
Evaluation of AEFI (Adverse Events Following Immunization)
Efficacy of Sinovac Vaccination Among Children Aged 6-11 Years in
Indonesia
Nuril Islami and Diana Laila Rahmatillah
Faculty of Pharmacy, Universitas 17 Agustus 1945 Jakarta, Indonesia
Keywords: COVID-19, Sinovac, AEFI (Adverse Events Following Immunization), Efficacy, Children Aged 6-11 Years.
Abstract: Although children are rarely diagnosed in the early days of COVID-19, vaccination of children is still carried
out to achieve herd immunity status in the world. The Indonesian government urges the use of the Sinovac
vaccine for children aged 6-11 years. This study was conducted to evaluate AEFI and the efficacy of the
Sinovac vaccination among children aged 6-11 years in Indonesia and other factors that influence these things.
456 participants who fulfilled the inclusion criteria were involved. This study uses the prospective cross-
sectional method. A valid questionnaire was used in this study with Cronbach alpha 0,8. Those participants
filled out the google form which was shared through social media. It was found that males 194 and females
262. A significant correlation was found between AEFI and Efficacy with gender, comorbidities, age, and
BMI (p value<0,05). 63,04% of participants had pain in the injection area and 44,88% of them had pain in the
upper arm after vaccination. Around 1,8% of participants were infected by Covid-19. From this study, it can
be concluded that Sinovac vaccination has high efficacy and most of them had pain in the injection area event
following immunization as adverse.
1 INTRODUCTION
Coronavirus is part of family Coronaviridae. They
spread amongst many hosts. Clinically, it provides a
diffusion of signs of breathing infections, from the
commonplace bloodless to severe and often even
fatal. The brand-new virus chargeable for this
outbreak turned into originally named "2019-ncov"
or "SARS-cov-2". Sars-cov-2 has recently been
carefully related to SARS-CoV, which has 80%
identification in the RNA sequence (Ramatillah &
Isnaini, 2021). The virus spreads through the air,
could be very easily transmitted between human
beings with a long and threatening incubation
duration, and spreads quickly.
Severe acute respiratory syndrome Coronavirus
2 (SARS-CoV-2), a new coronavirus, was first
detected in the city of Wuhan, China in December
2019 (Dhar et al., 2020). The virus spread to the
sector from China, prompting the sector fitness
agency (WHO) to claim an endemic on March 11,
2020. The national index case was introduced on
March 2nd, traced from a restaurant in South
Jakarta. Nine months later, extra than 7,000 new
cases and more than one hundred fifty each day
deaths had been stated as a seven-day moving
average in Indonesia, without signs and symptoms
of slowing or bending the curve (Ophinni et al.,
2020).
As for children, they are diagnosed exceptionally
not often at the start of the presence of Covid-19
inside the global. To start with, children have been
considered less prone to Covid-19. In a report
through Wu and mcgoogan2, which recorded
instances from December eight to February 20, 2020,
1% of youngsters and 1% of kids under 10 years of
age in China had been affected. Other researchers
said that the hazard of serious illness and death from
Covid-19 turned higher inside the older age
institution. However, more and more young human
beings are being identified because of the sickness
pandemic's progress and the fast Development of
Covid-19 checking out techniques. These days we
recognize that younger humans can also be critically
affected by SARS-cov-2 (Levy et al., 2021). Based
totally on the analysis of Covid-19 statistics on April
10, 2022, the wide variety of Covid-19 sufferers who
died at the age of 3-6 years is said to have been 131
232
Islami, N. and Rahmatillah, D.
Evaluation of AEFI (Adverse Events Following Immunization) Efficacy of Sinovac Vaccination Among Children Aged 6-11 Years in Indonesia.
DOI: 10.5220/0011979200003582
In Proceedings of the 3rd International Seminar and Call for Paper (ISCP) UTA â
˘
A
´
Z45 Jakarta (ISCP UTA’45 Jakarta 2022), pages 232-243
ISBN: 978-989-758-654-5; ISSN: 2828-853X
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

sufferers (0.16%) at the same time as they were aged
elderly 7-12 years had been 246 sufferers (0.14%)
(Per, 2022).
To grow the immunity of kids elderly 6-11
towards publicity to the Covid-19 virus, the
Indonesian government is vaccinating kids of that
age (Kementerian Pendidikan Dan Kebudayaan »
Republik Indonesia, n.d.). The vaccine presently in
use is of the Sinovac type and already has an
Emergency Use Authorization (EUA) (Vaksinasi
COVID-19 Untuk Anak Usia 6-11 Tahun Dimulai 14
Desember – Sehat Negeriku, n.d.). Similarly, the
Indonesian Technical Advisory Group on
Immunization (ITAGI) has also made hints for
implementing the Covid-19 vaccination for
youngsters aged 6-eleven.
The Corona Vac vaccine from Sinovac Biotech in
China is an inactivated SARS cov-2 virus and uses
aluminum hydroxide as an excipient. The maximum
common AEFI from the Covid-19 vaccination is
nearby pain on injection, followed with the aid of
malaise. There is no association between the
occurrence of AEFI symptoms and gender. Both
ladies and men have the same chance of experiencing
symptoms of AEFI. We observed some rare
symptoms which have been suggested, including
drowsiness and dysphagia. The immune response
may additionally play a function in the signs and
symptoms of dysphagia after vaccination (Supangat
et al., 2021).
Although the third clinical trial for the Sinovac
vaccine has been conducted using numerous
international locations thus far in Brazil, Turkey,
Indonesia, and Chile, there are nonetheless minimum
facts on AEFI (Adverse Events After Immunization)
and the efficacy related to the Sinovac vaccine for
children aged 6-11 years (Empat Negara Berbagi
Pengalaman Uji Klinis Tahap-3 Vaksin Sinovac,
n.d.). Based on these matters, further clinical
research to evaluate AEFI (Adverse Events After
Immunization) and the efficacy of the Sinovac
vaccination among children aged 6-11 years in
Indonesia is enormously wished. This takes a look at
becoming conducted with the aid of searching at
diverse elements along with sociodemography that
may link AEFI (Adverse Events Following
Immunization) and the efficacy of the Sinovac
vaccine in youngsters elderly 6-11 years in
Indonesia.
On December 31, 2019, the WHO China country
workplace received records of approximately a case
of pneumonia of unknown etiology detected in
Wuhan (Hubei Province of China), which would
later be considered the center of the spread of SARS-
cov-2. The emergence of Covid-19 is presently
already the third excessive epidemic resulting from -
cov in people during the last many years, after
excessive Acute respiration Syndrome (SARS) and
center East Respiratory Syndrome (MERS), in 2002
and 2012.
The pathogenesis of Covid-19 is complex.
However, it could be defined conceptually using
traditional models for three predominant
pathological methods associated with irritation of the
local manifestations of classical (canonical) general
irritation, acute systemic inflammation, and
continual systemic infection of low depth
(Matsuzawa et al., 2022).
