
Integrated View of the Cognitive, Cerebral and Cardiac Systems
During an Inhibition Task
C. De Faria
1
, M. Causse
2a
, B. Valéry
1b
and C. T. Albinet
1c
1
Laboratoire Sciences de la Cognition, Technologie, Ergonomie (SCoTE), INU Champollion, Albi, France
2
Institut Supérieur de l’Aéronautique et de l’Espace (ISAE-SUPAERO), Université Fédérale de Toulouse, Toulouse, France
Keywords: Flanker Task, Go/No-Go Task, Cardiac Pre-Ejection Period, Functional NIRS.
Abstract: This study examined the concomitant variations of cardiac (re)activity (pre-ejection period: PEP) and meta-
bolic brain activity (functional near infrared spectroscopy) during a cognitive task in young adults. Variants
of a flanker task involved different levels of inhibition control using a within-subject design and implied
neutral, congruent and incongruent conditions as well as conditions requiring a response (Go) or no response
(No-Go). Preliminary results showed that behavioral performance was significantly decreased when the re-
quired level of inhibitory control increased. PEP reactivity (the difference between PEP values during the task
and PEP values during the resting period) was significantly lower than 0 only during the first minute of each
experimental task condition (lasting about 4.5 minutes), going back to baseline level afterward. PEP reactivity
was most important during the most challenging Flanker Go/No-Go block. As a conclusion, PEP reactivity
was shown to be sensitive to different levels of inhibitory control requirement and to be a short lasting phe-
nomenon, demonstrating a possible rapid dynamic adaptation of the cardiac activity to task constraints.
1 INTRODUCTION
Maintaining optimal behavioral performance in
dynamic, complex and stressful situations is a
constant challenge. To better understand performance
fluctuations and prevent accidents, it is important to
have an integrated view of the cognitive, cerebral and
cardiac systems that control behavior and
physiological activity. However, these systems are
traditionally studied separately despite their strong
interdependence. Yet, a better understanding of the
fundamental mechanisms of the integrated
functioning of the central and peripheral nervous
system should ultimately allow the development of
new tools for promoting maximum cognitive
performance and safety in natural situations, such as
in civil or military aircraft.
Regarding the cognitive system, a key function
that allows adaptive behaviors and flexibility is
inhibition. It sustains the ability to stop, avoid or
ignore automatic, dominant or inappropriate
responses in certain situations and to focus attention
a
https://orcid.org/0000-0002-0601-2518
b
https://orcid.org/0000-0003-2642-0516
c
https://orcid.org/0000-0001-7743-4948
on relevant information (Miyake et al., 2000).
Behavioral paradigms allow to examine inhibition
ability such as in the Flanker task (Eriksen & Eriksen,
1974) or the Go/No-Go task (Heil et al., 2000).
Regarding the cerebral system, it is well known that
specific brain networks are activated in order to
support the processing of information during complex
tasks. In particular when tasks involve inhibition,
activated brain regions have notably been located in
the cingulate, prefrontal, and parietal cortices
(Collette et al. 2006). A technique for studying brain
activity is functional near infrared spectroscopy
(fNIRS). It makes it possible to noninvasively
monitor tissue oxygenation and hemodynamics of the
brain, particularly by monitoring the variations of
concentration in oxyhemoglobin and
deoxyhemoglobin. This brain imaging technique has
shown its interest in the evaluation of cerebral
metabolic activity, in particular according to
cognitive load in specific cortical regions (Fishburn
et al., 2014). Finally, regarding the cardiovascular
system, heart activity has been shown to adapt to
De Faria, C., Causse, M., Valéry, B. and Albinet, C.
Integrated View of the Cognitive, Cerebral and Cardiac Systems During an Inhibition Task.
DOI: 10.5220/0011946900003622
In Proceedings of the 1st International Conference on Cognitive Aircraft Systems (ICCAS 2022), pages 15-19
ISBN: 978-989-758-657-6
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
15

levels of complexity of a cognitive task, presumably
in order to support behavioral performance (Richter
et al., 2008). Cardiac activity is known to be
modulated by two branches of the autonomic nervous
system (ANS): the parasympathetic branch has an
inhibitory influence (decreases heart rate), while the
sympathetic branch has an excitatory influence
(increases heart rate) (Levy, 1990). Sympathetic
activity can be accurately evaluated by calculating the
cardiac pre-ejection period (PEP), which corresponds
to the time interval between the onset of ventricular
depolarization and the opening of the aortic valve
(Berntson et al., 1994). While still relatively new in
the field of cognitive neuroscience, PEP, as a marker
of the autonomic nervous system, has already been
used in studies on mental effort, where it was shown
that an increase in task difficulty resulted in a PEP
decrease (Richter et al., 2008; Silvestrini & Gendolla,
2013).
