
Evaluation Moderna Booster Vaccine Recipient Questionnaire for
Health Workers in Pharmacies and Hospitals Among Indonesian
Citizens
Seftian Putra Dwijaya Marpaung and Diana Laila Ramatillah
Faculty Pharmacy, Universitas 17 Agustus 1945 Jakarta, Indonesia
Keywords: Booster Vaccine, Moderna, Pharmacy/Clinical Health Workers, Hospital/Public Health center Health
Workeers
Abstract: Evaluating the compparison of the effectivennes and adverse effect following immunization of the Moderna
boster vaccine against Health workers in Hospital and Pharmaciesin Indonesia and other factors that influence
it. This study was a cross-sectional observational study using convenience sampling in 603 respondents
working as hospital workers and pharmacists in Indonesia vaccinated with the Moderna booster vaccine, data
analysis using SPSS version 25 and Cronbac'h I am using the result with the alpha of 0.600. It was found that
the efficacy of the Moderna Booster Vaccine on Hospital/Public health center Health Workers and
Pharmacy/Clinic Health Workers (87.42% and 96.4%). Other factors that influence adverse effects following
immunizations and vaccine efficacy are gender, age, and BMI (Body Mass Index) with a p-value of each
variable < 0.05. The study data show that the efficacy of Moderna booster vaccines varies in the mode of
action. The data show that the efficacy of the latest vaccine for HCWs working in hospitals/abscesses is 96.4%
higher than the efficacy of the latest vaccine for HCWs working in pharmacies/clinics. Common adverse
effects following immunizations after the Moderna booster injection are arm pain, fever, flu, and cough, but
the adverse effect following immunizations of nausea is more common in health workers working in
pharmacies/clinics 18.27%, compared to health workers working in hospitals/health centers 11.92%.
1 INTRODUCTION
The Novel Coronavirus (SARS-CoV-2) is spreading
from Wuhan city Hubei Province, China to the whole
corner of the world. Coronavirus is a sense RNA virus
positive with starting diameter from 60 nm to 140 nm
with a bulge-like nail on the surface that makes it look
like a crown below a microscope electron (Singhal,
2020). As of May 30, 2022 (11.13 GMT+7) total
Covid- 19 cases recorded worldwide have reached
more than 531 million cases soul with Dead more than
6.3 million souls. Vaccines could stimulate system
immunity receivers to prevent or reduce the
possibility of infection-exposed pathogens. The
vaccine is an attenuated virus, vaccine uses
pathogenic strains that have been weakened to become
non-infectious in non-human tissues, which are then
injected into the recipient and are called by the
immunity body.
With 6 different vaccines currently in use in
Indonesia, Moderna's vaccine has high efficacy, 94-
95% compared to other vaccines already in Indonesia,
making Moderna one of the best vaccines. (Araminda
& Ramatillah, 2022a). The high death toll associated
with Covid-19 is common in frontline energy
medicine to combat Covid-19. Therefore, medical
personnel will be prioritized for her Covid-19 vaccine
booster (Klugar et al., 2021). The Ministry of Health
of the Republic of Indonesia provides a Covid-19
booster vaccine that has passed clinical, which has
could be used for whole power health both those on
duty at pharmacies, clinics, health centers, and homes
Sick that is Moderna Booster Vaccine (Tenzin &
Dorji, 2020).
The US Food and Drug Administration (FDA)
and the European Medicines Agency (EMA) first
approved booster vaccines, they are in " group
priority " (parents 65 years old to top, people with
disease attendant, officer health, and travel. (Overseas
people) Residents of the " region risky high " will
accept the first booster dose (dose third) between
December 24 and 31, 2021. NITAG (National
Immunization Technical Advisory Groups)
Marpaung, S. and Ramatillah, D.
Evaluation Moderna Booster Vaccine Recipient Questionnaire for Health Workers in Pharmacies and Hospitals Among Indonesian Citizens.
