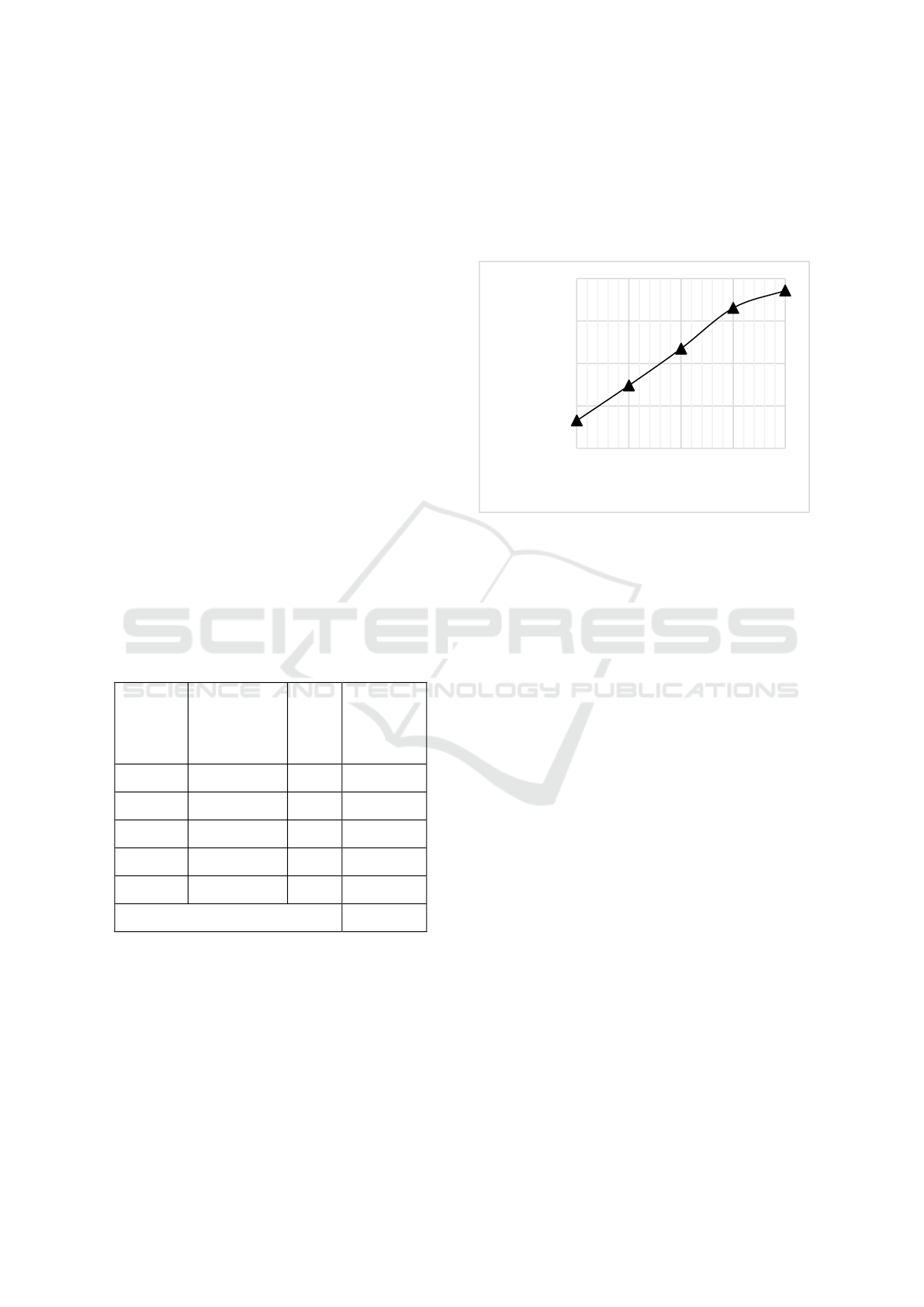
The results of the study in the table1 Shows that
the greater the air flow rate, the higher the average
temperature in the reactor produced. This is due to the
pyrolysis process using limited air, an oxidation
process that is exothermic (releasing heat). In this
oxidation zone, the large amount of air causes the
amount of oxygen present in the air to oxidize the
carbon contained in the material so that the heat
generated is also greater and causes an increase in
temperature. The increase in temperature generally
shifts with increasing air flow rate. The greater the air
flow rate, the maximum temperature (hot spot) will
occur the faster it will occur and then there will be a
decrease in temperature due to reduced carbon
contained in the raw material (Ramos, L.P., 20035).
