
The Main Features of the Formation and Chemical Composition of
the Lakes of Eastern Transbaikalia (Russia)
Svetlana Borzenko and Igor Fedorov
Institute of Natural Resources, Ecology and Cryology, Siberian Branch of the Russian Academy of Sciences,
Chita, Russia
Keywords: Lakes of Eastern Transbaikalia, Chemical Composition, Secondary Mineral Formation, Thermodynamic
Modelling.
Abstract: The concentrations of bicarbonate, carbonate, sulphate and chloride ions grow irregularly in the conditions
of an arid climate due to the evaporation of lake water. Basically, in the lake waters, the concentrations of
carbonate complexes (НСО
3
-
+СО
3
2-
) and chloride ions (Cl
-
) increase. The accumulation of sulphate (SO
4
2-
)
does not happen, mainly as a result of bacterial reduction. The rapid growth of НСО
3
-
+СО
3
2-
is also
associated with this process. The chloride ion content increases with increasing salinity, as well as because
no geochemical barriers exist to hinder its accumulation. As a result, soda or chloride-soda lakes are
generally formed in the considered territory. Sulphate lakes are extremely rare.
1 INTRODUCTION
This article presents the results of hydro-chemical
investigations, made in August 2019, of the
chemical composition of some lakes in Eastern
Transbaikalia. All of the considered lakes are
located in the Chita-Ingoda depression. This
depression is one of the largest Mesozoic basins in
Transbaikalia; it is up to 260 km long, with an
average width of 17 km and a total area of about
4400 km
2
(Florensov, 1960). There are about 20
lakes in the depression. The largest of the lakes is
Lake Doroninskoe, a soda lake. Its area is 5 km
2
, and
it has a maximum depth of 6,5 m. It is a meromictic
lake with a pronounced layered stratification of the
salinity of the water in its physical and chemical
characteristics. The salinity, depending on the
season, ranges from 10 to 35 g/L in the top layer of
the oxygen-encompassing depth of 3–5 m; in the
hydrogen sulphide layer, the salinity is in a narrower
range – 28–36 g/L.
The main feature of the lakes in the considered
territory is the considerable variability of their
salinity and chemical composition. Extreme
continental climate and interannual variations in the
total moisture of the territory lead to significant
changes in the hydrological regime of the lakes and
lake water transitions from one hydro-chemical type
to another. Directed transformations of the chemical
composition, with the change from carbonate to
sulphate and then to chloride, must occur in the
course of this change according to previous works
(Posokhov, 1981; Shvartsev, 1982; Drever, 1982).
Such a succession of the chemical composition
occurs due to the precipitation of salts as the lake
water is saturated; first, the least soluble minerals,
calcite and dolomite, precipitate, and then gypsum,
soda, etc. The actual distribution of chemicals in the