The destructive impact of Covid-19 during the
last year has led to a worldwide attempt to build herd
or network immunity, starting with immunity on the
individual degree to boost the population stage. Viral
immunity is mediated by immunological memory,
which develops after the number one immune
response is generated to viral antigens. The natural
immunity of SARS-cov-2 can increase from previous
infections; this could result in a speedy and powerful
immune response, accordingly, defending the
devotee. However, the presence and length of SARS-
cov-2- precise immune memory cells that offer
dependable protecting immunity in individuals with
beyond infections is still poorly understood.
Statistics concerning these responses can be useful in
figuring out whether received immunity will
successfully contribute to the Development of herd
immunity (Mistry et al., 2019).
As of August 29, 2020, it was expected that about
15.8 in keeping with 100,000 children ages 0-4 and
9.2 consistent with 100,000 in those elderly5- 17
years were hospitalized within the U.S. due to Covid-
19 virus infection. From these records, it is regarded
that there are fewer youngsters hospitalized because
of Covid-19 virus infection, but this still exceeds the
pre-vaccine technology hospitalization burden from
different viruses that can currently be averted via
vaccination. About a third of the kids hospitalized
with Covid-19 positive, 80% of them have the
multisystem inflammatory syndrome in kids (MIS-
C) and are admitted to extensive care devices (ICU).
Further, the deaths of youngsters from Covid-19 had
been no longer expected to be close to 110-188
deaths (Qona et al., 2022).
Some of the corona viruses can cause respiratory
infections in humans ranging from mild symptoms
such as coughs and colds to more serious symptoms.
Symptoms of COVID-19 are usually mild and appear
gradually. some infected individuals may be
asymptomatic and still feel well (Ramatillah et al.,
Evaluation of AEFI (Adverse Events Following Immunization) Efficacy of Sinovac Vaccination Among Children Aged 6-11 Years in
Indonesia
233

2021). People who get infected may also experience
asymptomatic viral shedding or fever, fatigue,
myalgia, arthralgia, rhinorrhea, sore throat, and/or
conjunctivitis at one point of the spectrum. But it can
also progress to fever, cough, hemoptysis, silent
hypoxia, chest pain or tenderness, respiratory failure,
or even multiorgan failure. Olfactory (hyposmia,
anosmia, and parosmia) or taste (dysgeusia)
problems have been identified as important
chemosensory problems in Covid-19. Non-
conductive olfactory disorder (OD) may be the only
manifestation. Different extrapulmonary
manifestations consist of diarrhea, lymphopenia,
thrombocytopenia, impaired liver and kidney
function, rhabdomyolysis, meningoencephalitis,
stroke, seizures, Guillain-Barré syndrome, cardiac
arrhythmias or heart block, pancreatitis, multisystem
vasculitis including Kawasaki disease, rash or
lesions including have a cold. chills,
thromboembolism, and acute thyroiditis.
Six months after the onset of symptoms of Covid-
19, more than 60% of the patients were observed to
have persistent symptoms of fatigue or muscle
weakness. Sleep problems (26%), anxiety, or
melancholy (23%) are also not uncommon. Other
symptoms namely disturbances in smell or taste,
palpitations, joint pain, dizziness, diarrhea, vomiting,
and chest pain, a series of signs known as "Covid-19
acute-delivery syndrome". Those with severe
diseases need assisted breathing to overcome
impaired diffusion from the lungs.
In general, children have shorter and milder
illnesses than adults. However, at some point in the
healing phase of the disorder, an extraordinary but
life-threatening Kawasaki-like disease is seen, called
early life multisystem inflammatory syndrome (MIS-
C) or pediatric inflammatory syndrome transiently
associated with SARS-cov-2. Children with MIS-C
tend to be older, have fewer lymphocytes and
platelets, and have higher CRP and ferritin levels
than children with Kawasaki disease (Kai-Wang To
et al., 2021).
Research for the expansion of a robust vaccine
for SARS-cov-2 began early in the Covid-19
pandemic (Rashedi et al., 2022). Several vaccines
have been developed to reduce the morbidity and
mortality associated with Covid-19 and prevent
transmission of the virus (Eid et al., 2021). More than
70 SARS-cov-2 vaccines developed from the unique
vaccine platform, including inactivated whole
manufacture virions, live attenuated viruses, nucleic
acid viruses, viral vectors, and recombinant S
proteins, are already in clinical trials (Kai-Wang To
et al., 2021).
Active immunization can produce herd immunity
and expanding the newly formulated and approved
Covid-19 vaccine using the US Food and Drug
Administration (FDA) is being rolled out. Covid-19
vaccines, such as messenger RNA (mRNA; Pfizer-
BioNTech Comirnaty - BNT162b2 & Moderna -
mrna-1273), protein-based (Novavax – NVX-
cov2373), and viral vector-based (Johnson &
Johnson Janssen - Ad26.COV2. S , Oxford-
AstraZeneca - AZD1222/chadox1, Sputnik V
vaccine - gamcovid-Vac- rad26/rad5), mainly goal
spike protein (S), while inactivated conventional
vaccines (Sinopharm-BBIBP-corv, Sinovac-
coronavac, Covaxin - BBV152) targets the complete
virus (Mistry et al., 2019).
Nine standard options can help guide whether a
Covid-19 vaccine for children should be mandated.
The criteria can be divided into three classes: four
criteria related to vaccines, 2 related to disease, and
3 related to implementation. Typically, each of these
criteria will be considered in determining whether a
vaccine should be mandated for children, although
those given for each criterion may be exceptional. At
a time of great demand for public wellness, together
with the current pandemic, it is proposed that every
criterion remains in vaccine policymaking. However,
five criteria should be prioritized. The criteria that
should be prioritized over relaxation are paramount:
there must be evidence that the Covid-19 vaccine is
safe for children at an acceptable stage of risk.
Assembly of these standards usually requires a pre-
licensed safety process and data from license-issuing
studies to reveal facet results after the vaccine has
been administered to more than one human. 4
different standards want to be prioritized in
considering whether the Covid-19 vaccine is
mandatory for young people. First, the severity of the
Covid-19 disorder must be large and sufficient, at
least for a portion of the population (the fifth
criterion). Second, vaccinating children must reduce
the threat of disease transmission (criterion 6). The
basis of a necessary vaccine policy regarding
children is that a higher proportion of those who are
immune saves you from harming others.
This criterion, rather than the role of children in
the transmission of SARS-cov-2 characters to
individuals, is still lacking. 1/3, the Covid-19 vaccine
must also be effective in protecting children from
disease (second criterion). It doesn't need to be 100%
strong, similar to other vaccines we currently order
for children. Fourth, because the benefits of the
Covid-19 vaccine will mostly be felt by adults who
are too threatened, not children, they should not feel
lazy for children; or, in place of, the parent or mother
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
234

or father of the child, to comply with the vaccine
mandate (criterion 9). This means that mandated
vaccines must be widely available, easy on the hands,
and inexpensive to anyone (Medical Association,
2020).