DOI: 10.5220/0011979100003582
In Proceedings of the 3rd International Seminar and Call for Paper (ISCP) UTA â
˘
A
´
Z45 Jakarta (ISCP UTA’45 Jakarta 2022), pages 225-231
ISBN: 978-989-758-654-5; ISSN: 2828-853X
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
225

recommends gift mRNA vaccine (Pfizer or Moderna)
for individual booster immunization heterologous. As
of January 1, 2022, about 170,000 people (93% of
group priority) get booster injections(Naully et al.,
2022)
Moderna booster It is a -based messenger RNA
(mRNA). The vaccine with packaged in lipid
nanoparticles (NP), material viral genetic material
(mRNA), in which a spike glycoprotein can give code
on the body that causes sticks to the cells host. That's
what causes the body to build immunity against the
SARS-CoV2 (S) virus, Ingredients vaccine Moderna
are ribonucleic acid (mRNA), and fats consisting of
(SM- 102, Polyethyleneglycol(PEG)2000,
dimyristoyl glycerol(DMG), Cholesterol, 1,2-
Distearoylsn-glycerol-3-phosphocholine(DSPC),
tromethamine, Tromehamin hydrochloride,
acid acetate, sodium acetate, and sucrose (6).
Due to amount cases that have increasingly been
exposed to Covid 19 increase so is the amount of
power health workers exposed to COVID-19.
University of Oxford Medical Evidence Center
(Driggin et al. 2020) states that 13.8% of cases
positive is worker key main in-service health and
sector other. On April 16, 2019, the number of
workers with key critical positive- increased to
16.2%. This has a significant impact on the load -of
work and stress of frontline workers main, the
weakened ability of system care health to handle
problems. Situation this implication is whether you
are serious to Keep going increase the amount of
power health infected with COVID-19. Remember
risk to personal, and professional health is often
considered Required to do it. Obligation this set in the
guiding Code of Ethics for professional health.
However, system-effective health- doesn’t only
depend on the service and skills of professional
medical like doctors and nurses. But it also depends
on the service professional other (Nyashanu et al.,
2020). Although the related data with efficacy and
adverse effect following immunizations about
Moderna booster vaccine already there is. Study
clinical more carry on required about comparison
evaluation Among power health work at home sick
and those at the clinic/pharmacy in the workforce
receiving medical Moderna booster vaccine with
seeing various other aspects such as possible
sociodemographics existence linkages with efficacy
nor adverse effect following immunization from
vaccines used.
2 MATERIALS AND METHODS
2.1 Design
This study used a prospective cross-sectional study to
evaluate the comparison of efficacy and adverse
effect following immunizations between health
workers working in hospitals/Public health centers
and health workers at pharmacies/clinics using a
questionnaire. This research was conducted for 3
months (September – November). For the sampling
technique using convenience, sampling through all
items that make up the research inclusive criteria.
Inclusion criteria were all hospital health
workers/health centers and pharmacy/clinic health
workers in Indonesia who had carried out the
Moderna Booster Vaccination and were willing to
give informed consent to be included in the study.
Exclusion criteria were all Hospital/Public health
center health workers and Pharmacy/Clinical health
personnel who had not performed the Moderna
booster vaccination, Indonesian people suffering
from cancer (stages 3&4), HIV/AIDS sufferers, TB
patients, autoimmune patients (Lupus patients).
2.2 Participants
The participants in this study were all Hospital/Public
health center health personnel and Pharmacy/Clinic
health workers who had been vaccinated with the
Moderna booster with a total of 600 respondents, and
adverse events. A p-value of 0.05 was considered
significant.
2.3 Instruments
This study uses a questionnaire distributed through
social media (WhatsApp, Twitter, Facebook,
Instagram, and Telegram). The number of
questionnaires in this study was 50 questions on
identity and comorbidities. The 50 questions were
about the adverse effect following immunizations
received after the first and second doses of
vaccination in the short and long term, as well as
monitoring the adverse effect following
immunizations of the vaccine for 1-6 months after
being vaccinated.
2.4 Statistical Analysis
The collected results were analyzed using the SPSS
version 25 application. Fisher, Chi-square, Mann-
Whitney, and Kruskal-Wallis tests were used to find
associations between risk factors (gender, age, BMI,
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
226

Negative
Positive
vaccine type) and adverse events. A p-value of 0.05
was considered significant.
2.5 Ethical Approval
As stated in Figure 1, ethical approval was obtained
before conducting the research from the health
research ethics committee of the University of 17
August 1945 Jakarta, with approval
letterNo.53/KEPK-UTA45JKT/EC/EXP/08/2022
3 RESULT AND DISCUSSION
3.1 Prevalence of Participants by
Domicile
This research was conducted spread throughout
Indonesia with the percentage of domicile of each
respondent 1.16% from West Sumatra Province,
3.48% from South Sumatra Province, 4.81% from
North Sumatra Province, 22.06% from DKI Jakarta,
Yogyakarta province, 0.17% came from Malaysia,
36.48% came from West Java province, 9.95% came
Figure 1: Prevalence of participants by domicile.
