
Decreased Connectivity in Left Frontal Orbital Cortex After Sleep
Deprivation
Aoke Zheng
1,2
, Yan He
2,*
and Hao Yan
2,*
1
College of English Studies, Xi’an International Studies University, Xi’an, China
2
Key Laboratory for Artificial Intelligence and Cognitive Neuroscience of Language, Xi’an International Studies
University, Xi’an, China
Keywords: Sleep Deprivation, Anger, gPPI, Brain Network.
Abstract: Men are reported to be likely to express more anger than women. Insufficient sleep may lead to cognitive
changes, and most adults are plagued by this problem. Researches have proved that lack of sleep can cause
the emotion of anger. However, the neural mechanisms of this phenomenon still need to be discovered.
Aiming to explore why men tend to experience anger after sleep deprivation (SD) by utilizing the method of
brain network science, a generalized PsychoPhysiological Interaction (gPPI) analysis was administered. A
total of 18 male participants were enrolled in this study. As a vital area for regulating emotions, the left frontal
orbital cortex (FOrb) showed decreased connectivity to the posterior cingulate gyrus (PC) and cerebellum,
while PC and cerebellum are known to involve in emotion regulation. Decreased connectivity in these areas
might provide a plausible explanation for how SD influences male to process the emotion of anger. Our results
provide further evidence that sleep deprivation is closely related to the emotion of anger and the adoption of
brain network science offers new insights in uncovering the neural mechanism for SD’s impact on men’s
processing of anger.
1 INTRODUCTION
Humans spend approximately one-third of their lives
asleep. (Farahani et al., 2019). Previous researches
indicate that emotional abilities are fairly disrupted
due to sleep deprivation (Krause et al., 2017).
According to Saghir et al (2018), sleep deprivation
leads to the bursts of anger. Anger is a syndrome of
relatively specific feelings; certain physiological
reactions are urged to damage some target (Berkowitz
et al., 2004). Although it has great impact on our daily
life, it remains one of the least studies of the basic
emotions (Alia-Klein et al., 2020). Men are more
emotionally reactive to anger, and this can trigger
more aggressive tendencies (Potegal et al., 2004;
Iverson et al., 2019, Kim et al., 2022; Fernández-
Modamio et al., 2020). Potegal and Archer (2004)
demonstrated that men were more frequently the
targets of anger than women. Iverson et al. (2019)
illustrated that a significant minority of middle-aged
men reported some degree of anger and aggression,
which are correlated with depression and anxiety.
Kim et al. (2022) illustrated that men tend to be more
aggressive than women when they experience the
same level of anger. Fernández-Modamio et al.
(2020) found that men recognized disgust and neutral
expressions better than women. Previous studies have
proved that men experience more anger, which leads
to acute consequences for their life quality. However,
most studies focus on questionnaire survey, it is
necessary to probe into the influence of anger by way
of objective measurements.
Previous neuroimaging researches based on
functional magnetic resonance imaging (fMRI), a
non-invasive technique to observe activation or
connectivity activity of the brain, also demonstrated
that emotion functions were greatly influenced by
sleep deprivation. By means of brain networks,
self-organized associations are revealed among
various brain regions in order to accomplish different
cognition functions. Under the condition of sleep
deprivation, the functional connectivity between the
amygdala and the ventral anterior cingulate cortex
(vACC) was significantly decreased, which resulted
in aggravation of subjective mood including anger
(Motomura et al., 2013). Resting-state functional
Zheng, A., He, Y. and Yan, H.
Decreased Connectivity in Left Frontal Orbital Cortex After Sleep Deprivation.
DOI: 10.5220/0011929300003612
In Proceedings of the 3rd International Symposium on Automation, Information and Computing (ISAIC 2022), pages 367-371
ISBN: 978-989-758-622-4; ISSN: 2975-9463
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
367

MRI (fMRI) was used to examine the changes in
functional connectivity of the basolateral amygdala
(BLA) and centromedial amygdala (CMA) following
sleep deprivation (Shao et al., 2014). The findings
indicated that a lack of sleep led to a significant
decrease in the functional connectivity between the
basolateral amygdala (BLA) and various executive
control regions. These studies demonstrated that sleep
deprivation may worsen anger emotional states.
In short, males are affected by anger to a great
extent, and sleep deprivation has effect on men’s
negative emotion like anger, while the underlying
neural mechanism is not clear. In this paper, we
adopted Stockholm Sleepy Brain Research to reveal
how sleep deprivation affect men’s angry emotion
processing.