Protein-based vaccines or inactivated vaccines
are currently used repeatedly in children in general
with added aluminum salts. Picovacc, also called
coronavac, is an alum-adjuvanted inactivated virus
vaccine (SARS-cov-2 pressure CN2) that produces
neutralizing antibodies extensively in rodents and
non-human primates and demonstrates safety in
established doses in demanding situations (Gao et al.,
2020).
AEFI can be classified as Serious Adverse Events
(SAE), which are events that require hospitalization,
endanger the patient (cause a risk of death and
require immediate clinical intervention to prevent
death), and cause significant and/or permanent
dysfunction. Disability, resulting in congenital
abnormalities or causing death; or Non-Serious
Adverse Events (NSAE), i.e., all events that do not
meet the SAE criteria. Any serious, undesirable, or
unexpected signs or symptoms that manifest in
individuals who have received any type of
immunobiologist are considered AEFI and can be
caused by several factors related to the
immunobiological component, the vaccination
process, or people who have been vaccinated (da
Silva et al., 2021).
Clinical trials of vaccines before distribution
permission received crucial information
approximately AEFI and due to the fact Covid-19
vaccines were advanced the usage of new
technology, publish-advertising surveillance has
become critical to detecting uncommon or lengthy-
time period side consequences (Kant et al., 2022; Tan
et al., 2022). Most of the discovered AEFI had been
expected and associated with local reactions at the
site of vaccine administration (Živanović et al.,
2021). AEFI, which was recorded right away after
the injection of the vaccine, couldn't expressly
display a causal dating c (Gianfredi et al., 2021).
Non-allergic AEFI that is regularly related to the
covid-19 vaccine usually appears to be the same as
other existing vaccines (Kim et al., 2021).
Mechanically, most AEFI is caused by using organ
hypersensitive reactions or immune responses to
vaccines (Fu et al., 2021). Participants who had
Covid-19 (confirmed with a positive test)
experienced one or more of the well-known systemic
extra frequently after the primary dose than after the
second dose (Kant et al., 2022). Primarily based on
previous research conducted in Indonesia, it is said
that side outcomes skilled by way of recipients of the
overall dose of the Sinovac vaccine are expected,
which include ache on the injection website online,
fever, drowsiness, and pain in the upper limbs (Kezia
& Ramatillah, 2022).
2
MATERIALS AND METHODS
2.1 Design
This is a prospective cross-sectional study. The
method was carried out to evaluate AEFI (Adverse
Events Following Immunization) and the efficacy of
the Sinovac vaccination among children aged 6-11
years in Indonesia using a questionnaire. This
research was conducted for three months, starting
from March to June 2022. The data collection
technique carried out in this study is convenience
sampling. Sampling takes all the items that make up
the research inclusion criteria. The inclusion criteria
in this study include all children aged 6-11 years in
the country who received a full dose of the Sinovac
vaccine and provided informed consent to be included
in this study. Then the exclusion criteria are children
aged 6-11 years who have not been vaccinated with a
full dose, suffering from cancer, HIV/AIDS,
tuberculosis (TBC), and autoimmune patients.
2.2 Participants
Participants in this study were all children in
Indonesia aged 6 to 11 years that have been
conducting a full dose of vaccination with the vaccine
Sinovac. The total of participants in the study was 456
respondents.
2.3 Instruments
This study uses a questionnaire distributed through
social media, such as WhatsApp, Twitter, Facebook,
Instagram, and Telegram. The total of questions in the
questionnaire in this study were 85 questions
consisting of questions about identity, efficacy of
Sinovac vaccine after the first and second
vaccinations, AEFI (Adverse Event Following
Immunization) vaccines obtained after the first and
second doses of vaccination in a short time. and long-
term vaccines and combine efficacy and AEFI for 3
months after vaccination.
Evaluation of AEFI (Adverse Events Following Immunization) Efficacy of Sinovac Vaccination Among Children Aged 6-11 Years in
Indonesia
235

2.4 Statistical Analysis
AEFI of the vaccine. The value of p<0.05 is
considered significant.
2.5 Ethical Consent
This research has received ethical approval from the
ethics committee of health research at the University
of August 17 1945 Jakarta, with approval letter
No.35/KEPK-UTA45JKT/EC/FB/07/2022.
3
RESULTS AND DISCUSSION
In this study, the number of respondents obtained was
456 respondents. All these respondents were children
aged 6-11 years who had done a full dose of Covid-
19 vaccination with Sinovac which was the inclusion
criterion in this study.
It is known that in this study there were more
female respondents with a percentage of 57.5%
compared to male respondents (42.5%). (Figure 1)
Respondents in this study were spread almost all
over Indonesia. The percentage of respondents'
domicile is 83.1% for the island of Java. This shows
that in this study most of the respondents live on the
island of Java such as West Java, Banten, DKI
Jakarta, Central Java, DI Yogyakarta, and East Java.
Then Sumatra Island 12.5%, Kalimantan Island 2.4%,
and the least proportion is Nusa Tenggara Island and
Bali at 2.0%. In this study, the researcher did not find
respondents who live in Sulawesi Island and Maluku
& Papua Island (Lemhannas RI, 2020). This may be
due to the lack of power in social media that
researchers have. (Figure 2)
Of 456 respondents, only 3 respondents had
comorbidities, namely one respondent with
comorbidity of sinusitis and 2 respondents with
comorbidities asthma. The percentage of those
comorbities can be seen in figure 3.
3.1 AEFI (Adverse Events Following
Immunization) Vaccine
Based on kipi.covid-19.go.id, the AEFI of the Covid-
19 vaccine is usually mild and only temporary. These
mild AEFI reactions include arm pain in the injection
area, headache, muscle pain, joint pain, chills, nausea,
vomiting, fatigue, fever, and also flu (Informasi
Tentang KIPI Atau Reaksi Setelah Vaksinasi COVID-
19, n.d.). Regarding monitoring, although there has
been no official research related to AEFI menstrual
cycle disorders, in some studies it is known that the
Covid- 19 vaccine can affect changes in the menstrual
cycle in reproductive women (Mahasing et al., 2022).
In this study, it was found that there were many
relationships between sociodemographics and the
AEFI of vaccines.
3.1.1 Relationship Between Gender with
AEFI Vaccine
After data analysis using fisher and chi-square tests,
it was found that there was a relationship between
gender and vaccine AEFI. (Table 1).