Figure 1: Effect of air flow rate on the average temperature
of pyrolysis.
In Figure 1 it can be seen that at a flow rate of 20
L/min the activated charcoal product produced is at
the maximum yield, which is 29.8%, while at a flow
rate of 40 L/min the minimum yield of activated
charcoal product produced is, which is 19%. It can be
seen that the yield continues to decrease along with
the addition or flow of air into the reactor, namely the
more oxygen and nitrogen gas that is circulated, the
yield of activated charcoal obtained is also relatively
decreased (Hasan et al., 2020). In addition,
temperature is also very influential on the pyrolysis
process. The higher the temperature, the better the
decomposition/decomposition process, but the less
amount of charcoal obtained while the more liquid
and gas results, due to the large number of
decomposed and evaporated substances. The
maximum yield was obtained at an average
temperature of 182.68 °C at 29.8% and the minimum
yield was obtained at a temperature of 448.98 °C at
19%, this is in accordance with the statement of Haji
et al., 2010 that due to the high temperature some
charcoal turns into ash and volatile gases, so the yield
tends to be low. It can be concluded that the oxygen
and nitrogen that are flowed into the reactor help the
pyrolysis process occur perfectly, the incoming
oxygen reacts with the activated charcoal to become
CO2 which causes the amount of solids to decrease.
The function of oxygen here is to oxidize the material
while nitrogen is a physical activating agent (Gao and
Li, 2008).
400,00
450,00
500,00
550,00
600,00
20 25 30 35 40
Temperature Average (oC)
Air Flow Rate (Liter/Minute)
The Effect of Air Flow Rate on Temperature, Yield and Water Content in the Production of Active Charcoal from Palm Oil Shells Using
Partial Oxidation Method
425

Figure 2: Effect of air flow rate on activated charcoal yield.
Comparison of Figure 1 and Figure 2 From this
data, it can be concluded that the yield of activated
charcoal produced relatively decreased as air was
added or flowed into the reactor during the pyrolysis
process. The calculation of the water content of
activated charcoal aims to determine the hygroscopic
nature (water absorption) of activated charcoal.
Activated charcoal is hygroscopic so it is very easy to
bind moisture from the air. From this very
hygroscopic nature, activated charcoal is used as an
adsorbent (Ikawati & Melati, 2010).
Figure 3: Effect of air flow rate on water content.
The results of the water content are shown in
comparison of Figure 1 and Figure 3 , from the figure
it can be seen that the higher the air flow rate, the
combustion process is close to complete and the
temperature tends to be higher, the less water vapor
trapped in the pores than the low air flow rate. At air
flow rate of 40 L/min the average temperature
obtained is 448.98 °C and the lowest water content is
5.80%, while at air flow rate of 20 L/min the average
temperature obtained is 182.68 ° C and the highest
water content of 6.94%. This increase in water
content is not only caused by an increase in the
hygroscopic nature of activated charcoal to water
vapor, it is also due to the binding of water vapor
molecules (Hasan et al., 2020). The moisture content
of charcoal can be affected by the amount of water
vapor in the air, the length of the cooling, grinding,
and sifting processes. From Figure 4.2 it can be seen
that the water content of all samples of activated
charcoal produced has met the quality standard of
activated charcoal according to SNI 06-3703-1995.