Figure 2: Vaccine Recipient .
3.81% from the Province of Kalimantan, 0.33% came
from Sulawesi Province, 1.66% came from from
Central Java province, 15.75% came from East Java
province, and 0.33% came from the province of Bali,
as shown in figure 1. Domicile is not one of the factors
that influence the adverse effect following
immunizations and vaccine efficacy, this can be seen
from the absence of significance between these two
varieties.
3.2 Vaccine Recipient and Presentation
of Positive Covid - 19
The relationship between the Moderna booster
vaccination status carried out on health workers
working in hospitals/health centers and
pharmacy/clinic health workers with the incidence of
Covid-19 shows that the analysis of the data that has
been obtained includes health workers in
hospitals/health centers who are more exposed to
covid after being hospitalized. Vaccination compared
to pharmacy/clinic health workers ‘(Pratama et al.,
2022) which in hospitals/public health centers as
many as 12.58% (n = 302) have been confirmed
positive for Covid-19, while in pharmacies or clinics
as many as 3.6% (n = 301) have been confirmed
positive for Covid-19. This is because health workers
who work in hospitals and health centers directly
interact with Covid- 19 patients so they are more
susceptible to being exposed to Covid-19 than health
workers who work in pharmacies/clinics (Pharmd,
2021).
Figure 3: Presentation of Positive Covid – 19.
Presentation of positive covid-19
120.%
100%
80%
96.34%
87.41%
60%
40%
20%
12.6%
3.65%
0%
Pharmacy Hospital Healt/
Health/ Clinic Public Health Workcare
Evaluation Moderna Booster Vaccine Recipient Questionnaire for Health Workers in Pharmacies and Hospitals Among Indonesian Citizens
227
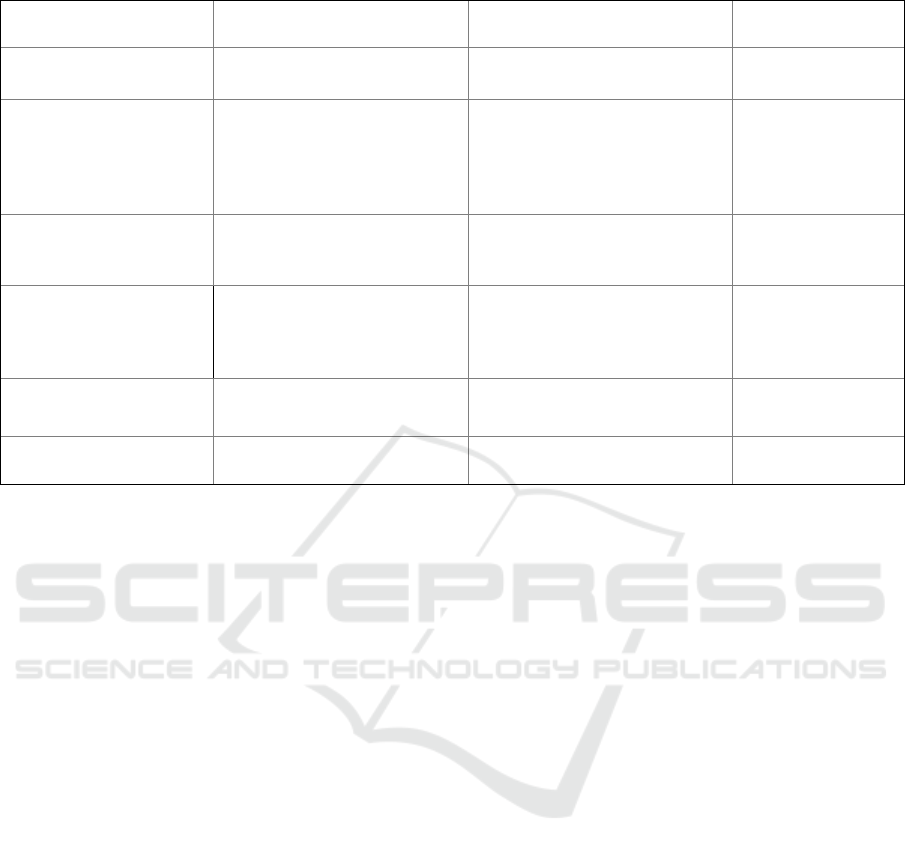
Table 1: Association between Work Place with Covid Status.