57,5%
42,5%
Figure 1. The percentage of gender among vaccinate
d
children with Sinovac (6-11 years)
Figure 3. The percentage of comorbidities among
vaccinated children with Sinovac (6-11 years)
0,4%
0,2%
99.4%
2.0%
2,4%
12,5%
83,1%
Figure 2. The percentage of domicile among vaccinate
d
children with Sinovac (6-11 years)
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
236
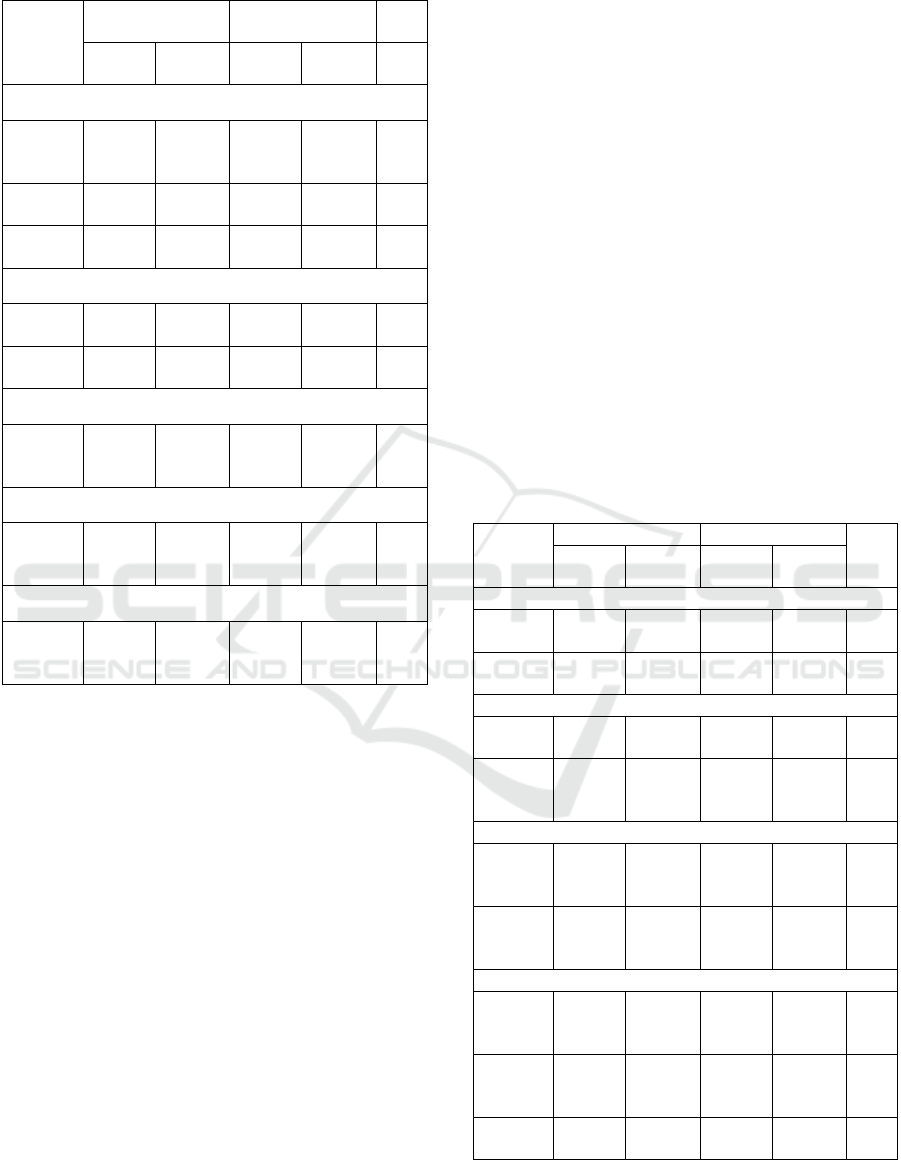
Table 1: Relationship of gender with AEFI vaccine.
Variable Male (n=194) Female (n=262) p-
value
Frequen
c
y
Percenta
g
e
Frequen
c
y
Percenta
g
e
AEFI after the first vaccination
Thirst or
dehydrati
on
29 14,95% 60 22,90% 0,03
4*
Diarrhea
1
0,52%
9
3,44% 0,03
5*
Cough 14 7,22% 35 13,36% 0,03
6*
AEFI vaccine after the second vaccination
Cough 20 10,31% 12 4,58% 0,01
8*
Fever 46 23,71% 39 14,89% 0,01
7*
Monitoring after 1 month of vaccination
Menstrua
l cycle
p
roblems
-
-
2
0,76% 0,00
0 #
Monitoring after 2 months of vaccination
Menstrua
l cycle
p
roblems
-
-
1
0,38% 0,00
0 #
Monitoring after 3 months of vaccination
Menstrua
l cycle
p
roblems
-
-
1
0,38% 0,00
0 #
Chi-Square test #Fisher test
It is known that vaccine recipients with a female
gender experience AEFI more than male vaccine
recipients. After the first vaccination, 22.90% of
female respondents experienced thirsty AEFI or
dehydration (p=0.034) after doing the first dose of
vaccination, while for male respondents, only 14.95%
experienced AEFI. After the second vaccination, it
was found that there was a difference that male
respondents who received the vaccine experienced
more AEFI than female respondents. Likewise, with
AEFI diarrhea (p=0.035) and cough (p=0.018),
female respondents who experienced AEFI diarrhea
were 3.44%, and for AEFI, the cough was 13.36%
while male respondents were 0.52% for AEFI diarrhea
and 7.22% for AEFI cough. (Table 1)
After the chi-square test was carried out to see if
there was a relationship between gender and vaccines
on monitoring 1-3 months after vaccination, it was
found that there was a relationship between gender
and the AEFI vaccine, namely, female respondents
experienced menstrual cycle problems with a p-
value<0.05. The above is by research that has been
carried out previously in Thailand. The study stated
that the AEFI of the Covid-19 vaccine was more
experienced by women. AEFI is usually spelled out at
the time of introducing a new vaccine. Similarly, in
this study, the Sinovac- CoronaVac vaccine is the first
Covid-19 vaccine and has only been introduced to the
Indonesian people since the beginning of the
vaccination recommendation for Covid-19. The
study also mentioned that the Covid-19 vaccination
can affect the menstruation cycle in reproductive
women which may be caused by the stress
experienced in these women (Mahasing et al., 2022).
(Table 1)
3.1.2 Relationship Between Comorbidities
with AEFI Vaccine
After data analysis using fisher and chi-square tests,
it was found that there was a relationship between
comorbidities and vaccine AEFI. (Table 2)
Table 2: Relationship between comorbidities with AEFI
vaccine.
