
Evaluation Comparison of the Effectiveness of Full Dose Pfizer
Vaccine with Pfizer Booster Society in Indonesia
Marthius Putra Yehezkie and Diana Laila Ramatillah
Faculty Pharmacy, Universitas 17 Agustus 1945 Jakarta, Indonesia
Keywords: Vaccine Effectiveness, the Complete Dose of Pfizer, Pfizer Booster, and Society in Indonesia.
Abstract: Efforts to socialize vaccination programs to achieve herd immunity worldwide are increasingly being
carried out. The vaccination program is aimed at all people in the world, especially in Indonesia. In addition
to full-dose vaccination, now booster vaccination is also shown to all people in the world, especially in
Indonesia. The Indonesian government provides several types of full-dose vaccines as well as boosters, one
of which is Pfizer. This study was conducted to evaluate the effectiveness comparison between the complete
dose of Pfizer vaccine and Pfizer Booster in the community in Indonesia. This type of research is
observational with a prospective invitational method. This study involved 600 respondents, of which 300
respondents were Pfizer boosters and 300 respondents were Pfizer complete doses. In this study, there were
372 males and 228 females. A significant correlation was found between the type of vaccine with side
effects, age with side effects, and BMI with side effects. The effectiveness of each vaccine was 99.3% for
the Pfizer booster and 99.1% Pfizer full dose vaccine. From this study, it can be concluded Pfizer booster
more effective compare to full dose Pfizer vaccine.
1 INTRODUCTION
In late 2019, the coronavirus 2019 (COVID-19)
outbreak became a public health threat to people
worldwide. The lower respiratory tract is the main
target for severe respiratory disease (SARS-CoV-2)
infections (Lee et al., 2020) It was recorded on the
official Covid- 19 website regarding the
Development of Covid-19 Cases in Indonesia in the
March 2022 update, and there were 5,630,096
confirmed positive patients and 4,944,237 recovered
patients, and 149,036 deaths. Caused by
Coronavirus Disease 2019 (COVID19) Severe Acute
Respiratory Syndrome Coronavirus 2 (SARSCoV2)
has been declared a new pandemic by the World
Health Organization (WHO) ‘(Anka et al., 2021).
Vaccines are a way to control outbreaks of infectious
diseases and reduce the risk of pandemics and
epidemics. The sooner the vaccine is distributed, the
sooner the outbreak can be brought under control
‘(Excler et al., 2021). The Ministry of Health of the
Republic of Indonesia provides six types of
clinically passed Covid-19 vaccines for people in
Indonesia that can be used by people in Indonesia,
namely Sinovac, Astrazeneca, Moderna, Pfizer,
Sinopharm, Janssen.
The first Covid 19 vaccine approved by the
world's
regulatory agencies to prevent respiratory
syndrome
coronavirus 2 (SARSCov2) is a comirnaty
vaccine developed by Pfizer and BioNTech
‘(Nittner-Marszalska et al., 2021). The Pfizer-
Biotech Covid-19 vaccine is an mRNA vaccine in
which lipid nanoparticles are modified utilizing
nucleosides. Encoding the spike glycoprotein of
SARS-CoV-2, the cause of the coronavirus disease
(COVID19) (Sutardi and Ramatillah, 2022)
The Pfizer-BioNTech (BNT162b2) vaccine is an
mRNA- vaccine containing modified lipid
nanoparticles made using a nucleoside, which
encodes the SARS-CoV-2 spike glycoprotein and is a
coronavirus disease (coronavirus disease) (Sutardi
and Ramatillah, 2022). According to research by The
New England journal of medicine on Safety and
Efficacy of the BNT162b2 mRNA Covid-19
Vaccine. The efficacy of the Pfizer Vaccine against
COVID-19 is 95% (Polack et al., 2020).
Acquired Herd Immunity is formed at the
individual level by natural infection by pathogens or
by vaccines. Several current clinical trials are
underway to evaluate new vaccine candidates and
drug replacement strategies for preventing and
treating SARS-CoV-2 disease. However, it is
206
Yehezkiel, M. and Ramatillah, D.
Evaluation Comparison of the Effectiveness of Full Dose Pfizer Vaccine with Pfizer Booster Society in Indonesia.