2 METHODS
2.1 Study Design
Neuroimaging data used in this study were obtained
from the Stockholm Sleepy Brain Project which is
publicly available as one OpenNEURO database
(Tamm, 2019). The study was a randomized
crossover design with the interval of one month. They
underwent fMRI scanning under full sleep and 3-hour
sleep deprivation with the interval of one month in a
counterbalanced order. The experiment employed
three different emotional paradigms, and two resting
state sessions.
2.2 Participants
Participants filled out an online screening form after
being recruited through the website, posters and a
newspaper advertisement (Nilsonne et al., 2017). In
this study, a total of 18 males are enrolled. All of them
passed the inclusion criteria, including no psychiatric
or neurological illness, no working or studying
experience in psychology, behavioral science or
medicine, no color blind, and all right-handed. They
also completed the insomnia severity index (ISI) to
test the insomnia symptoms, and the Karolinska Sleep
Questionnaire (KSQ) to test the sleep patterns.
Besides, the Hospital Anxiety and Depression Scale
(HADS) was used to test the depressive symptoms.
2.3 Experimental Paradigm
The Karolinska Directed Emotional Faces (KDEF)
database served for the source of the experiment
materials (Lundqvist et al., 1998). The study
employed happy, neutral and angry emotional
pictures in a block design. Every block lasted for 20
seconds and 20 faces were included, each of which
was displayed for 0.5 seconds. In total, there were 12
blocks of images (4 happy, 4 angry and 4 neutral).
They were organized in groups of three, such as
happy-neutral-happy or angry-neutral-angry.
Participants were required to grade how happy and
angry they felt on a visual analog scale of 0-100 after
each set of three (Nilsonne et al., 2017).
2.4 Data Acquisition
fMRI data were obtained from a 3T Discovery 750
MRI scanner (General Electric) with an 8-channel
head coil. T1-weighted anatomical scans were
acquired with a sagittal BRAVO (brain volume
imaging) sequence, TR=6.4ms, TE=2.81ms,
inversion time=0.45ms, FA=11°, FOV=240mm ◊
240 mm◊180mm, 180 slices, slice thickness=1 mm,
interleaved bottom-up. Functional scans were
acquired in a gradient echo-planar-imaging (EPI)
sequence, TR=3000ms, TE=34ms, FA=80°,
FOV=220 mm◊220 mm◊110mm, 46 slices, slice
thickness=2.3mm, interleaved bottom-up.
2.5 fMRI Preprocessing and Analysis
To investigate task-modulated changes in
connectivity, we performed generalized
PsychoPhysiological Interaction (gPPI) measures.
gPPI is a special type of multiple regression that
includes a psychological regressor, a physiological
regressor, and a condition specific interaction
regressor, which reveals whether the functional
connectivity between separate nodes depending on
what task the participant is currently doing. Its
computation comes from multiple regression model
corresponding with each individual ROI (Region of
Interest) time series. Each time course was
deconvolved and then its output was regarded as
physiological regressors. In detail, the experimental
conditions of emotion contagion were taken as
psychological regressors. The interactions between
the time-courses of ROI seeds and the experimental
conditions were used as PPI regressors, which were
convolved with HRF, and different β values were
generated, as presented in Figure 1.
Data were analyzed in CONN toolbox V.20.b.