4 CONCLUSIONS
The best results in the process of making activated c
From the research that has been carried out on
variations in air flow rates of 20 L/min, 25 L/min, 30
L/min, 35 L/min, and 40 L/min, it can be concluded
that the air flow rate reaches the optimum condition
at a speed of 30 L/min. min. with Optimum results
shown at air flow rate of 30 L/min, with a product
yield of 24%, water content of 5.85%,
ACKNOWLEDGEMENTS
The author would like to thank the Research and
Development Center of the Samarinda State
Polytechnic which has funded this research, and also
to the Chemical Engineering Laboratory of the
Samarinda State Polytechnic as the research site. and
special thanks to Razak Faculty of Technology and
Informatics, Universiti Teknologi Malaysia, UTM
Kuala Lumpur, Malaysia who helped push this article
REFERENCES
Abnisa, F., Daud, W. M. A. W., Husin, W. N. W., & Sahu,
J. N. (2011). Utilization possibilities of palm shell as a
source of biomass energy in Malaysia by producing
bio-oil in pyrolysis process. Biomass and Bioenergy,
35(5), 1863-1872. doi:10.1016/j.biombioe.2011.01.033
Bansal, R.C., Donnet, J.B., Stoeckli, H.F. Active carbon.
Marcel Dekker, (1988), New York.
BPS. (2020). Statistik Kelapa Sawit Indonesia 2019. In N.
P. 05130.2002 (Ed.), (Nomor Publikasi: 05130.2002
ed., pp. 137).
Bansal, R.C., Donnet, J.B., Stoeckli, H.F. Active carbon.
Marcel Dekker, (1988), New York.
Gao, N., Li, A., (2008), “Modeling and Simulation of
combined Pyrolysis and Reduction Zone for a
downdraft Biomass Gasifier”, Energy Conversion and
Management 49, 3483-3490
Huang, Y., Liu, H., Yuan, H., Zhan, H., Zhuang, X., Yuan,
S., Wu, C. (2018). Relevance between chemical
structure and pyrolysis behavior of palm kernel shell
17
19
21
23
25
27
29
31
20 25 30 35 40
Yield (%)
Air Flow Rate (Liter/Minute)
5,70
5,85
6,00
6,15
6,30
6,45
6,60
6,75
6,90
7,05
20 25 30 35 40
Water Content (%)
Air Flow Rate (Liter/Minute)
iCAST-ES 2022 - International Conference on Applied Science and Technology on Engineering Science
426

lignin. Sci Total Environ, 633, 785-795.
doi:10.1016/j.scitotenv.2018.03.238
Hasan, S., Aladin, A., Syarif, T., & Arman, M. (2020).
Pengaruh Penambahan Gas Nitrogen Terhadap Kualitas
Charcoal Yang Diproduksi Secara Pirolisis Dari
Limbah Biomassa Serbuk Gergaji Kayu Ulin
(Euxideroxylon Zwageri). Journal of Chemical Process
Engineering, 5(1), 61–68. https://doi.org/10.33536/
jcpe.v5i1.472
Ikawati, & Melati. (2010). Pembuatan Karbon Aktif Dari
Kulit Singkong UKM Tapioka Kabupaten Pati. Area,
1–8.
Mohd Salleh MA, Nsamba HK, Yusuf HM, Idris A, Ghani
WAWAK. Effect of equivalence ratio and particle size
on EFB char gasification. Energy Sources, Part A
Recover Util Environ Eff 2015;37:1647e62.
https://doi.org/10.1080
Prahas, D., Kartika, Y., Indraswati, N., Ismadji, S.
(2008),Activated carbon from jackfruit peel waste by
H3PO4 chemical activation: Pore structure and surface
chemistry characterization. Chemical Engineering
Journal., 140, 32-42.
Ramos, L.P., (2003). The chemistry involved in the stram
treatment of lignocellulosic materials. Quim. Nov. 26,
863–871.
Shuit SH, Tan KT, Le KT, Kamaruddin AH, (2009). Oil
palm biomass as a sustainable source: a Malaysian case
study. Energy;34:1225–35.
Yuliusman. (2015). Production of activated carbon from oil
palm shells with KOH and N
2
/CO
2
as activating
ingredients. Seminar on Technology and Engineering
(SENTRA), 978–979.
Nahrul Hayawin, Z., Astimar, A. A., & Idris, J. (2020). Nor
Faizah. J., Ropandi, M., Astimar, AA, Noorshamsiana,
AW, & Abd-Aziz, S.
The Effect of Air Flow Rate on Temperature, Yield and Water Content in the Production of Active Charcoal from Palm Oil Shells Using
Partial Oxidation Method
427