DOI: 10.5220/0011978800003582
In Proceedings of the 3rd International Seminar and Call for Paper (ISCP) UTA â
˘
A
´
Z45 Jakarta (ISCP UTA’45 Jakarta 2022), pages 206-212
ISBN: 978-989-758-654-5; ISSN: 2828-853X
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

unclear whether these studies will result in effective
interventions, and although the optimistic estimate
of each vaccine study is at least 12-18, these studies
are considered effective ‘(Randolph and Barreiro,
2020).
1.1 Covid-19
Coronavirus (CoV) is part of the Coronaviridae
family. Coronavirus (COVID19) began to spread in
December 2019 and was detected in early January
2020. The virus began to spread in China from mid-
January to late January. The virus spreads in the host
and clinically shows several symptoms, such as the
common cold to respiratory infections ‘(Randolph
and Barreiro, 2020). Covid-19 is an RNA infection
in the form of a royal attribute, having a diameter of
approximately 60- 140 nm. It is transmitted through
inhalation, namely coughing and sneezing. The
Covid-19 virus enters the human nasal system by
inhalation and begins to replicate. The main receptor
for the Covid-19 virus is ACE2. Then the virus starts
to spread with a limited immune response and can be
detected with a feeling of discomfort in the nose.
The COVID-19 virus then spreads and reaches the
human respiratory tract, and the virus faces a more
robust innate immune response. Most Covid-19
patients are elderly/elderly patients, such as lung
disease, hypertension, malignant tumors, coronary
heart disease, and chronic kidney disease
(Ramatillah and Isnaini, 2021).
The Covid-19 virus belongs to the category of
betacoronavirus viruses, such as MERS-CoV and
SARS-CoV-1. The virus comes from animals,
namely bats. The patients in the United States were
similar to those obtained in China, suggesting the
possible emergence of this new virus from animal
reservoirs. There were reports that several pets had
previously tested positive for SARS-CoV-2 in
several countries such as Hong Kong, Belgium, and
New York City zoos (López, de Casas Moreno and
Mera, 2021)
The Covid-19 virus attacks the immunity of
humans. Immunity in humans is the best fortress.
The individual's immune system is divided into three
categories, namely native immunity (quickly),
adaptive immunity (long term), and passive
immunity. The human immune system can detect
and support antibodies, defending against viruses or
invading germs. However, when it first attacks the
body, the immune system cannot work correctly, and
we become sick. Precisely what happened like
Covid-19 (Ramatillah and Isnaini, 2021)
1.2 Symptom
Symptoms in patients with COVID-19 are similar to
Middle East Respiratory Syndrome (MERS),
starting from 2-14 days of exposure to SARS-CoV-2.
Causes such as high body temperature, fatigue, and
phlegm. In a sick person found, there are symptoms
such as weakness, flu, inflammation, and excretion.
These causes are usually easy and start slowly. Some
patients contracted SARS- CoV-2 infection but did
not show any reason and did not feel unwell(López,
de Casas Moreno and Mera, 2021)
A retrospective analysis was performed on
patients in Jinan and Rizhao, Shandong Province,
from January 23 to February 15. There were 53 cases,
including 67 cases with an average age of 41.47 years
(range 21-65 years), and 26 cases (49.1%) were men.
Most cases were mild (8 cases, 15.1%) and
conventional (44 cases, 83%), with only one severe
case (1.9%). The most common symptoms at the
beginning of the illness were fever (60.4%), dry
cough (54.7%), phlegm (37.7%), pharyngitis
(35.8%), and fatigue (32.1%). Less common
symptoms were headache (20.8%), anorexia
(15.1%), myalgia (13.2%), chest pain, (11.3%),
nausea (5.7%), diarrhea (3 .8%), dizziness
(3.8%), and vomiting (1.9%) (Asaduzzaman et al.,
2020).