Factor Hospital/Health Care
(n=302)
Pharmacy/Clinic (n = 301) p-value
Positive Covid-19 Yes = 38
No = 264
Yes = 11
No = 290
0.0001
*
Symptoms No Symptoms = 16
Mild Symptoms = 25
Moderate Symptoms = 6
Severe Symptoms = 1
N
ever Ge
t
Infected = 254
No Symptoms = 4
Mild Symptoms = 8
Moderate Symptoms = 1
Severe Symptoms = 1
N
ever Ge
t
Infected = 287
0.0001
#
Increase D- Dimer Yes = 6
No = 46
N
ever Ge
t
Infected = 250
Yes = 1
No = 21
N
ever Ge
t
Infected = 279
0.0001
#
Hospitalized Home Isolation = 35
Hospital Non- ICU = 5
Hospital ICU = 1
N
ever Ge
t
Infected = 261
Home Isolation = 13
Hospital Non- ICU = 0
Hospital ICU= 1
N
ever Ge
t
Infected = 287
0.0001
#
Positive Covid-19 (1
to 3 months)
Yes = 20
No = 282
Yes = 4
No = 297
0.0001
*
Positive Covid-19 (4
to 6 months)
Yes = 15
No = 287
Yes = 5
No = 296
0.038 *
*
= Chi-squared,
#
= Fisher's Exact
3.3 Association Between WorkPlace
with Covid Status
The results of the weekly morbidity and mortality
report on the effectiveness of the third dose of the
Moderna booster vaccine show that
immunocompromised adults received the third dose
of the mRNA vaccine 28 days after the second dose
and the currently recommended third dose (booster) 5
months after the second dose. Emphasizes the
importance of people immunocompromised adults
who are immunocompromised (10). COVID-19
booster vaccine was introduced in the UK on
September 14, 2021. Of course, only, using proof
from COV- BOOST studies showing that mRNA
vaccines have a powerful booster effect with
reactivity low miss from vaccine main given, English
for immunizations and immunizations The Joint
Committee recommends BNT162b2 or health centers
and health workers in pharmacies or clinics, are fever
(55.6% and 56.14%), and pain in the injection area
(75.8% and 77.4%, Cough (13.9% and 13.2%), Flu
(17.8% and 18.9%), Nausea (11.9% and 18.2%),
Headache (41% and 48.5%), Drowsiness (44.3% and
43.5), Bleeding (1.6% and 1.6), the upper shoulder
joint (64.2% and 62.4%), Diarrhea (5.9% and 5.3%)
or vaccine half dose (50g) mRNA-1273 (Moderna) as
a booster dose in 6 months after finishing the series
vaccination main. (Andrews et al., 2022). From a
comparative study of symptoms and antibody
response after administration of the Moderna vaccine
or SARS-CoV-2 Pfizer, it was found that the antibody
response decreased at 6 months, an 84% reduction in
Moderna but still higher than in convalescent donors.
Antibody response did not correlate with gender or
symptom severity (Kelliher et al., 2022).
In the study of absorption of booster doses of
COVID-19 among residents of Saudi Arabia, the
results of the occurrence of adverse effects following
immunizations at the second dose (p<0.01), COVID-
19 infection (p=0.006), the severity of COVID-19
symptoms (p<0.01), had COVID-19 after receiving
both doses (p<0.01) ), and the type of presenting
symptom (p = 0.028) were significantly associated
with decreased absorption of the COVID-19 booster
dose (Kelliher et al., 2022) the most common adverse
effect following immunizations that occur after
Moderna booster vaccination, with a comparison of
health workers in hospitals.