(Whitfield-Gabrieli, 2012). Functional images were
first
realigned, where all scans are coregistered and
ISAIC 2022 - International Symposium on Automation, Information and Computing
368
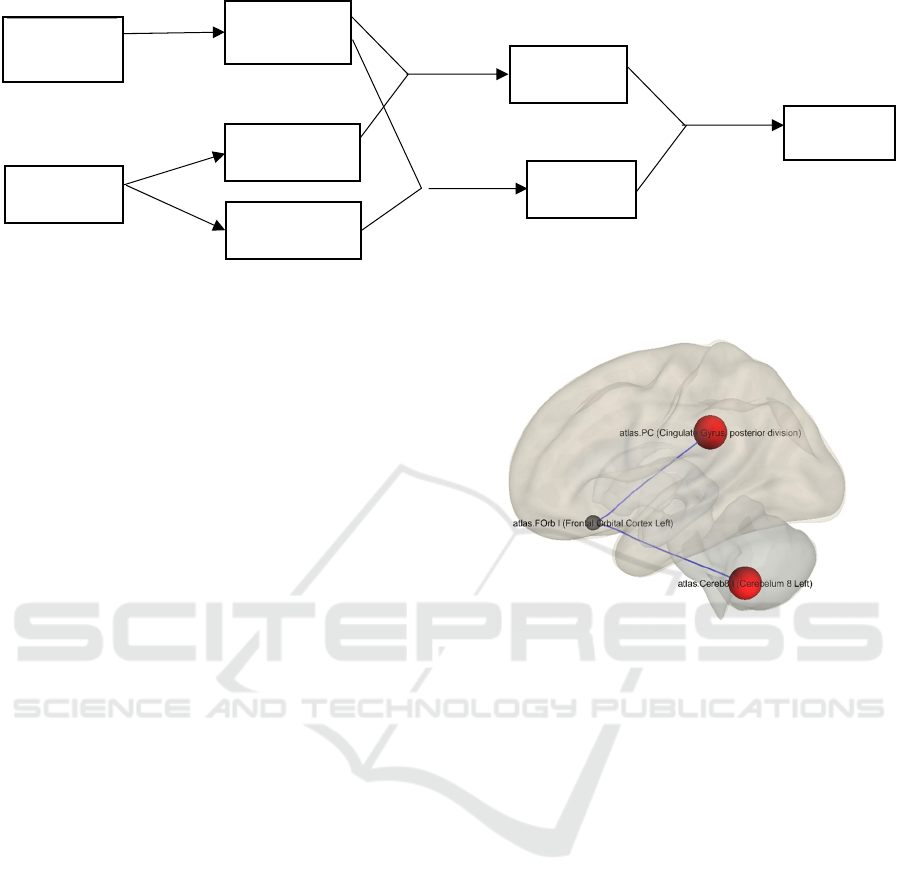
Figure 1: gPPI analysis process.
resampled to a reference image. Next, temporal
misalignment was corrected by slice-timing. Outlier
identification was then applied to identify potential
outlier scans. Later, structural and functional data
were segmented into grey matter, white matter, and
CSF tissue classes and normalized into standard MNI
space (Ashburner et al., 1997). The functional data
was then smoothed using spatial convolution with a
Gaussian kernel of 8mm full width half maximum
(FWHM). Subsequently, the denoising step, which
applies linear regression and band-pass filtering, was
used to remove unwanted motion, physiological, and
other artifactual effects from the BOLD signal (Nieto-
Castanon, 2020). In the end, a paired t-test were
conducted to compare functional connectivity
between full sleep and sleep-deprived conditions.
3 RESULTS
According to the paired t-test, significant effects
emerged in the left frontal orbital cortex (LFOrb). As
demonstrated in Figure 2, the functional connectivity
between LFOrb and the posterior cingulate gyrus
(PC) was decreased after sleep deprivation. In
addition, the functional connectivity between LFOrb
and left Cerebellum was decreased. Decreased
connectivity was also found between left amygdala
and right hippocampus. Moreover, some visual areas
such as right occipital pole and right frontal eye fields
showed decreased connectivity. Increased
connectivity was found between right rostral
prefrontal cortex and left frontal pole. Besides, the
right occipital fusiform gyrus showed increased
connectivity with left temporal occipital fusiform
cortex. The same results were not found under happy
condition.
Figure 2: Decreased functional connectivity between left
frontal orbital cortex and posterior cingulate gyrus (LPC),
left Cerebellum 8.
4 DISCUSSION
According to the experimental results, the paired t-
test showed significant effects in the left frontal
orbital cortex (LFOrb) and posterior cingulate gyrus
(PC). Previous researches indicated that men were
facing with sleep problems and had difficulties in
dealing with anger emotion simultaneously. This
study intended to decipher the neural mechanisms
underlying these phenomena. Results suggested
significant effects in the left frontal orbital cortex
(LFOrb) seed. Specifically, after sleep deprivation,
the functional connectivity between LFOrb and
posterior cingulate gyrus (PC) was decreased. In
addition, the functional connectivity between LFOrb
and left Cerebellum 8 was decreased.
As a crucial area for regulating emotion, the
orbitofrontal area represents one critical structure in a
neural system serving decision making (Arnsten et al.,
2012; Bechara et al., 2000). Decreased connectivity
ROI BOLD
timecourse
deconvolve
physiological
regressors
interaction
PPI
regressors
convolved with
HRF
β values
task
psychological
regressors
psychological
regressors
interaction
PPI
regressors
Decreased Connectivity in Left Frontal Orbital Cortex After Sleep Deprivation
369

of OFC would impair stimulus‐reward reversal
learning, response inhibition, and ability to balance
the appropriateness of their behavior in the social
context (Viskontas et al., 2007). Research has shown
that there are changes in risk-taking behavior
following sleep deprivation, bringing about more
dangerous or risky decisions (Womack et al., 2013).