These three systems are thus essential to the
adaptive capacities of the individual to face the
demands of the environment. The link between
inhibition and the cardiovascular system (Kuipers et
al., 2016) and the link between inhibition and the
cerebral system (Herrmann et al., 2005) have been
studied in the past, but very few studies have
examined the three systems altogether. Our
understanding of their interactions or their integration
into a functional system is therefore very limited. The
aims of the present study are 1) to systematically
examine the way these three systems react to a
challenging task involving different levels of
inhibitory control and 2) to examine whether they are
functionally integrated to manage behavior
adaptation.
2 METHODS
2.1 Participants
Thirty young adults (M
age
= 20.23 ± 2.36; 15 females)
participated in the study and received a compensation
of 10€. They reported no neurological or
cardiovascular disorders. All participants had normal
or corrected vision. They all gave their written
consent at the beginning of the study, which was
approved by the local ethics committee (IRB - N°
00011835-2021-0928-418).
2.2 Measures
2.2.1 Behavioral Measures from the
Modified Flanker Task
The behavioral task is a modified version of the
Eriksen Flanker task (Eriksen & Eriksen, 1974; Heil
et al., 2000) involving neutral, congruent and
incongruent conditions as well as conditions
requiring a response (Go) or requiring no response
(No-Go). The modified Flanker task was presented on
a computer screen and the participant responded by
pressing one of the two keys on a response box. The
task consisted in responding as quickly and precisely
as possible to a central stimulus, the target, by
indicating the direction of the arrow (< or >) while
ignoring stimuli placed on either side of the target (>>
or << for the congruent and incongruent conditions,
or □□ for the neutral condition). The task was
organized around three experimental blocks
following training blocks. A first block, the “neutral
block”, involving only neutral trials (e.g., □□<□□)
corresponded to a choice reaction time task, involving
no or very little executive control. A second block,
the “flanker block”, corresponded to the classical
Flanker task with half congruent trials (e.g., <<<<<)
and half incongruent trials (e.g., <<><<). This design
allowed to assess interference management ability
(inhibition of irrelevant information) by comparing
performance on incongruent trials with that of
congruent trials. A third block, the “flanker no-go
block”, corresponded to the modified flanker task
with additional Go trials (70%) and No-Go trials
(30%) depending on the nature of a preparatory
signal. Each trial was preceded by a preparatory
signal (-----), which could be either of the same color
as the following target (Go trial) or of a different color
(No-Go trial). This allowed to evaluate the
interference management ability, but also the
response inhibition ability during No-Go trials
requiring to stop (inhibit) the response normally
expected. Thus, these three blocks differed in the
amount of inhibitory control necessary for their
successful execution. Each block lasted
approximately 4 minutes 30 seconds and was
repeated twice. The order of presentation of the
blocks was counterbalanced between the participants.
A 3-minute rest period was allowed between two
blocks to ensure a return to the baseline level of
cardiac activity (Czarnek et al., 2021). The dependent
variables were percentage of correct responses and
response time (RT) in ms for correct responses.
ICCAS 2022 - International Conference on Cognitive Aircraft Systems
16

2.2.2 Cardiovascular Measures
The measurement of cardiac activity was carried out
using the Biopac MP160 system at an acquisition
frequency of 2000 Hz. Once the training was finished,
the electrocardiogram (ECG) and impedance
cardiogram (ICG) electrodes were placed on neck and
torso of the participant. Blood pressure (BP)
measurements (Omron Carescape V100) were also
recorded during each rest period in order to monitor
BP evolution for the interpretation of ECG/ICG
signals (Sherwood et al., 1990). The data collected
were pre-processed on Matlab for ECG/ICG
measurements using an in-house tool. PEP was
calculated as the time interval between R-onset and
B-point (Sherwood et al., 1990). R-onset is defined as
the lowest deflection before R peak on the ECG
signal. R-peaks were found using a threshold peak
detection algorithm and visually inspected. The first
derivative of the ICG signal was computed and the
resulting dZ/dt signal was averaged over 1 minute
epochs. B-point is located based on the RZ interval
(Lozano et al., 2007). Resting PEP was calculated
over the 3 minute rest period. To examine the
dynamic of the cardiac activity during task blocks,
mean PEP in ms was calculated on 4 successive
windows of 1 minute. Dependent variables are mean
PEP in ms and PEP reactivity in ms (task PEP minus
resting PEP).