Variable Sinusitis
(
n=1
)
Asthma
(
n=2
)
p-
value
Frequen
cy
Percenta
ge
Frequen
cy
Percenta
ge
AEFI after the first vaccination
Nauseou
s
- - 2 100% 0,00
0*
Heart
attac
k
1 100% - - 0,00
0*
AEFI vaccine after the second vaccination
Cough 1 100% - - 0,00
1*
Thirst or
dehydrati
on
1 100% 1 50% 0,00
5*
Monitoring 1 month after vaccination
Menstrua
l cycle
p
roblems
1 100% - - 0,00
0 #
Thirst or
dehydrati
on
1 100% 1 50% 0,01
4 #
Monitorin
g
2 months after vaccination
Menstrua
l cycle
p
roblems
1 100% - - 0,00
0 #
Thirst or
dehydrati
on
1 100% - - 0,00
0 #
Tired 1 100% - - 0,00
3 #
Chi-Square test #Fisher test
Evaluation of AEFI (Adverse Events Following Immunization) Efficacy of Sinovac Vaccination Among Children Aged 6-11 Years in
Indonesia
237
It is known that respondents who have sinusitis
disease experience more AEFI than respondents who
have asthma-borne diseases. Just like the
Relationship of comorbidities with a vaccine after the
first and second vaccinations, in monitoring after 1-3
months after vaccination, respondents with
concomitant diseases,
an
d sinusitis experienced more
AEFI than respondents
with asthma comorbidities.
On monitoring 3 months after vaccination, no
relationship was found between comorbidities and
AEFI vaccines. The above can occur because sinusitis
patients are susceptible to the virus (Marin et al.,
2023). Another possibility is due to the presence of
invasive fungal infections in respondents receiving
the vaccine. Based on invasive fungal infection
sinusitis case reports seen in the literature, the fungal
infection is very influential on the severity of Covid-
19 (Borrelli et al., 2022). More research is needed on
the Relationship between comorbidities and AEFI
covid-19 vaccines, especially Sinovac. (Table 2)
3.1.3 Relationship Between Age and AEFI
Vaccine
Table 3. Relationship between age with AEFI vaccine.
Variable
A
g
e
(
n=456
)
p-value
Frequenc
y
Percentage
AEFI afte
r
the first vaccination
Nauseous 46 10,09% 0,014*
Upper arm
p
ain
232 50,88% 0,041*
AEFI afte
r
the secon
d
vaccination
Feve
r
85 18,64% 0,020*
Dizz
y
76 16,67% 0,004 #
Easy to get
sleep
y
150 32,89% 0,007 #
Thirst or
dehydration
52 11,40% 0,044 #
Upper arm
p
ain
179 39,25% 0,005 #
Monitorin
g
1 month afte
r
vaccination
Menstrual
cycle
p
roblems
2
0,44% 0,009 #
Monitoring 2 months afte
r
vaccination
Menstrual
cycle
p
roblems
1
0,22% 0,036 #
* Mann-Whitney test #Kruskal Wallis test
Figure 4. Diagram of the relationship between age with
AEFI Vaccine
After data analysis using the Mann-Whitney Test for
questions related to the AEFI vaccine and the Kruskal
Wallis test for monitoring questions 1-3 months after
vaccination, it was found that there was a relationship
between age and AEFI vaccine. This is indicated with
a p-value of less than 0.05. The most experienced
AEFI of respondents was upper arm pain after the
first vaccination (p=0.041) with a percentage of
50.88% and the least was at the time of AEFI
monitoring until 3 months after vaccination, namely
changes in the menstrual cycle after 2 months of
vaccine acceptance (p=0.036) with 0.21%. (Table 3)
It is known that younger children have less AEFI.
This can happen because the immune systems of
younger people are Stronger and more efficient than
those of older people. Another possibility that can
occur is the clinical symptoms of the inflammatory
response to the lung injury of the younger children are
milder than those older children (Qona et al., 2022).
(Figure 4)
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
238
3.1.4 Relationship between BMI and AEFI
Vaccine
Table 4: Relationship of gender with vaccine efficacy.
Variable
BMI (n=456)
p-value
Frequency Percentage
AEFI after the first vaccination
Fever 203 42,38% 0,021*
Pain in the injection
area
338 70,56% 0,00*
Nauseous 48 10,02% 0,036*
Dizzy 145 30,27% 0,003*
Easy to get sleepy 228 47,60% 0,000*
Upper arm pain 242 50,52% 0,036*
AEFI after the second vaccination
Pain in the injection
area
266 55,53%
0,050*
Flu
26 5,43%
0,039*
Easy to get sleepy
162 33,82%
0,026*
Upper arm pain
188 39,25%
0,004*
Monitoring 2 months after vaccination
Menstrual cycle
problems
1
0,21%
0,007 #
Easily Tired
30 6,26%
0,000 #
Upper arm pain
31 6,47%
0,000 #
Thirst or dehydration
20 4,18%
0,000 #
Bleeding
3
0,63%
0,000 #
Monitoring 3 months after vaccination
Easily Tired
6
1,25% 0,016 #
Upper arm pain
6
1,25%
0,016 #
*Mann-Whitney test #Kruskal Wallis test
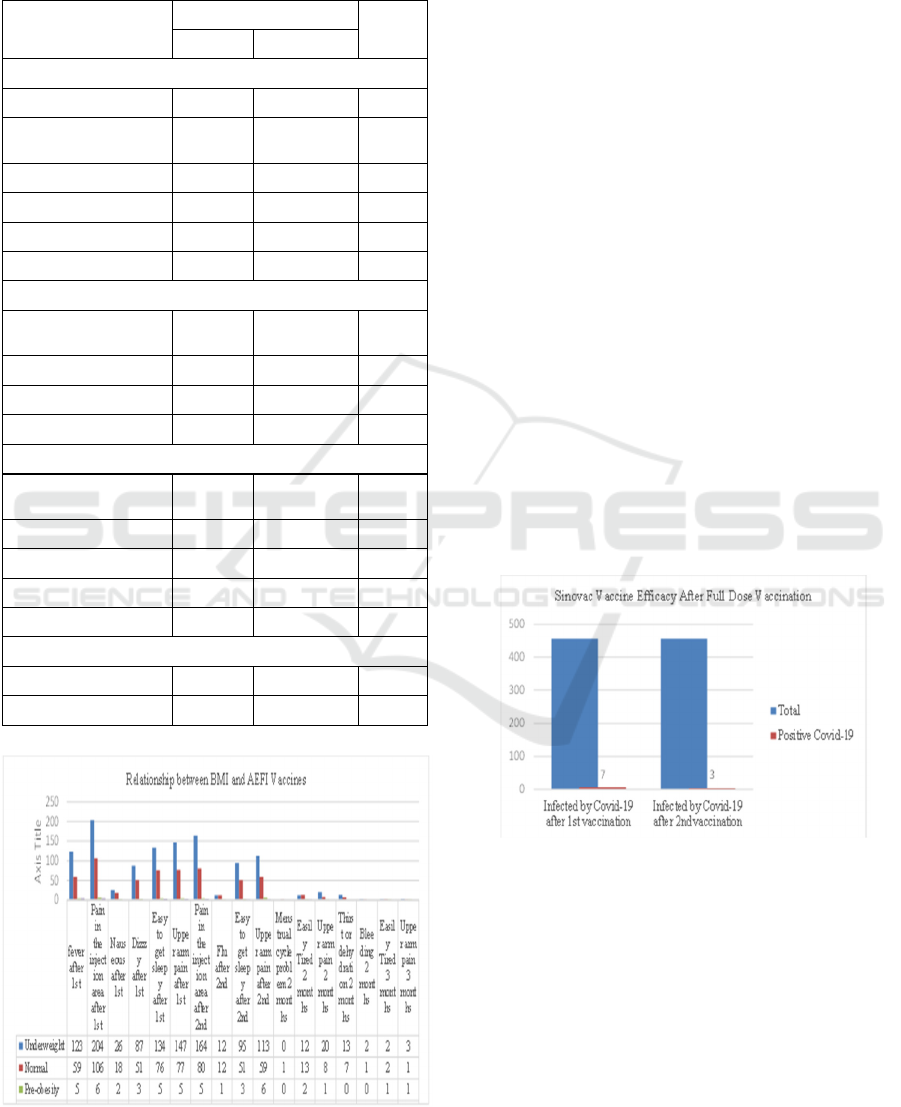
Figure 5: Relationship between BMI and AEFI vaccine.