Lack of sleep enhances the sensitivity of reward
system. Negative emotional experiences are
associated with the activation of the reward network,
including orbitofrontal cortex. Posterior cingulate
cortex plays a critical role in the default mode
network. It receives strong feedback from areas
involved in emotion processing and social behavior,
including the orbital frontal cortex (Maddock et al.,
2003). Cerebellum is in control of the arousal and
reward system. The abnormal activation in this area
might also influence the ability to deal with the
negative emotion. Decreased connectivity in these
areas might be able to explain why SD significantly
influenced the emotion of anger of men.
Our results also found decreased connectivity
between left amygdala and right hippocampus.
Amygdala-hippocampus interactions allow for
emotional processing in the amygdala to influence
memory storage in the hippocampus, thereby
mediating emotional memories’ consolidation and
retrieval. (Kirby et al., 2018; Yang et al., 2017;
Roesler et al., 2021; Fastenrath et al., 2014). Yang et
al. (2017) proposed that the amygdala and
hippocampus can act synergistically to regulate
emotion-based memories. The findings of a fMRI
study suggest that the amygdala may be instrumental
in regulating how emotional information is stored in
the hippocampus. The study found that the connection
between the amygdala and hippocampus is much
stronger when emotionally positive or negative
pictures are being encoded (Fastenrath et al., 2014).
Decreased functional connectivity between the
amygdala and hippocampus may indicate that sleep
deprivation impairs male’s ability of angry emotional
memory retrieval and consolidation.
Sleep is an essential part of our life. Insufficient
sleep could correlate with anger, which may lead to
aggressive behaviors. It is of great importance to
study the effect of sleep deprivation on anger. This
study took males as participants and focused on how
sleep deprivation influences angry emotion, which
shed light on the study of male’s emotion and sleep
problems. However, this study did not investigate
whether the same results exist in females. Further
studies can make a comparison between males and
females to depict gender differences in angry emotion
processing under the condition of sleep deprivation.
ACKNOWLEDGMENTS
This work was supported partly by the Basic Research
Program for the Natural Science of Shaanxi Province
of China under Grant 2019JQ861 and Grant
2022JM134. This work was partly supported by the
start-up foundation from Xi’an International Studies
University (Grant no. KYQDF202138).
REFERENCES
Alia-Klein, N., Gan, G., Gilam, G., Bezek, J., Bruno, A.,
Denson, T. F., ... & Verona, E. (2020). The feeling of
anger: From brain networks to linguistic expressions.
Neuroscience & Biobehavioral Reviews, 108, 480-497.
Arnsten, A. F., & Rubia, K. (2012). Neurobiological circuits
regulating attention, cognitive control, motivation, and
emotion: Disruptions in neurodevelopmental
psychiatric disorders. Journal of the American Academy
of Child & Adolescent Psychiatry, 51(4), 356-367.
Ashburner, J., & Friston, K. (1997). Multimodal image
coregistration and partitioning--a unified framework.
NeuroImage, 6(3), 209–217.
Bechara, A., Damasio, H., & Damasio, A. R. (2000).
Emotion, decision making and the orbitofrontal cortex.
Cerebral cortex, 10(3), 295-307.
Berkowitz, L., & Harmon-Jones, E. (2004). Toward an
understanding of the determinants of anger. Emotion,
4(2), 107-130.
Farahani, F. V., Fafrowicz, M., Karwowski, W., Douglas,
P. K., Domagalik, A., Beldzik, E., ... & Marek, T.
(2019). Effects of chronic sleep restriction on the brain
functional network, as revealed by graph theory.
Frontiers in Neuroscience, 13, 1087.
Fastenrath, M., Coynel, D., Spalek, K., Milnik, A.,
Gschwind, L., Roozendaal, B., …& de Quervain, D. J.
F. (2014). Dynamic modulation of amygdala–
hippocampal connectivity by emotional arousal.
Journal of neuroscience. The Journal of Neuroscience,
34(42), 13935-13947.
Fernández Modamio, M., Gil Sanz, D., Arrieta Rodríguez,
M., Gómez de Tojeiro-Roce, J., Bengochea Seco, R., &
González Fraile, E. (2020). Emotion recognition in
patients with schizophrenia: The role of sex.