3.4 Correlation Between WorkPlace
with Adverse Effects Following
Immunizations
According to the WHO, a common adverse effect
following immunizations after vaccination is
injection site pain, fever, fatigue, headache, muscle
aches,
chills, diarrhea, and a less common adverse
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
228
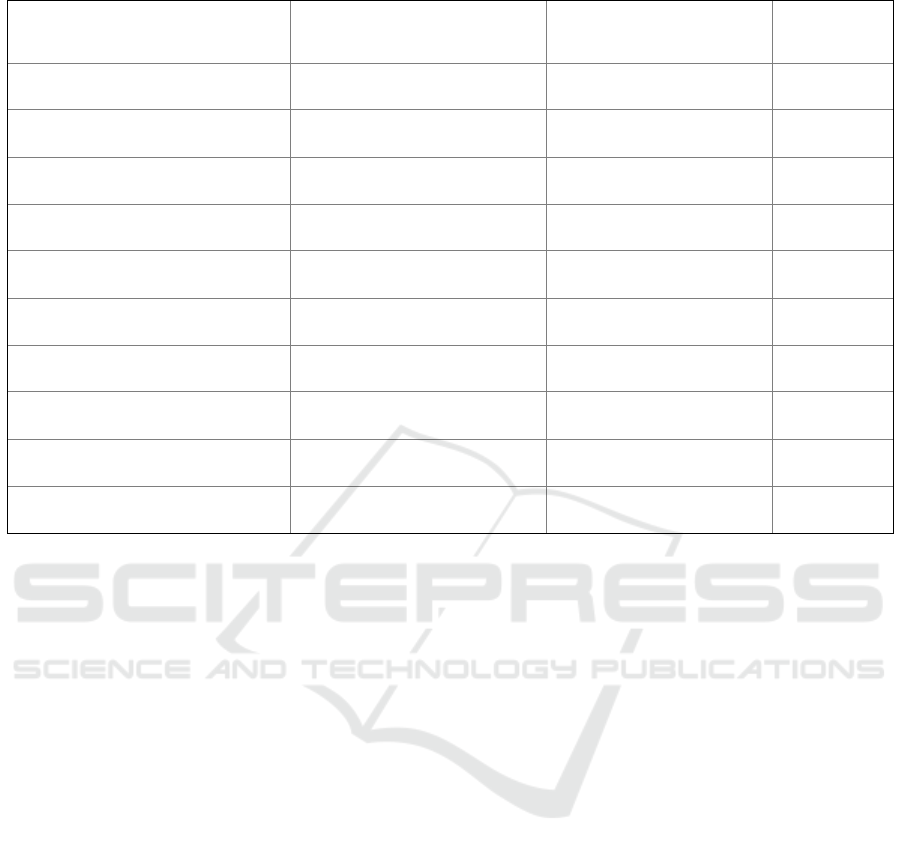
Table 2: Correlation between Workplace with Adverse effects following immunizations.
Adverse effects following
immunizations
Hospital/Healthh Care (n =
302)
Pharmacy/Clinic (n =
301)
p-value
Fever Yes = 168
N
o = 134
Yes = 169
N
o = 132
0.935 *
Pain at Injection Yes = 229
N
o = 73
Yes = 233
N
o = 68
0.701 *
Cough Yes = 42
N
o = 260
Yes = 40
N
o = 261
0.906 *
Flu Yes = 54
N
o = 248
Yes = 57
N
o = 244
0.754 *
Nausea Yes = 36
N
o = 266
Yes = 55
N
o = 246
0.031 *
Headache
Yes = 124
N
o = 178
Yes = 146
N
o = 155
0.072 *
Sleepy Yes = 134
N
o = 168
Yes = 131
N
o = 170
0.870 *
Bleeding Yes = 5
N
o = 297
Yes = 5
N
o = 297
1,000 *
Upper arm Yes = 194
N
o = 108
Yes = 188
N
o = 133
0.673 *
Pain In area Injection Yes = 18
N
o = 284
Yes = 16
N
o = 285
0.860 *
effect following immunization is a severe allergic
reaction (14). In a study conducted in the United
States, the adverse effect following immunizations of
the Moderna vaccine was fever (35.65%), headache
(59.26%), arm pain (94.21%), nausea (26.62%), and
chest pain (1.85%) ‘(Araminda & Ramatillah,
2022b).
The most commonly reported adverse effect
following immunizations of the Moderna booster
vaccine include injection site pain, headache, malaise,
muscle aches, malaise, chills, arthralgia, mucosal
lesions, oral paresthesias, taste disturbances, itching,
rash, itchy mouth and throat, and throat blockage.
reported, muscle cramps, loss of appetite, poor sleep
quality, diarrhea, hot flashes, nasal congestion, and
difficulty breathing. Local reactions at the injection
site, such as urticaria and urticarial rash, have also
been reported (Ananth et al.,2021)
In several other studies, adverse effects following
immunizations were more common in the Moderna
group after the first and second doses. Mild pain at the
injection site is common and lasts about 3 days.