1.3 Vaccine
Vaccination against (SARS-CoV-2) remains the
main hope in controlling the pandemic (COVID-
19). (WHO) Approves COVID19 Vaccine
Emergency Use List (EUL) contains (mRNA) Pfizer
BNT162b2. BioNTech (Pfizer, Inc; Philadelphia,
PA, USA) and Moderna mRNA vaccine 1273
(MODER) naTX, Inc.; Cambridge, Mass. USA);
Viral vector vaccines (AstraZeneca, Cambridge,
UK) and Janssen Ad26.COV2.S (Janssen Biotech,
Inc; a pharmaceutical company owned by Janssen,
Johnson & Johnson; New Brunswick, NJ, USA);
and
Sinopharm, Inactivated Virus Vaccine (China
National Pharmaceutical Group) and Sinovac
(Sinovac Biotech Ltd.; ‘(Al-Awwal et al., 2022)
AntiCoV2 vaccine was developed in a much shorter
time than previous vaccinations. Previous
vaccinations took 8 to 10 years for human use, while
the antiSARSCoV2 vaccine took 8 to 10 months
(Han et al., 2020)
Most Covid-19 vaccines need to be given in two
doses at intervals of 3-12 weeks to provide sufficient
immunity for recipients of the Janssen vaccine,
currently used as a single dose. The SARSCoV2
Evaluation Comparison of the Effectiveness of Full Dose Pfizer Vaccine with Pfizer Booster Society in Indonesia
207
spike protein mRNA vaccine was retrieved and
transcribed after being injected into host cells. It
produces spike proteins, is presented to B and T cells
on the cell surface, and induces an immune
response. The vaccines used are already weakened
germs, so they do not cause illness but create
immunity (Al-Awwal et al., 2022)
There are four types of vaccines, namely mRNA
vaccines developed by PfizerBioNTech and
Moderna. RNA and DNA vaccines are closer to
using RNA or DNA made as if it were safe to build
immune capabilities—vector vaccine virus
(adenovirus) developed by AstraZeneca, Johnson &
Johnson, Litera, Sputnik. Viral vector vaccines do
not cause disease but use genetically modified
viruses that detect viral germs to capture immunity
better. Inactivated viral immunization was
developed by Sinovac (Han et al., 2020.)
2 MATERIALS AND METHODS
2.1 Design
This study used a prospective cross-sectional study to
evaluate the comparative effectiveness of the Pfizer
full-dose and Pfizer booster vaccines in the
Indonesian population. A validated questionnaire
was used to collect the data. For the sampling
technique using convenience, the sampling follows
the inclusion criteria. Inclusion criteria are people in
Indonesia over 17 years who have received a total
dose of Pfizer vaccine or Pfizer Booster and are
willing to participate in the study. The exclusion
criteria were all Indonesians over 18 years of age
suffering from cancer, autoimmune diseases,
HIV/AIDS patients, pregnant women, and
Indonesians who only followed the 1st dose of the
Pfizer vaccine.
2.2 Participant
Participants in this study were all people in
Indonesia aged over 17 years who had been
vaccinated with a total dose of Pfizer or Pfizer
booster with a total of 600 respondents.
2.3 Data Analysis
The results obtained were analyzed using the SPSS
version 25 application. Fisher, Chi-square, Mann-
Whitney, and Kruskal-Walis tests were used to find
the relationship between risk factors (gender, age,
BMI, vaccine type) and side effects. A p-value of
0.05 was considered significant.
2.4 Ethical Approval
Ethical approval was obtained prior to conducting
the study. The ethical approval was sourced from the
health research ethics committee of the Universitas
17 Agustus 1945, Jakarta, with the approval letter
No.38/KEPK- UTA45JKT/EC/EXP/07/2022.
3 RESULTS AND DISCUSSION
3.1 Prevalence of Participants by
Domicile
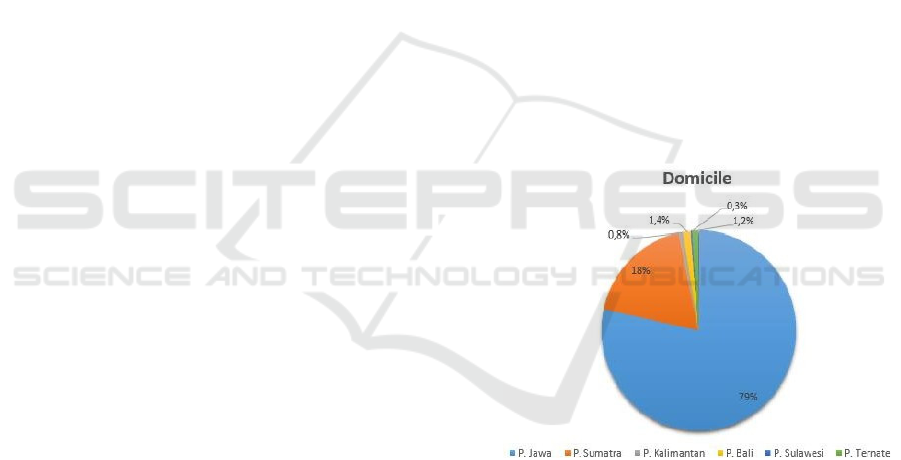
The study this conducted in Indonesia with a
percentage domicile for each respondent 79% from
the island of Java, 18% from the island of Sumatra,
0.8% from the island of Kalimantan, 1.4%from the
island of Bali, 0.3% from the island of Sulawesi and
1.2% from the island of Ternate (figure 1). Domicile
is not one of the factors affecting vaccine
effectiveness and side effects.