waters of the studied lakes (table 1) differs from the
above scheme of metamorphism.
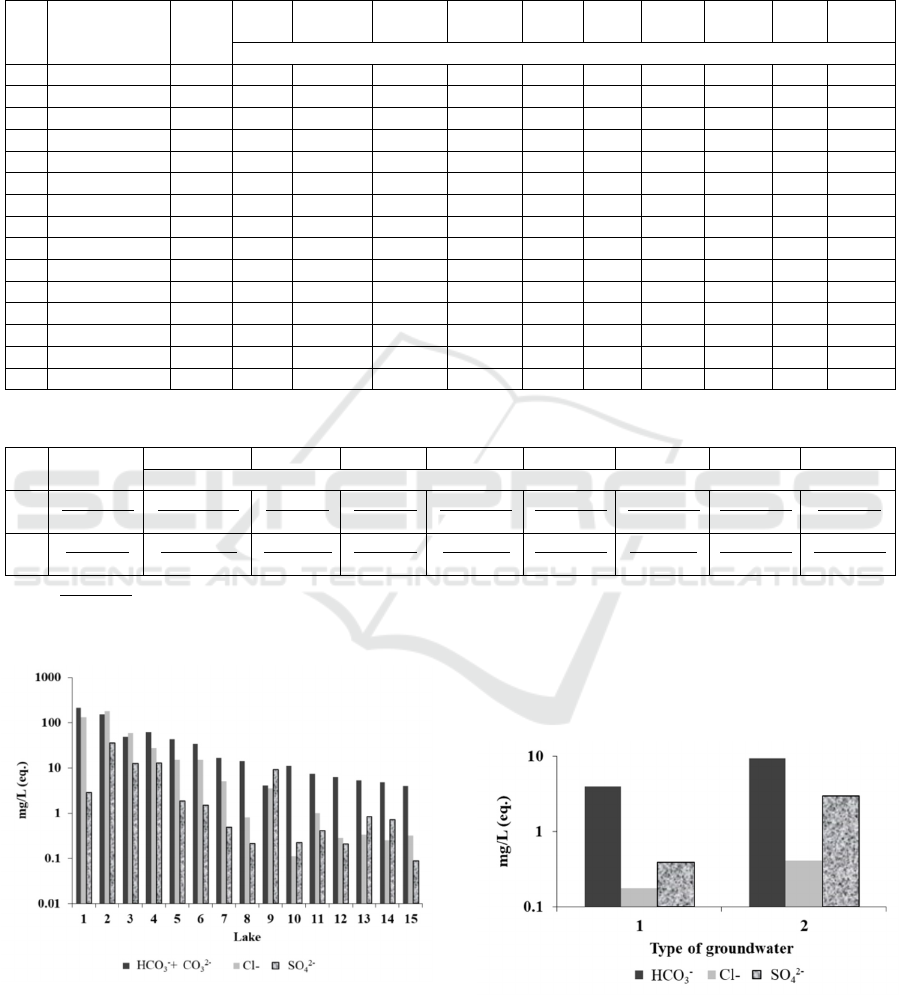
Only in freshwater lakes with an anionic
composition was carbonate actually the most
abundant anion, and the second most abundant ion
was chloride one in the salt lakes; in some cases,
chloride held the lead position. Sulphates dominated
only in one case; in the other cases, the sulphate
concentration was below 20 % (eq.). The advanced
growth of the chloride ion concentration compared
to that of sulphate ions in the lake water does not
correspond to the ratio of these anions in the
groundwater, which provides the bulk of the salt
supply for these lakes (table 2, figure 1). The
equivalent concentration of chloride is on average
lower than that of sulphate. Therefore, to saturate the
water with sulphate minerals and precipitation,
generally, chloride and sulphate ions must
accumulate in equal proportions. Actually, in the
studied lake waters, not only is the accumulation of
sulphates proportional to that of chlorides, but it lags
96
Borzenko, S. and Fedorov, I.
The Main Features of the Formation and Chemical Composition of the Lakes of Eastern Transbaikalia (Russia).
DOI: 10.5220/0011912000003536
In Proceedings of the 3rd International Symposium on Water, Ecology and Environment (ISWEE 2022), pages 96-100
ISBN: 978-989-758-639-2; ISSN: 2975-9439
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
behind the rate of accumulation and the contents of
bicarbonate and carbonate, which, in comparison to
sulphate and chloride, in most cases grow more
intense. Such a situation is not in line with the
general ideas about these minerals and requires
special consideration.
Table 1: Chemical composition of lakes water.
No Lake pH
DOC
HCO
3
-
+ CO
3
2-
SO
4
2-
Cl
-
F
-
Ca
2+
Mg
2+
Na
+
K
+
TDS
mg/L
1 Doroninskoe 9,88 34,2 12952 133,4 4715 11,4 7,37 35,8 10209 127 28191
2 Che
p
che
k
-2 9,59 83,4 9308 1690 6326 29,4 10,2 27,5 8647 37,4 26076
3 Torm 8,96 18,8 2935 590 2090 12,8 4,81 347 2388 13,8 8381
4 Che
p
che
k
-3 9,25 24,1 3770 610 960 10,0 11,0 20,0 2610 20,1 8011
5 Balm 8,96 73,1 2600 89,6 540 4,10 9,50 19,8 1432 23,6 4719
6 Hunduyskoe 9,53 29,7 2096 70,0 536 4,50 2,00 93,6 1024 42,2 3868
7 Che
p
che
k
-1 9,05 36,7 1024 23,6 178 4,60 14,2 30,1 494 10,0 1778
8 Lebedinsko
y
e 9,90 23,9 871 10,2 28,2 0,69 11,1 25,6 317 4,55 1268
9 Bolvanka 9,88 16,8 249 435 126 0,38 23,1 18,3 338 1,74 1191
10 Hun
d
-1 8,50 28,1 667 10,7 4,00 0,95 25,3 52,0 129 3,05 891
11 Tanga 9,07 14,5 460 19,4 35,5 0,83 16,7 27,8 132 11,7 703
12 Gorekatsan 9,09 19,8 377 10,0 10,0 1,26 13,8 14,4 112 2,81 540
13 Hun
d
-2 9,04 19,5 323 40,2 11,8 0,57 26,3 18,0 88,6 4,08 512
14 Great 9,05 2,60 295 33,6 8,91 0,95 13,2 23,8 74,8 2,38 451
15 Nikolaev 9,10 34,2 246 4,11 11,2 0,42 19,4 15,3 52,0 1,87 349
Table 2: Groundwater chemical composition of district Chita-Ingoda depression on dedicated types.