After analyzing the data using Mann-Whitney for
questions related to the vaccine AEFI and the Kruskal
Wallis test to find out 3 months after vaccination it
was found that there was a relationship between BMI
and vaccine AEFI with a p-value of 0.05, which can
be seen in table 1. the data that can be used in the
graph in Figure 2, it is known that the respondents
who received the vaccine the most experiencing AEFI
were children aged 6-11 years who had low body
weight. (Table 4)
The results obtained in this study are following
previous studies related to the side effects of Sinovac
in adolescents aged 12-18 years that have been
carried out by Qona et al. In this study, it was found
that respondents who received vaccines with BMI
<18.5 (less body weight) had the majority of AEFI
vaccines (Qona et al., 2022). The results obtained in
this study may be caused by the respondents obtained
by the researchers having a BMI of less than 18.5
(less body weight). (Figure 5)
3.2 Vaccine Efficacy
The efficacy of the Sinovac vaccine was 98.46% after
all respondents had the first vaccination and 99.34%
in the second vaccination. Based on this, it can be
known that the efficacy of the Sinovac vaccine after
all respondents have been vaccinated in the first and
second doses or the full dose is 98.2%. (Figure 6)
Figure 6: Diagram of vaccine efficacy.
3.2.1 Relationship Between Gender and
Vaccine Efficacy
After data analysis using fisher tests, it was found that
there was a relationship between comorbidities and
vaccine efficacy. (Table 5)
Evaluation of AEFI (Adverse Events Following Immunization) Efficacy of Sinovac Vaccination Among Children Aged 6-11 Years in
Indonesia
239

Table 5: Relationship of gender with vaccine efficacy.
Variable
Male (n=194) Female (n=262)
p-
value
Frequency Percentage Frequency Percentage
Infected with Covid-19 after vaccination
Second
vaccination
3
1,55%
-
-
0,043*
Self-
isolation
3
1,55%
-
-
0,043
#
Mild
symptoms
3
1,55%
-
-
0,043
#
*Fisher Test #Chi-Square Test
It is known that in this study, only a relationship was
found between gender and vaccine efficacy after
vaccination respondents carried out the second dose
of vaccination. Likewise, it can also be seen that only
respondents of the male gender were infected with
covid-19 after receiving the second dose
of
vaccination. Based on the data above, it can be
concluded that in female respondents the Sinovac
vaccine works more effectively against Covid-19.
The possibility that can happen because in general,
women practice more preventive behaviors and avoid
behaviors at risk of contracting Covid-19
(Khubchandani et al., 2021). (Table 5).
3.2.2 Relationship Between Comorbidities
and Vaccine Efficacy
After data analysis using fisher tests, it was found that
there was a relationship between comorbidities and
vaccine efficacy. (Table 6).
Table 6: Relationship between comorbidities and vaccine
efficacy.
Variable Sinusitis (n=1) Asthma (n=2) p-
value
Frequenc
y
Percenta
g
e
Frequenc
y
Percenta
g
e
After the first vaccination
Loss of
sense of
disengageme
nt an
d
taste
1
100%
-
-
0,000
*
Respiratory
distress
1
100%
1
50% 0,000
*
Cough and
sore throat
-
-
2
100% 0,002
*
Diarrhea
1
100%
-
-
0,000
*
Skin rash
1
100%
-
-
0,000
*
After the second vaccination
Loss of
sense of
disengageme
nt an
d
taste
1
100%
-
-
0,000
*
Respiratory
distress
1
100%
-
-
0,000
*
Headache
1
100%
-
-
0,019
*
*Fisher test
It is known that respondents who have congenital
sinusitis ailment revel in extra symptoms of being
inflamed with Covid-19. That is inconsistent with
preceding research on continual Rhinosinusitis and
Covid-19. Inside the observation, it became said that,
although CRS patients are vulnerable to exacerbation
of viral infections, CRS has now not been said chief
comorbidity and isn't a dangerous aspect for Covid-
19, there can also be a defensive role towards SARS-
cov-2 infection, and CRS is not related to severe
Covid- 19. Even though the observation also referred
to that in CRS sufferers who were unresponsive to
medical remedies and carried out surgical processes,
the treatment could affect the danger of SARS-Cov-2
infection with the aid of modulating the host’s
immune response to viral infections, ACE2
expression in airway epithelial cells, and pre-present
syntonic irritation resulting from CRS (Marin et al.,
2023). (Table 6)
3.2.3 Relationship Between Age and Vaccine
Efficacy
After data analysis using the Mann-Whitney test, it
was found that there was a relationship between age
and vaccine efficacy. (Table 7)
Table 7: Relationship between age with vaccine efficacy.
Variable Age (n=456) p-value
Frequency Percentage
After the first vaccination
Respiratory
distress
21 4,61% 0,000*
After the second vaccination
Cough and sore
throat
32 7,02% 0,010*
Fever 64 14,04% 0,032*
Headache 53 11,62% 0,015*
*Mann-Whitney test
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
240

Figure 7. Diagram of Relationship between age and vaccine
efficacy
It can be concluded that there is a relationship
between age and vaccine efficacy. The most common
symptoms of Covid-19 experienced by respondents
receiving the Sinovac vaccine were fever with
14.04% (p = 0.032). The aforementioned are
supported with a p-value of less than 0.05. There was
no relationship between age and vaccine efficacy on
monitoring for 3 months after vaccination. (Table 7)
It is known that respondents receiving vaccines
with older age experience more symptoms of Covid-
19 than younger vaccine respondents. This is not
much different from previous studies preliminary
reports show that only a small percentage of Covid-
19 cases occur in children and adolescents (aged 0–
18 years), with the incidence increasing with age
(European Centre for Disease Prevention and
Control, 2020). (Figure 7)
3.2.4 Relationship Between BMI and
Vaccine Efficacy
Table 8: Relationship of BMI with vaccine efficacy.