Figure 1: Domicile.
3.2 Prevalence of Participants by
Comorbid
Figure 2 shows that from a total of 600 respondents,
each of the 300 respondents who received the total
dose of Pfizer vaccine and 300 respondents who
received the Pfizer booster, the percentage of
complications from the total was 0.3% diabetes,
0.3% hypertension, and 99.4%. Without
comorbidities.
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
208

Figure 2 : Comorbidities
3.3 Relationship Between Types of
Vaccines and Side Effects
From the fisher test results, where the variable type
of vaccine as categorical data was analyzed with
side effects questions as other categorical data, the
results are as presented in table 1.
Significant results were obtained between the
type of vaccine and the side effects experienced by
vaccine participants after receiving the full dose of
Pfizer vaccine and Pfizer Booster. The results
showed that the Pfizer booster vaccine had more
side effects than the complete dose of the Pfizer
vaccine. It is known that from a total of 300
respondents who received the full dose of the
vaccine, 18% felt side effects such as fever, 32% felt
pain in the injection area, 8.6%.
Experienced diarrhea, 17% felt dizzy, 23% felt
drowsy after the vaccine, and 20.6% felt pain in the
upper arm. In contrast, in the other 300 respondents
who received the booster vaccine, 42.6% felt fever,
74% felt pain in the injection area, 4 .3% felt
diarrhea, 37.3% felt dizzy, and 45.6 felt drowsiness,
and 54% felt pain in the upper arm. In addition to
the type of vaccine, another variable that has a
significant correlation with the side effects that
appear is age.
3.4 Correlation Between BMI and
Vaccine Side Effects
Variables of age, side effects, and the efficacy of
each vaccine also have a significant correlation. In
this study, the range of respondents aged 17-66 years
with an average of 27 years. The details of the data
are presented in table 2.
From the results of the Man-Whitney test for
questions about side effects after the vaccine and test
results. Kruskal Wallis for questions regarding
monitoring side effects after 1-3 months after
receiving the vaccine.
3.5 Relationship Between Age and Side
Effects
The last variable that correlates with side effects and
efficacy is BMI. The average BMI obtained is 23.7,
shown in table 3.
Table 1: Relationship between types of vaccines and side effects.
Variable
Percentage %
p-value
Complete Dose (n=300)
Boosters
(n=300)
Fever after vaccine 54/18 128/42.6 0.000
Painful in area injection after vaccine 97/32.3 222/74 0.000
Diarrhea after vaccine
Dizzy after vaccine
Feel sleepiness after vaccine
Painful in arm bag. on after vaccine
26/8.6
51/17
69/23
62/20,6
13/4.3
112/37.3
137/45.6
162/54
0.020
0.000
0.000
0.000
Evaluation Comparison of the Effectiveness of Full Dose Pfizer Vaccine with Pfizer Booster Society in Indonesia
209

Table 2: Relationship between age and side effects.