2.2.3 Cerebral Activity Measures
Cerebral hemodynamics was monitored by near
infrared spectroscopy using NIRScout system. A 16
sources and 14 detectors mapping was used, covering
the orbitofrontal cortex, the dorsolateral prefrontal
cortex, the inferior frontal gyrus, the supplementary
and pre-motor area and parts of the parietal cortex.
Eight short-channels were also used to remove
systemic physiological activity. fNIRS data was
processed using the BrainAnalyzIR toolbox (Santosa
et al., 2018). First, the raw data signal was converted
into optical density, then using the modified Beer-
Lambert Law, optical density data was converted into
oxyhemoglobin (HbO
2
) and deoxyhemoglobin (HHb)
concentrations. Then, a general linear model was used
to process the data, using the autoregressive
iteratively reweighted least squares (AR-IRLS)
model, and using the short-channels data as
regressors following the procedure recommended by
Santosa et al. (2020). Dependent variables were beta
values for HbO
2
and HHb.
Behavioral, ECG, ICG and NIRS data were
synchronously recorded throughout the experiment to
examine their concurrent evolution.
3 RESULTS
Data analysis is still ongoing at the moment of
submission of this abstract and thus not all results can
be presented here. Only PEP and behavioral results
will be presented and discussed.
3.1 Flanker Task Results
Overall, the percentage of correct responses was
significantly greater in the neutral block (M = 99.52
± 0.73) than in the flanker block (M = 98.07 ± 1.82),
which was higher than in the flanker no-go block (M
= 96.97 ± 1.83). Similarly, overall, RT significantly
differed between the three blocks. RT were lower for
the neutral block (M = 400.32 ± 58.24) comparing to
the flanker block (M = 474.16 ± 69.18) and the
flanker no-go block (M = 505.76 ± 82.82).
In the flanker block, mean RT of congruent trials
(M = 413.11 ± 45.94) was significantly lower than
mean RT of incongruent trials (M = 540.60 ± 97.36).
Also, the percentage of correct responses for
congruent trials (M = 99.65 ± 1.34) was significantly
higher than the one for incongruent trials (M = 92.63
± 7.13). Similarly, in the flanker no-go block, mean
RT of congruent trials (M = 440.95 ± 58.83) was
significantly lower than the one of incongruent trials
(M = 571.59 ± 120.96). Also, the percentage of
correct responses for congruent trials (M = 99.49 ±
1.95) was significantly higher than the one for
incongruent trials (M = 93.33 ± 8.01). Moreover, the
percentage of correct responses for Go trials, the
percentage of correctly answered trials, (M = 94.41
±6.56) was significantly higher than the one for No-
Go trials, percentage of correctly not answered trials,
(M = 88.61 ± 13.55).
3.2 PEP Results
For each task block, mean PEP of the first 1-minute
window was significantly lower than mean PEP for
the 3 other windows, which did not differ each other.
PEP was thus shorter during the first minute of the
task and then rapidly went back to baseline value and
stabilized at this level. Mean PEP during each resting
block varied from 113 ms to 115 ms and mean PEP
during each task block varied from 108 ms to 114 ms.