No рН
НСО
3
-
SO
4
2-
Cl
-
Ca
2+
Mg
2+
Na
+
К
+
TDS
m
g
/L
1
7,07–7,72
7,56
69,6–497,1
241,8
1,5–43,5
18,5
1,4–12,2
6,33
12,1–65,9
38,6
6,42–49,1
19,7
2,43–46,1
22,2
0,03–3,17
1,09
109–686
348
2
7,58–8,1
7,83
378,7–778
575
32,7–443
143
3,6–29,1
14,5
80,1–115
72,0
34,7–55,8
43,6
94,6–189
127,9
0,05–8,24
3,0
855–1365
980
Note.
Min–Max
Av
g
a
b
Figure 1: Distribution of major anions in the lake water (а) and groundwater (b). (Numeration of lakes and groundwater
types according to the tables 1 and 2, respectively).
The Main Features of the Formation and Chemical Composition of the Lakes of Eastern Transbaikalia (Russia)
97

2 RESULTS AND DISCUSSION
The calculation of the change of the composition of
water due to its evaporation was executed according
to the thermodynamic modelling program HG32
(Bukaty, 2002). The soda lake Doroninskoe (Q1)
was chosen as a model. The calculations used the
water chemical composition feeding the
Doroninskoye lake (Q2) (table 3). The chloride, as
the most conservative component, was used as an
indicator of the degree of evaporation; it does not
enter into reactions concerning the formation of
hydro-minerals in the studied range of TDS.
The residual solution composition (Q3) was
estimated using the concentration of water up to the
level of chloride in the lake water over the ions,
resulting in the percent equivalent of the presented
relations: CO
3
2-
+ HCO
3
-
– 45; Cl
-
– 27; SO
4
2-
– 26;
Na
+
– 97 %. The model solution were supersaturated
by calcite, dolomite, fluorapatite and gypsum were
the main components of the equilibrium phases
(table 4). The result of the thermodynamic
calculation corresponds to a scheme in which
metamorphic water evaporated, but it differs
significantly from the data on the lake. In this case,
the estimated sulphate content (5945 mg/L) was
higher, and the estimated bicarbonate and carbonate
content (9056 mg/L) is lower than in the lake water.
The causes of various theoretical and actual
concentrations of anions can be explained as
follows.
Table 3: Components and physico-chemical parameters of model solutions.
Solution pH
CO
3
2-
+HCO
3
-
SO
4
2-
Cl
-
F
-
Ca
2+
Mg
2+
Na
+
K
+
TDS
m
g
/L
Q
1
9,98 12952 133,4 4715 11,4 7,37 35,8 10209 127 28190
Q
2
7,55 19,6 1,55 0,81 0,02 3,15 1,67 1,83 0,25 28,9
Q
3
9,45 905
6
5945 4715
0
,73 1‧1
0
-6
0
,6
0
10628 511 3085
6
Table 4: Saturation of model solutions with minerals (per liter).
No Mineral name Formula Mineral mass, mg
1 Calcite CaCO
3
3836
2 Dolomite CaM
g(
CO
3
)
2
133
3 Ma
g
nesite M
g
CO
3
-
4 Gypsum CaSO
4
‧ 2H
2
O 1627
5 Chlorite Mg
2,25
Al
1,5
Si
1,25
O
5
(OH)
4
0,57
6 Montmorillonite MgAl
2
Si
4
O
11
(OH)
2
0,49
7 Montmorillonite Ca
0,15
Al
1,9
Si
4
O
10
(
OH
)
2
1,36
8 Montmorillonite
K
0,33
Al
1,9
Si
4
O
10
(
OH
)
2
9,33
9 Montmorillonite Na
0,33
Al
2,33
Si
3,67
O
10
(
OH
)
2
0,02
10 Montmorillonite KMgAlSi
4
O
10
(OH)
2
0,70
11 Natron Na
2
CO
3
‧10H
2
O-
12 Analcime NaAlSi
2
O
5
(
OH
)
2
-
13 К-Illite
(
total
)
2,05
14 Illite M
g
2,75
Al
1,5
Si
3
O
10
(
OH
)
2
0,04
15 Fluorapatite Ca
5
(PO
4
)
3
F 125
16 Сhlorapatite Ca
5
(PO
4
)
3
Cl 1,23
17 Troilite (Fe
2+
)S 0,37
Note. Dash
–
water is not saturated.