Variable
Percentage
p-value
Age (n=600 mean – average=27)
Effect side pain in the area
injection after vaccine
319/52.1 0.006
Infection Covid after 3
month vaccination
8/1.3 0.001
Monitoring change
cycle menstruation 1-3 month
after vaccine
29/4.7 0.011
Monitoring easy feel
tired 2-3 months after
vaccine
49/7.9 0.013
Monitoring painful in arm
2-3 month after vaccine
94/15.3 0.017
Monitoring easy
dehydration after 2-3 month
after vaccine
78/12.7 0.006
*Man-Whitney test, Kruskal Wallis test
Table 3 : Correlation between BMI and vaccine side effects
Variable
Percentage
p-value
BMI (n=600 mean=23,7)
Monitoring fatigue after 1-3 month vaccine
47/7.6 0.001
Monitoring pain in arm after 1-3 month vaccine
52/6.4 0.000
Monitoring easy ehydration after 1-3 month vaccine
49/7.9 0.000
*Kruskal Wallis Test
4 DISCUSSION
4.1 Efficacy and Side Effects
From the results of the study, it was found that
people in Indonesia aged 17 years and over who
received the complete dose of Pfizer and Pfizer
booster vaccines, recipients of Pfizer booster vaccine
were higher than the Pfizer complete dose vaccine
levels of 99.3% for the Pfizer booster and
99.1 % for the full Pfizer dose. The results
showed that the sponsoring group had the option of
concurrently building up the level of the counter-
acting agent seen after two doses, implying that the
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
210

decline in resistance after the third part would be
slower even though a shortfall of time had passed to
decide if the problem was.
Preliminary studies also show that antibody
quality is higher after getting a booster. This
immune system continues to create antibodies at
specific sites and results in the experience of
infection or immunization and exhibits a broader and
stronger immune response after the third dose. The
body's defense system has a second line of
protection in T cells to fight off infected cells.
Therefore, if antibodies are insufficient to prevent
contamination, T cells can intervene to control the
disease that previously contributed to the
disease/disease (Andrews et al., 2022).
Table 1 shows that the Pfizer booster vaccine has
more side effects than the Pfizer whole dose vaccine.
This is supported by the p-value of the variable,
which shows the number <0.005. Some significant
side effects were obtained, such as fever, pain in the
injection site, diarrhea after the vaccine, dizziness,
feeling drowsy, and pain in the upper arm. This is
because the booster vaccine uses a larger dose.
4.2 Correlation Between Age and Side
Effects
As shown in Table 2, age impacts vaccine side
effects. The results of this study indicate that the
average age of the vaccine participants is 27 years,
and several side effects arise, namely pain in the
area of the injection site, changes in the menstrual
cycle in women, easy feeling tired, and easy
dehydration. According to research in Saudi Arabia,
differences in the immune system at age greatly
affect the side effects after the vaccine, where the
immune system of younger people is stronger and
more efficient than the immune system of older
people(Alamer et al., 2021)
4.3 Correlation Between BMI and Side
Effects
As shown in table 3, BMI impacts vaccine side
effects. The results showed several side effects such
as fatigue, arm pain, and dehydration. It is known
that people with a high BMI due to obesity are
overweight and easily feel tired to carry out their
activities. According to research in Saudi Arabia, the
vaccine's side effects can be exhausting because the
vaccine has worked and reacted on the immune
system (El-Shitany et al., 2021).
The next side effect is pain in the arm. From
writing in Iran various aspects can encourage pain
after injection such as the size of the needle and the
length of the injection needle used. It is known that
people with a high BMI have excess fat.
The following side effect is that you feel thirsty
after
the vaccine. It is known that people with a high
BMI require a lot of body fluids than people with a
normal BMI. According to another study, lymph
nodes and adenoids become active after a booster
vaccine, which causes thirst.
5 CONCLUSION
From the writing that has been done, it can be
observed that the effectiveness of the Pfizer booster
vaccine is higher than the Pfizer full dose vaccine,
which is 99.3% for the Pfizer booster vaccine and
99.1% for the Pfizer complete dose vaccine.
However, these two types of vaccines have a fairly
high level of efficacy for people in Indonesia. For
side effects, a significant correlation was found,
indicating that the Pfizer booster vaccine had higher
side effects than the Pfizer full dose vaccine. While
the side effects that
REFERENCES
Al-Awwal, N. et al. (2022) ‘A Review of SARS-CoV-2
Disease (COVID-19): Pandemic in Our Time’,
Pathogens, 11(3), pp. 1–14. Available at:
https://doi.org/10.3390/pathogens11030368.