Variable
BMI (n=456)
p-value
Frequency Percentage
After the first vaccination
Cough and sore throat 67 14,69% 0,007*
Diarrhea 19 4,17% 0,047*
AEFI after the second vaccination
Loss of sense of
disengagement
an
d
taste
7
1,54% 0,033*
Cough and sore throat 32 7,02% 0,005*
Diarrhea
9
1,97% 0,049*
*Mann-Whitney test
Figure 8: Diagram of Relationship BMI with vaccine
efficacy.
After data analysis using the Mann-Whitney test, it
was found that there was a relationship between BMI
and vaccine efficacy with a p-value of less than 0.05
(Table 8). In the data that has been described in the
graph, it is known ones who experience the most
symptoms of Covid-19 are children who have low
body weight. (Figure 8)
A cohort study conducted by previous researchers
stated that people with very low BMI risk of being
hospitalized or dying from Covid-19 than people with
normal weight (Wilder-smith & Frahsa, 2022).
4 CONCLUSIONS
The efficacy of the Sinovac vaccine among children
aged 6-11 years is very high at 98.2%. The
sociodemography that results in the most severe
AEFI
is comorbidities. The vaccine AEFI that most
respondents felt was upper arm pain and pain in the
injection area after the first vaccination. Meanwhile,
the vaccine AEFI that most respondents felt at 3
months of monitoring was upper arm pain after 2
months after vaccination.
REFERENCES
Borrelli, M., Nasrollahi, T., Ulloa, R., Raskin, J., Ference,
E., & Tang, D. M. (2022). Invasive Fungal Sinusitis
During Active COVID-19 Infection. 0(0), 1–3.
https://doi.org/10.1177/01455613221112337
da Silva, R. B., da Silva, T. P. R., Sato, A. P. S., Lana, F. C.
F., Gusmâo, J. D., Souza, J. F. A., & Matozinhos, F. P.
(2021). Adverse events following immunization against
SARS-CoV-2 (covid-19) in the state of Minas Gerais.
Revista de Saude Publica, 55, 01–10.
https://doi.org/10.11606/s1518-8787.2021055003734
Dhar, J., Samanta, J., & Kochhar, R. (2020). Corona Virus
Disease-19 pandemic: The gastroenterologists’
Evaluation of AEFI (Adverse Events Following Immunization) Efficacy of Sinovac Vaccination Among Children Aged 6-11 Years in
Indonesia
241

perspective. Indian Journal of Gastroenterology, 39(3),
220–231. https://doi.org/10.1007/s12664-020-01075-2
Eid, E., Abdullah, L., Kurban, M., & Abbas, O. (2021).
Herpes zoster emergence following mRNA COVID-19
vaccine. Journal of Medical Virology, 93(9), 5231–
5232. https://doi.org/10.1002/jmv.27036
Empat Negara Berbagi Pengalaman Uji Klinis Tahap-3
Vaksin Sinovac. (n.d.). Retrieved June 28, 2022, from
https://kemlu.go.id/beijing/id/news/11971/empat-
negara-berbagi-pengalaman-uji-klinis-tahap-3-vaksin-
sinovac
European Centre for Disease Prevention and Control.
(2020). COVID-19 in children and the role of school
settings in COVID-19 transmission. Ecdc, Stockholm,
August, 31.
Fu, Q., Xie, H., Zhou, L., Li, X., Liu, Y., Liu, M., Wang,
C., Wang, X., Wang, Z., Tang, J., Xiao, H., Xiao, Z.,
Zhou, J., Feng, C., Wang, L., Ao, Z., Chen, X., Su, C.,
Wu, X., … Jiang, L. (2021). Auricular acupressure for
adverse events following immunization related to
COVID-19 vaccine injection: study protocol for a
multicenter, three-arm, blinded randomized controlled
trial. Trials, 22(1), 1–12.
https://doi.org/10.1186/s13063-021-05837-x
Gao, Q., Bao, L., Mao, H., Wang, L., Xu, K., Yang, M., Li,
Y., Zhu, L., Wang, N., Lv, Z., Gao, H., Ge, X., Kan, B.,
Hu, Y., Liu, J., Cai, F., Jiang, D., Yin, Y., Qin, C., …
Qin, C. (2020). Development of an inactivated vaccine
candidate for SARS-CoV-2. Science, 369(6499), 77–
81. https://doi.org/10.1126/science.abc1932
Gianfredi, V., Minerva, M., Casu, G., Capraro, M.,
Chiecca, G., Gaetti, G., Mazzocchi, R. M., Musarò, P.,
Basteri, P., Bertini, B., Ferri, C., Odone, A., Signorelli,
C., Alberti, V. F., & Gastaldi, G. (2021). Immediate
adverse events following covid-19 immunization. A
cross-sectional study of 314,664 Italian subjects. Acta
Biomedica, 92(7).
https://doi.org/10.23750/abm.v92iS6.12365
Informasi Tentang KIPI atau Reaksi Setelah Vaksinasi
COVID-19. (n.d.). Retrieved July 28, 2022, from
https://kipi.covid19.go.id/
Kai-Wang To, K., Sridhar, S., Hei-Yeung Chiu, K., Ling-
Lung Hung, D., Li, X., Fan-Ngai Hung, I., Raymond
Tam, A., Wai-Hin Chung, T., Fuk-Woo Chan, J., Jian-
Xia Zhang, A., Chi-Chung Cheng, V., & Yuen, K.-Y.
(2021). Lessons learned 1 year after SARS-CoV-2
emergence leading to COVID-19 pandemic.
https://doi.org/10.1080/22221751.2021.1898291
Kant, A., Jansen, J., Balveren, L. Van, & Hunsel, F. Van.