In lakes, the balance of inorganic carbon is
formed due to the intake of bicarbonate ion with
underground and surface runoff and mineralization
of dissolved organic matter (DOC). The values of
DОС indicate their high concentration in the lake
waters (table 1). Detritus is one of the sources of
DOC, which comes from the watershed and is
formed as a result of the mineralization of algae.
Although it is believed that most of the detritus is
allochthonous (Hutchinson, 1957), when it is
introduced to the watershed, in many cases the bulk
of it is likely to accumulate in the reservoirs
themselves with withering algae. The extensive
development of the coastal strip is observed in most
lakes. Perhaps photosynthesis in the water column is
the main process of organic carbon formation. In the
surface layers of the salt lakes, the total production
of DOC bacteria and algae in the summer period is
ISWEE 2022 - International Symposium on Water, Ecology and Environment
98

estimated to be 60 mg C/(m
2
‧ d) (Namsaraev and
Namsaraev, 2007).
The lag in the accumulation in the lake waters,
and often the sulphate ion output, occur due to the
associated microbiological processes as well. This
happens only because of the processes of sulphate
reduction. The intensity of the sulphate reduction in
lakes in the Transbaikal region in the summer is
estimated to be 30 mg S/dm
3
per day on average
(Gorlenko et al., 2010). More than 100 mg/L of
hydrogen sulfide was recorded in the bottom layers
of Doroninskoye soda lake, tested at different times
(Borzenko and Zamana, 2011).
However, the role of sulphate reduction is not
limited to the transformation of forms of sulphur,
since electron transport is provided by the oxidation
of carbon, which is part of the dissolved organic
matter in the water column. It is known that
sulphate-reducing bacteria (SRB) include agents
containing the light isotope
12
C in their metabolism
(Galimov, 1968). The mirror-symmetrical
distribution curves of the concentrations of S
2-
and
δ
13
C in the water column demonstrate the
relationship of the sulphate reduction process with
the carbon isotopic composition clearly (figure 2).
The most carbon was present (δ
13
C = -0.17 ‰) in
the chemocline zone, where the concentration of
hydrogen sulphide in the period under review was
the greatest. The oxygen isotopic composition of the
carbonates involved in the oxidation of biogenic
carbon also points to oxygen’s entry into carbonate
ions from sulphates. The parallel curves of isotope
distribution and the concentrations of sulphate are
proof.
Figure 2: Distribution of dissolved S
6+
, S
2-
, δ
13
S and δ
18
O
in the Doroninskoe lake water.
The sediment associated with the saturation of
water in certain hydrogenic minerals is no less of an
important factor in the formation of the
hydrochemistry of lakes. According to
thermodynamic calculations (table 5), for lakes,
typically the carbonate type of sedimentation in the
hydrogenic deposition may contain in small amounts
calcium and magnesium carbonates, clay minerals
(montmorillonite, illite), zeolites (chlorite), metal
sulphides, etc.
Table 5: Saturation of water with minerals in lakes considered (mg/L). (Numeration of minerals according to the table 4).
Lake
Mineral
1 2 3 5 8 14 15 17
Doroninskoe 6‧10
-6
5,32 54,9 5‧10
-2
0,046 2,9‧10
-3
13,3 0,37
Chepchek-1 2‧10
-6
0,48 2‧10
-4
1‧10
-3
5‧10
-5
2‧10
-6
1‧10
-4
-
Chepchek-2 2‧10
-7
17,4 3,21 2‧10
-6
4‧10
-3
2‧10
-3
1,94 -
Chepchek-3 1‧10
-7
5,23 1‧10
-4
4‧10
-6
1‧10
-5
3‧10
-8
0,14 -
Torm 2‧10
-7
32,4 5,02 6‧10
-5
2,0 1‧10
-4
2,03 -
Balm 9,33 26,2 55,7 1‧10
-8
1‧10
-10
9‧10
-6
0,07 0,07
Hunduyskoe 1‧10
-6
8,47 28,6 6‧10
-7
0,04 2‧10
-6
0,09 -
Great 1,35 18,6 5,11 1‧10
-12
2‧10
-5
9‧10
-7
0,06 -
Nikolaev 9‧10
-7
43,0 2‧10
-4
1‧10
-12
3‧10
-8
2‧10
-6
0,09 1‧10
-4
Lebedinskoye - 45,9 39,5 3‧10
-12
2‧10
-5
2‧10
-5
0,08 0,25
Hund-1 9‧10
-7
11,2 1‧10
-3
2‧10
-10
1‧10
-7
1‧10
-5
0,07 -
Hund-2 2,2 6,11 1‧10
-3
1‧10
-8
1‧10
-7
1‧10
-5
0,07 -
Tanga 9‧10
-7
7,13 5‧10
-4
5‧10
-8
1‧10
-7
2‧10
-6
0,09 -
Gorekatsan 8,6 4,80 2‧10
-4
2‧10
-10
1‧10
-5
9‧10
-8
0,07 0,23
Bolvanka 9‧10
-7
4,41 4‧10
-9
1‧10
-8
1‧10
-8
4‧10
-8
0,05 0,02
Note. Dash – water is not
saturated.