Alamer, E. et al. (2021) ‘Side effects of covid-19 pfizer-
biontech mrna vaccine in children aged 12–18 years in
saudi arabia’, Vaccines, 9(11), pp. 1–11. Available at:
https://doi.org/10.3390/vaccines9111297.
Andrews, N. et al. (2022) ‘Effectiveness of COVID-19
booster vaccines against COVID-19-related
symptoms, hospitalization and death in England’,
Nature Medicine, 28(4), pp. 831–837. Available at:
https://doi.org/10.1038/s41591-022-01699-1.
Anka, A.U. et al. (2021) ‘Coronavirus disease 2019
(COVID-19): An overview of the immunopathology,
serological diagnosis and management’, Scandinavian
Journal of Immunology, 93(4), pp. 1–12. Available at:
https://doi.org/10.1111/sji.12998.
Asaduzzaman, M. et al. (2020) ‘Since January 2020
Elsevier has created a COVID-19 resource centre with
free information in English and Mandarin on the novel
coronavirus COVID- 19 . The COVID-19 resource
centre is hosted on Elsevier Connect , the company ’ s
public news and information ’, PLoS ONE,
39(January), pp. 667–677. Available at:
http://dx.doi.org/10.1371/journal.pone.0239254.
El-Shitany, N.A. et al. (2021) ‘Minor to moderate side
effects of pfizer-biontech COVID-19 vaccine among
Evaluation Comparison of the Effectiveness of Full Dose Pfizer Vaccine with Pfizer Booster Society in Indonesia
211

saudi residents: A retrospective cross-sectional study’,
International Journal of General Medicine, 14, pp.
1389–1401. Available at:
https://doi.org/10.2147/IJGM.S310497.
Excler, J.L. et al. (2021) ‘Vaccine development for
emerging infectious diseases’, Nature Medicine, 27(4),
pp. 591–600. Available at:
https://doi.org/10.1038/s41591-021-01301-0.
Han, Y. nan et al. (2020) ‘A comparative-descriptive
analysis of clinical characteristics in 2019-
coronavirus-infected children and adults’, Journal of
Medical Virology, 92(9), pp. 1596–1602. Available at:
https://doi.org/10.1002/jmv.25835.
Lee, P.I. et al. (2020) ‘Are children less susceptible to
COVID-19?’, Journal of Microbiology, Immunology
and Infection, 53(3), pp. 371–372. Available at:
https://doi.org/10.1016/j.jmii.2020.02.011.
López, A.M., de Casas Moreno, P. and Mera, J.M.B.
(2021) ‘Informative treatment and media
competencies on COVID-19 in Ecuador’, Revista de
Comunicacion, 20(1), pp. 137–152. Available at:
https://doi.org/10.26441/RC20.1-2021-A8.
Menni, C. et al. (no date) ‘Prep rin ot p ee r r we d Pr ep t
n p r r’.
Nittner-Marszalska, M. et al. (2021) ‘Pfizer-biontech
COVID-19 vaccine tolerance in allergic versus non-
allergic individuals’, Vaccines, 9(6), pp. 1–8.
Available at: https://doi.org/10.3390/vaccines9060553.
Polack, F.P. et al. (2020) ‘Safety and Efficacy of the
BNT162b2 mRNA Covid-19 Vaccine’, New England
Journal of Medicine, 383(27), pp. 2603–2615.
Available at: https://doi.org/10.1056/nejmoa2034577.
Ramatillah, D.L. and Isnaini, S. (2021) ‘Treatment profiles
and clinical outcomes of COVID-19 patients at private
hospital in Jakarta’, PLoS ONE, 16(4 April), pp. 1–11.
Available at:
https://doi.org/10.1371/journal.pone.0250147.
Randolph, H.E. and Barreiro, L.B. (2020) ‘Herd
immunity: Understanding Covid-19 by Haley etal’,
Immunity, 52(January), pp. 737–741.
Sutardi, A.Q.I. and Ramatillah, D.L. (2022) ‘Evaluation
Comparison Between Sinovac and Pfizer Vaccine
Among Indonesian Children and Teenager Under 18
Years Old’, International Journal of Applied
Pharmaceutics, 14(Special issue 2), pp. 22–30.
Available at:
https://doi.org/10.22159/ijap.2022.v14s2.44745.
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
212