(2022). Description of Frequencies of Reported
Adverse Events Following Immunization Among Four
Different COVID ‑ 19 Vaccine Brands. Drug Safety,
45(4), 319–331. https://doi.org/10.1007/s40264-022-
01151-w
Kementerian Pendidikan dan Kebudayaan » Republik
Indonesia. (n.d.). Retrieved June 1, 2022, from
https://www.kemdikbud.go.id/main/blog/2021/12/vaks
inasi-covid19-bagi-anak-usia-611-tahun-dorong-
optimalisasi-pembelajaran-tatap-muka-terbatas
Kezia, V., & Ramatillah, D. L. (2022). Intensive
Monitroing of Sinovac Vaccine for Safety and Efficacy
Among Indonesian Population. International Journal
of Applied Pharmaceutics, 14(Special issue 2), 44–48.
https://doi.org/10.22159/ijap.2022.v14s2.44748
Khubchandani, J., Sharma, S., Price, J. H., Wiblishauser,
M. J., Sharma, M., & Webb, F. J. (2021). COVID-19
Vaccination Hesitancy in the United States: A Rapid
National Assessment. Journal of Community Health,
46(2), 270–277. https://doi.org/10.1007/s10900-020-
00958-x
Kim, M. A., Lee, Y. W., Kim, S. R., Kim, J. H., Min, T. K.,
Park, H. S., Shin, M., Ye, Y. M., Lee, S., Lee, J., Choi,
J. H., Jang, G. C., & Chang, Y. S. (2021). COVID-19
vaccine-associated anaphylaxis and allergic reactions:
Consensus statements of the KAAACI
urticaria/angioedema/anaphylaxis working group.
Allergy, Asthma and Immunology Research, 13(4),
526–544. https://doi.org/10.4168/aair.2021.13.4.526
Lemhannas RI. (2020). Daftar 34 Provinsi Beserta Ibukota
di Indonesia. 1–15.
http://www.lemhannas.go.id/images/2020/08/PPID/4_
Daftar_Nota_Kesepahaman_2020.pdf
Levy, E. R., Blumenthal, J., & Chiotos, K. (2021).
Coronavirus disease 2019 in children. Current Opinion
in Infectious Diseases, 34(5), 500–509.
https://doi.org/10.1097/QCO.0000000000000762
Mahasing, C., Yasopa, O., Sansilapin, C.,
Rattanathumsakul, T., Thammawijaya, P.,
Suphanchaimat, R., & Doung-Ngern, P. (2022).
Investigation of a Cluster of Immunization Stress-
Related Reactions after Coronavirus Disease 2019
(COVID-19) Vaccination, Thailand, 2021. Vaccines,
10(3), 1–12. https://doi.org/10.3390/vaccines10030441
Marin, C., Hummel, T., Liu, Z., & Mullol, J. (2023).
Chronic Rhinosinusitis and COVID-19. The Journal of
Allergy and Clinical Immunology in Practice, 10(6),
1423–1432. https://doi.org/10.1016/j.jaip.2022.03.003
Matsuzawa, A., Vaccarezza, M., Gusev, E., Sarapultsev,
A., Solomatina, L., & Chereshnev, V. (2022). SARS-
CoV-2-Specific Immune Response and the
Pathogenesis of COVID-19.
https://doi.org/10.3390/ijms23031716
Medical Association, A. (2020). Should We Mandate a
COVID-19 Vaccine for Children?
https://doi.org/10.1001/jamapediatrics.2020.3019
Mistry, P., Barmania, F., Mellet, J., Peta, K., le Strydom,
A., Viljoen, I. M., James, W., Gordon, S., Pepper, M.
S., Martin Centre, L., & William Dunn, S. (2019).
SARS-CoV-2 Variants, Vaccines, and Host Immunity.
https://doi.org/10.3389/fimmu.2021.809244
Ophinni, Y., Hasibuan, A. S., Widhani, A., Maria, S.,
Koesnoe, S., Yunihastuti, E., Karjadi, T. H., Rengganis,
I., & Djauzi, S. (2020). COVID-19 Vaccines: Current
Status and Implication for Use in Indonesia. Acta
Medica Indonesiana, 52(4), 388–412.
Per, U. (2022). ANALISIS DATA COVID-19 INDONESIA.
April
.
Qona, A., Ibnatus, A. H., & Ramatillah, D. L. (2022).
Original Article EVALUATION COMPARISON
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
242

BETWEEN SINOVAC AND PFIZER VACCINE
AMONG INDONESIAN CHILDREN AND TEENAGER
UNDER 18 YEARS OLD. 14(2), 22–30.
Ramatillah, D. L., Gan, S. H., Sulaiman, S. A. S., Puja, D.,
Abubakar, U., Jaber, A. A. S., Lukas, S., & Jusnita, N.
(2021). Evaluation of treatment outcome for pneumonia
among pre-vaccinated covid-19 patients with/without
comorbidity in a public hospital in Bengkulu,
Indonesia. Vaccines, 9(12), 1–9.
https://doi.org/10.3390/vaccines9121411
Ramatillah, D. L., & Isnaini, S. (2021). Treatment profiles
and clinical outcomes of COVID-19 patients at private
hospital in Jakarta. PLoS ONE, 16(4 April), 1–11.
https://doi.org/10.1371/journal.pone.0250147
Rashedi, R., Samieefar, N., Masoumi, N., Mohseni, S., &
Rezaei, N. (2022). COVID-19 vaccines mix-and-
match: The concept, the efficacy and the doubts.
Journal of Medical Virology, 94(4), 1294–1299.
https://doi.org/10.1002/jmv.27463
Supangat, Sakinah, E. N., Nugraha, M. Y., Qodar, T. S.,
Mulyono, B. W., & Tohari, A. I. (2021). COVID-19
Vaccines Programs: adverse events following
immunization (AEFI) among medical Clerkship
Student in Jember, Indonesia. BMC Pharmacology and
Toxicology, 22(1), 1–7.
https://doi.org/10.1186/s40360-021-00528-4
Tan, A. Y., Chang, C. T., Yu, Y. K., Low, Y. X., Fatehah,
N., Razali, M., Tey, S. Y., Wen, S., & Lee, H. (2022).
Adverse Events Following BNT162b2 mRNA COVID-
19 Vaccine Immunization among Healthcare Workers
in a Tertiary Hospital in Johor , Malaysia. 2, 1–8.
Vaksinasi COVID-19 untuk Anak Usia 6-11 Tahun dimulai
14 Desember – Sehat Negeriku. (n.d.). Retrieved June
1, 2022, from
https://sehatnegeriku.kemkes.go.id/baca/rilis-
media/20211212/1938972/vaksinasi-covid-19-untuk-
anak-usia-6-11-tahun-dimulai-14-desember/
Wilder-smith, A., & Frahsa, A. (2022). Impact of BMI on
COVID-19 vaccine effectiveness Second brain tumours
after pituitary irradiation : lower risk than once thought.
THE LANCET Diabetes & Endocrinology, 10(8), 551–
552. https://doi.org/10.1016/S2213-8587(22)00170-X
Živanović, D., Jovin, V. M., Javorac, J., Ilić, M., & Zelić,
P. (2021). Commentary: Registered adverse events
following COVID-19 immunization in Serbia.
European Review for Medical and Pharmacological
Sciences, 25(20), 6408–6410.
https://doi.org/10.26355/eurrev_202110_27014
Evaluation of AEFI (Adverse Events Following Immunization) Efficacy of Sinovac Vaccination Among Children Aged 6-11 Years in
Indonesia
243