PEP reactivity calculated for the first 1-minute
window of each block was significantly different
from 0, indicating that task PEP was systematically
lower than resting PEP during the first minute of each
task. After that, PEP reactivity was not different from
0, except for w3 and w4 of the flanker block which
were significantly higher than 0. Comparison of the
Integrated View of the Cognitive, Cerebral and Cardiac Systems During an Inhibition Task
17
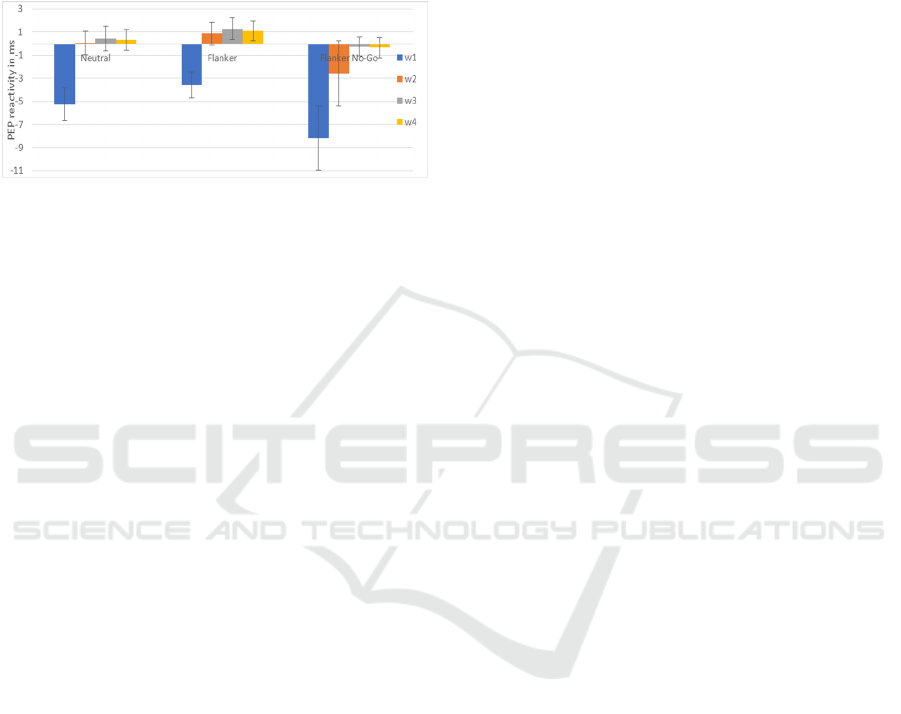
PEP reactivity of the first 1-minute window for each
block showed that while PEP reactivity for the flanker
No-Go block was significantly lower than the one for
the flanker block, PEP reactivity did not significantly
differ between the flanker No-Go block and the
neutral block or between the neutral block and the
flanker block.
Figure 1: PEP reactivity (in ms) for each block and each 1-
min window (w1, w2, w3 and w4) with standard error.
Larger negative PEP reactivity score reflects greater sym-
pathetic activation.
4 DISCUSSION
The preliminary results show that cognitive
performance decreased with the increase of the
amount of required inhibitory control in the task and
that PEP reactivity was significantly lower than 0 for
all block conditions, but only during the first minute.
These results partly agree with past research but may
highlight the rapid dynamic adaptation of the cardiac
activity to task constraints. The flanker no-go block,
which involves two kinds of inhibition (inhibition of
irrelevant information and response inhibition),
showed the most important PEP reactivity. This may
reflect that the increase of inhibitory control required
by the task generated an increase of sympathetic
activity to sustain effort and cognitive performance.
However, contrary to what was expected, this effect
on PEP reactivity was not linear, as the flanker block
had the lowest PEP reactivity. The next step is to
analyze the cerebral hemodynamic data as a function
of required inhibitory control and ultimately to
examine whether the variations in cardiac reactivity
and cerebral activity during the cognitive tasks are
functionally related and related to behavioral
performance. If they were actually functionally
connected, the integration of these dynamical cardiac
and cerebral markers into an online control system
could be used to detect and alert for performance and
attention fluctuations in pilot activity.
REFERENCES
Berntson, G. G., Cacioppo, J. T., Binkley, P. F., Uchino, B.
N., Quigley, K. S., & Fieldstone, A. (1994). Autonomic
cardiac control. III. Psychological stress and cardiac re-
sponse in autonomic space as revealed by pharmacolog-
ical blockades. Psychophysiology, 31(6), 599-608.
https://doi.org/10.1111/j.1469-8986.1994.tb02352.x
Collette, F., Hogge, M., Salmon, E., & Van der Linden, M.