The Main Features of the Formation and Chemical Composition of the Lakes of Eastern Transbaikalia (Russia)
99

Gypsum formation does not occur, mainly due to
the low content of sulfate ions in lake waters. The
chloride ion has no restrictions in the form of
hydrogenic minerals in this salinity range.
Therefore, its content increases with increasing
salinity of water.
3 CONCLUSIONS
Therefore, the formation and transformation of the
chemical composition of the water of the saline
lakes of East Transbaikalia is a multifactor process,
which, along with evaporative concentration,
involves hydrobiological processes and
sedimentation inside the basin. The content in the
salt lakes’ waters of carbonate and sulphate
components depends on the functioning of the
microbial community in a particular basin (body of
water). Evaporative sedimentation limits the
accumulation in water of many components, but the
bacterial production of carbonate complexes along
with conjugate sulphate reduction allows them to
concentrate in substantial amounts, up to the
formation of soda or chloride-soda waters. The
hydro-chemical variety of salt lakes is determined by
the dominance of a particular process in the
formation of the composition of the salt.
Obviously, differences in the composition of host
rocks, the intensity of water exchange, the
thermodynamic conditions and other factors
determine the variety of water-rock interactions. The
composition of secondary products can provide
isotope fractionation during certain stages in a
water-rock-gas system.
ACKNOWLEDGEMENTS
The research was carried out at the expense of the
Russian Science Foundation grant No. 22-17-00035,
https://rscf.ru/project/22-17-00035/
REFERENCES
Borzenko, S. V. and Zamana, L. V. (2011). Reduced
forms of sulfur in the brine of saline-soda lake
Doroninskoe, Eastern Transbaikal region.
Geochemistry International, 49(3): 253–261.
Bukaty, M. B. (2002). Software engineering for the
solution of hydrogeological problems. Bulletin of the
Tomsk polytechnic university, 305(6): 348–365
Drever, J. I. (1982). The geochemistry of natural waters,
Prentice-Hall. Englewood Cliffs.
Florensov, N. A. (1960). Mesozoic and Cenozoic
depressions of the Baikal region, Academy of Science
USSR. Moscow–Leningrad.
Galimov, E. M. (1968). Geochemistry of stable carbon
isotopes, Nedra. Moscow.Gorlenko, V. M.,
Namsaraev, Z. B., Bryantseva, I. A., Boldareva, E. N.,
Sorokin, D. Y., Buryukhaev, S. P., Namsaraev, B. B.,
Matyugina, E. B. and Borzenko S. V. (2010).
Microbial communities of the stratified soda lake
Doroninskoe (Transbaikal region). Microbiology,
79(3): 390–401.
Hutchinson, G. E. (1957). A treatise on limnology, Jonh
Wiley. New York.
Namsaraev, B. B. and Namsaraev, Z. B. (2007). Microbial
processes of carbon cycling and habitat conditions in
alkaline lakes of Transbaikalia and Mongolia. In T. N.
Zhilina, L. M. Gerasimenko and G. A. Zavarzin,
Proceedings of the S.N. Vinogradsky Institute of
Microbiology. Issue 14. Alkalophilic microbial
communities. (pp. 299–322). Moscow: Nauka.
Posokhov, E. V. (1981). Chemical evolution of the
hydrosphere, Gydrometeoizdat. Moscow–Leningrad.
Shvartsev, S. L. (Ed.). (1982). Fundamentals of
hydrogeology. Hydrogeochemistry, Nauka.
Novosibirsk.
ISWEE 2022 - International Symposium on Water, Ecology and Environment
100