However, delayed injection site reactions such as
erythema, tenderness, and induration are rare and
generally resolve within 4-5 days. Fatigue, myalgia,
arthralgia, and headache increased after the second
dose of the Moderna vaccine and lasted for
approximately 3 days. The adverse event rate in the
Moderna arm was independent of age and had no
sequelae.
In 18 other studies, adverse effects following
immunizations included localized axillary swelling or
tenderness ipsilateral to the injection site and
generalized rash (Francis et al., 2022). In a study of
adverse effects following immunizations of Moderna
vaccines in Bangladesh, it was stated that the most
common were pain at the injection site (97%), fever
(91%), headache (68.29%), and redness/swelling at
the injection site (70%) ‘(Mahmudetal.,2022) From a
study evaluating the prevalence of adverse effects
following immunizations associated with booster
doses of mRNA-based Covid-19 vaccines among
health workers in the Eastern Province, Saudi Arabia.
Get results from study ie, 81. 84% (401/490) of
professionals participating in health report at least one
effect side after accepting of COVID-19 vaccine. 3
(1-4) effects side reported (median (IQR)), (88. 73%)
reported, effect most frequent side reported is the sick
head (28.68%), painful joints or bone (27.18%),
myalgia (26.43%), nausea or vomiting (21.2%), fever
(18.95%), and rash (10.22%), whereas effect the
rarest side reported is redness the place injections
(8.23%), chills (7.98%), fatigue (7.73%), and
swelling the place injection. delayed menstruation
(Ali et al., 2022)
In the COVID-19 phase 4 vaccine candidate
study, the effectiveness of SARS- CoV-2 variants,
Evaluation Moderna Booster Vaccine Recipient Questionnaire for Health Workers in Pharmacies and Hospitals Among Indonesian Citizens
229

neutralizing antibodies, rare adverse effect following
immunizations, and traditional and nano-based
vaccine platforms were demonstrated by the
participant who accept two doses of mRNA-1273 28
days after the dose first. All participants were -shaped
into three groups (n = 15) according to doses of 25
g,100 g, or 250 g (Jackson et al. 2020). Response
more antibodies strongly reported(Naully et al., 2022)
after the dose first vaccine, 5 participants (33%) in the
25-gram group, 10 participants (67%) in the 100-gram
group, and 8 participants (53%) in the 250-gram
group reported effects of side light or medium. After
the dose second, 7 of 13 (54%) participants were in a
group of 25 g, 15 of 15 (100%) participants in the 100
g group, and the g group in a group of 250 grams.
Three participants in the 250-gram group reported one
or more effect serious side. For fever, no some
participants experienced fever after the dose first
(Zarreen et al., 2022)
4 CONCLUSION
The research data shows that there are differences in
the effectiveness of the Moderna booster vaccine in
the typpe of work; where in the data shows the
effectiveness of Moderna vaccine is found to be
higher in health workers working in hospitals/health
centers by 96.4% compred to the effectiveness of
Moderna vaccine in health workers who work in
Pharmacies/clinic by 87.43%. The adverse effect
following immunization nausea is the highest for
health workers who work in
pharmacies/clinics,which is 18.27%,compered to
health workers who work in hospital/helath centers by
11.92%.
REFERENCES
Ali, M. D., Almadan, L. Z., Alghamdi, R. A., Alghamdi, A.
S., Almarhoon, S. A., Hassan, Y. A. M., Ahmad, A.,
Ghosn, S. A., & Banu, N. (2022). Evaluation of
Prevalence of Side-Effects Associated with Booster
Dose of mRNA-Based COVID-19 Vaccine Among
Healthcare Workers in Eastern Province , Saudi
Arabia : A Descriptive Cross-Sectional Study. August,
4335–4346.
Ananth, R., Kadali, K., Janagama, R., Peruru, S., Gajula,
V., Madathala, R. R., Chennaiahgari, N., & Malayala,
S. V. (2021). Non ‐ life ‐ threatening adverse effects
with COVID ‐ 19 mRNA ‐ 1273 vaccine : A randomized
, cross ‐ sectional study on healthcare workers with
detailed self ‐ reported symptoms. March, 4420–4429.
https://doi.org/10.1002/jmv.26996
Andrews, N., Stowe, J., Kirsebom, F., Toffa, S., Sachdeva,
R., Gower, C., Ramsay, M., & Lopez Bernal, J. (2022).