(2006). Exploration of the neural substrates of execu-
tive functioning by functional neuroimaging. Neurosci-
ence, 139(1), 209-221. https://doi.org/10.1016/j.neuro-
science.2005.05.035
Czarnek, G., Richter, M., & Strojny, P. (2021). Cardiac
sympathetic activity during recovery as an indicator of
sympathetic activity during task performance. Psycho-
physiology, 58(2), e13724. https://doi.org/10.1111/
psyp.13724
Eriksen, B. A., & Eriksen, C. W. (1974). Effects of noise
letters upon the identification of a target letter in a
nonsearch task. Perception & Psychophysics, 16(1),
143-149. https://doi.org/10.3758/BF03203267
Fishburn, F., Norr, M., Medvedev, A., & Vaidya, C. (2014).
Sensitivity of fNIRS to cognitive state and load. Fron-
tiers in Human Neuroscience, 8. https://www.fron-
tiersin.org/article/10.3389/fnhum.2014.00076
Heil, M., Osman, A., Wiegelmann, J., Rolke, B., & Hen-
nighausen, E. (2000). N200 in the Eriksen-Task: Inhib-
itory Executive Processes? Journal of Psychophysiol-
ogy, 14(4), 218-225. https://doi.org/10.1027//0269-
8803.14.4.218
Herrmann, M. J., Plichta, M. M., Ehlis, A.-C., & Fallgatter,
A. J. (2005). Optical topography during a Go–NoGo
task assessed with multi-channel near-infrared spec-
troscopy. Behavioural Brain Research, 160(1),
135-140. https://doi.org/10.1016/j.bbr.2004.11.032
Kuipers, M., Richter, M., Scheepers, D., Immink, M., Sjak-
Shie, E., & van Steenbergen, H. (2016). How effortful
is cognitive control? Insights from a novel method
measuring single-trial evoked beta-adrenergic cardiac
reactivity. International Journal of Psychophysiology.
https://doi.org/10.1016/j.ijpsycho.2016.10.007
Levy, M. N. (1990). Autonomic Interactions in Cardiac
Control. Annals of the New York Academy of Sciences,
601(1 Electrocardio), 209-221. https://doi.org/10.1111/
j.1749-6632.1990.tb37302.x
Lozano, D. L., Norman, G., Knox, D., Wood, B. L., Miller,
B. D., Emery, C. F., & Berntson, G. G. (2007). Where
to B in dZ/dt. Psychophysiology, 44(1). https://doi.org/
10.1111/j.1469-8986.2006.00468.x
Miyake, A., Friedman, N. P., Emerson, M. J., Witzki, A. H.,
Howerter, A., & Wager, T. D. (2000). The unity and
diversity of executive functions and their contributions
to complex « Frontal Lobe » tasks: A latent variable
analysis. Cognitive Psychology,
41(1), 49-100.
https://doi.org/10.1006/cogp.1999.0734
Richter, M., Friedrich, A., & Gendolla, G. H. E. (2008).
Task difficulty effects on cardiac activity. Psychophys-
iology, 45(5), 869-875. https://doi.org/10.1111/j.1469-
8986.2008.00688.x
ICCAS 2022 - International Conference on Cognitive Aircraft Systems
18

Santosa, H., Zhai, X., Fishburn, F., & Huppert, T. (2018).
The NIRS Brain AnalyzIR Toolbox. Algorithms, 11(5),
73. https://doi.org/10.3390/a11050073
Santosa, H., Zhai, X., Fishburn, F., Sparto, P. J., & Huppert,
T. J. (2020). Quantitative comparison of correction
techniques for removing systemic physiological signal
in functional near-infrared spectroscopy studies. Neu-
rophotonics, 7(3), 035009. https://doi.org/10.1117/
1.NPh.7.3.035009
Sherwood, A., Allen, M. T., Fahrenberg, J., Kelsey, R. M.,
Lovallo, W. R., & Doornen, L. J. P. van. (1990). Meth-
odological Guidelines for Impedance Cardiography.
Psychophysiology, 27(1), 1-23. https://doi.org/10.1111/
j.1469-8986.1990.tb02171.x
Silvestrini, N., & Gendolla, G. H. E. (2013). Automatic ef-
fort mobilization and the principle of resource conser-
vation: One can only prime the possible and justified.
Journal of Personality and Social Psychology, 104(5),
803-816. https://doi.org/10.1037/a0031995
Integrated View of the Cognitive, Cerebral and Cardiac Systems During an Inhibition Task
19