Psicothema, 32(2), 197-203.
ISAIC 2022 - International Symposium on Automation, Information and Computing
370

Iverson, G. L., Terry, D. P., Luz, M., Zafonte, R., McCrory,
P., Solomon, G. S., & Gardner, A. J. (2019). Anger and
depression in middle-aged men: Implications for a
clinical diagnosis of chronic traumatic encephalopathy.
The Journal of Neuropsychiatry and Clinical
Neurosciences, 31(4), 328-336.
Krause, A. J., Simon, E. B., Mander, B. A., Greer, S. M.,
Saletin, J. M., Goldstein-Piekarski, A. N., & Walker, M.
P. (2017). The sleep-deprived human brain. Nature
Reviews Neuroscience, 18(7), 404-418.
Kim, D., Liu, Q., Quartana, P. J., & Yoon, K. L. (2022).
Gender differences in aggression: A multiplicative
function of outward anger expression. Aggressive
Behavior. 48(4), 393-401.
Kirkby, L. A., Luongo, F. J., Lee, M. B., Nahum, M., Van
Vleet, T. M., Rao, V. R., ... & Sohal, V. S. (2018). An
amygdala-hippocampus subnetwork that encodes
variation in human mood. Cell, 175(6), 1688-1700.
Lundqvist, D., Flykt, A., & Öhman, A. (1998). The
Karolinska Directed Emotional Faces – KDEF, CD
ROM from Department of Clinical Neuroscience,
Psychology section, Karolinska Institutet.
Maddock, R. J., Garrett, A. S., & Buonocore, M. H. (2003).
Posterior cingulate cortex activation by emotional
words: fMRI evidence from a valence decision task.
Human Brain Mapping, 18(1), 30-41.
Nieto-Castanon, A. (2020). Handbook of functional
connectivity Magnetic Resonance Imaging methods in
CONN. Boston, MA: Hilbert Press.
Nilsonne, G., Tamm, S., d'Onofrio, P., Thuné, H. Å.,
Schwarz, J., Lavebratt, C., ... & Åkerstedt, T. (2016). A
multimodal brain imaging dataset on sleep deprivation
in young and old humans.
Potegal, M., & Archer, J. (2004). Sex differences in
childhood anger and aggression. Child and Adolescent
Psychiatric Clinics, 13(3), 513-528.
Roesler, R., Parent, M. B., LaLumiere, R. T., & McIntyre,
C. K. (2021). Amygdala-hippocampal interactions in
synaptic plasticity and memory formation.
Neurobiology of Learning and Memory, 184, 107490.
Saghir, Z., Syeda, J. N., Muhammad, A. S., & Abdalla, T.
H. B. (2018). The amygdala, sleep debt, sleep
deprivation, and the emotion of anger: A possible
connection?. Cureus, 10(7).
Shao, Y., Lei, Y., Wang, L., Zhai, T., Jin, X., Ni, W., ... &
Yang, Z. (2014). Altered resting-state amygdala
functional connectivity after 36 hours of total sleep
deprivation. PloS One, 9(11), e112222..
Tamm, S. (2019). A Neuroimaging perspective on the
emotional sleepy brain (Doctoral dissertation,
Karolinska Institutet (Sweden)).
Tamm, S., Schwarz, J., Thuné, H., Kecklund, G., Petrovic,
P., Åkerstedt, T., ... & Nilsonne, G. (2020). A combined
fMRI and EMG study of emotional contagion following
partial sleep deprivation in young and older humans.
Scientific Reports, 10(1), 1-13.
Viskontas, I. V., Possin, K. L., & Miller, B. L. (2007).
Symptoms of frontotemporal dementia provide insights
into orbitofrontal cortex function and social behavior.
Annals of the New York Academy of Sciences, 1121(1),
528-545.
Whitfield-Gabrieli, S., & Nieto-Castanon, A. (2012). Conn:
A functional connectivity toolbox for correlated and
anticorrelated brain networks. Brain Connectivity, 2(3),
125-141.
Womack, S. D., Hook, J. N., Reyna, S. H., & Ramos, M.
(2013). Sleep loss and risk-taking behavior: A review
of the literature. Behavioral Sleep Medicine, 11(5), 343-
359.
Yang, Y., & Wang, J. Z. (2017). From structure to behavior
in basolateral amygdala-hippocampus circuits.
Frontiers in Neural Circuits, 11.
Decreased Connectivity in Left Frontal Orbital Cortex After Sleep Deprivation
371