Effectiveness of COVID-19 booster vaccines against
COVID-19-related symptoms, hospitalization and
death in England. Nature Medicine, 28(4), 831–837.
https://doi.org/10.1038/s41591-022-01699-1
Araminda, G. N., & Ramatillah, D. L. (2022a). Evaluation
Comparison Between Astrazeneca and Moderna
Vaccine’S Side Effects and Efficacy Among Indonesia
Society Based on Sociodemography. International
Journal of Applied Pharmaceutics, 14(Special Issue 2),
37–43. https://doi.org/10.22159/ijap.2022.v14s2.44747
Araminda, G. N., & Ramatillah, D. L. (2022b). Evaluation
Comparison Between Astrazeneca and Moderna
Vaccine’S Side Effects and Efficacy Among Indonesia
Society Based on Sociodemography. International
Journal of Applied Pharmaceutics, 14(Special Issue 2),
37–43. https://doi.org/10.22159/ijap.2022.v14s2.44747
Francis, A. I., Ghany, S., Gilkes, T., & Umakanthan, S.
(2022). Review of COVID- 19 vaccine subtypes ,
efficacy and geographical distributions. 389–394.
https://doi.org/10.1136/postgradmedj-2021-140654
Kelliher, M. T., Levy, J. J., Nerenz, R. D., Poore, B.,
Johnston, A. A., Rogers, A. R., Stella, M. E. O., Snow,
S. E., Cervinski, M. A., & Hubbard, J. A. (2022).
Comparison of Symptoms and Antibody Response
Following Administration of Moderna or Pfizer SARS-
CoV-2 Vaccines. 146(June).
https://doi.org/10.5858/arpa.2021-0607-SA
Klugar, M., Riad, A., Mohanan, L., & Pokorná, A. (2021).
COVID-19 vaccine booster hesitancy (VBH) of
healthcare workers in czechia: National cross-sectional
study. Vaccines, 9(12), 1–29.
https://doi.org/10.3390/vaccines9121437
Mahmud, S., Mian, A. U., Hasan, P., Muyeed, A., Ali, T.,
Ahmed, F. F., Islam, A., & Maliha, M. (2022). Side
Effects of COVID-19 Vaccines and Perceptions about
COVID-19 and Its Vaccines in Bangladesh. February
2021.
Naully, P. G., Jenderal, U., & Yani, A. (2022). 4 th
International Seminar on Global Health. October 2021.
Nyashanu, M., Pfende, F., & Ekpenyong, M. (2020).
Exploring the challenges faced by frontline workers in
health and social care amid the COVID-19 pandemic :
experiences of frontline workers in the English
Midlands region , UK ABSTRACT. Journal of
Interprofessional Care, 34(5), 655–661.
https://doi.org/10.1080/13561820.2020.1792425
Pharmd, W. V. (2021). SARS-CoV-2 vaccine breakthrough
infections among healthcare workers in a large Belgian
hospital network. 9, 1755–1757.
https://doi.org/10.1101/2021.05.22.21257658.7.
Pratama, T. D., Hasbie, N. F., Farich, A., & Yulyani, V.
(2022). Analisis faktor yang berhubungan dengan
kejadian infeksi Covid-19 pada tenaga kesehatan.
16(2), 172–181.
Singhal, T. (2020). A Review of Coronavirus Disease-2019
(COVID-19). Indian Journal of Pediatrics, 87(4), 281–
286. https://doi.org/10.1007/s12098-020-03263-6
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
230

Tenzin, S., & Dorji, T. (2020). Since January 2020 Elsevier
has created a COVID-19 resource centre with free
information in English and Mandarin on the novel
coronavirus COVID- 19 . The COVID-19 resource
centre is hosted on Elsevier Connect , the company ’ s
public news and information . January.
Zarreen, F., Dibyangshee, S., & Ramneet, S. (2022).
COVID - 19 phase 4 vaccine candidates , effectiveness
on SARS - CoV - 2 variants , neutralizing antibody ,
rare side effects , traditional and nano - based vaccine
platforms : a review. 3 Biotech, 12(1), 1–30.
https://doi.org/10.1007/s13205-021-03076-0
Evaluation Moderna Booster Vaccine Recipient Questionnaire for Health Workers in Pharmacies and Hospitals Among Indonesian Citizens
231
