
Evaluation of Exposure to COVID-19 From Participants in the
Complete Dose of Sinovac and AstraZeneca Vaccinations for Online
Drivers
Lia W. Manurung and Diana Laila Ramatillah
Faculty Pharmacy, Universitas 17 Agustus 1945 Jakarta, Indonesia
Keywords Covid-19, Sinovac, AstraZeneca, Online Drivers in Indonesia
Abstract: The type of this research is observational with a cross-sectional design using convenience sampling for all
online drivers in Indonesia who have received the full dose of the Sinovac and AstraZeneca vaccine. To
evaluate the comparison of the Sinovac vaccine and AstraZeneca vaccine on a driver online in Indonesia who
has received the full dose of the Sinovac and AstraZeneca vaccine. It was found that the efficacy of the
Sinovac and Pfizer vaccines was almost the same. Other factors affecting the side the effect and efficacy of
vaccines are gender, age, and BMI, with the p-value of each variable <0.05. The following is the relationship
between the type of vaccine, sex with a mean of 1.49, age with a mean of 24.27, and BMI with a mean of
22.3. For the side effects of the AstraZeneca vaccine and the Sinovac vaccine, the symptoms are almost the
same.
1 INTRODUCTION
Covid 19 which emerged at the end of 2019 has
become a threat to public health around the world.
(Sutardi and Ramatillah 2022)
Coronavirus is a large
family of viruses that can cause illnesses ranging
from mild, moderate to severe
symptoms. WHO-,
mentions that almost a year since the
first report of
cases of severe acute respiratory syndrome
coronavirus-2 in Wuhan province in China, more than
57 million cases have been diagnosed, thus WHO
declared coronavirus disease 2019 a pandemic on
March 9, 2020.
’
(Blum and Neumärker 2021) (WHO,
2020) In humans, it mainly infects cells in the airways
lining the alveoli. SARS- CoV-2 will bind to the
receptor and enter the cell. (Zhang et al. 2020) The
ability of the virus to overpower the immune response
determines the severity of the infection.
Dysregulation of the immune system then plays a role
in tissue damage in SARS-CoV-2 infection. (Susilo et
al. 2020) Meanwhile, the first case was confirmed on
March 2, 2020. To date, there is no effective drug to
reduce the burden of infection and the pandemic. The
Covid-19 pandemic will not only result in enormous
mortality but will also continue to burden the burden
of morbidity that severely disrupts communities
pandemic was first announced on March 11, 2020,
indicating that the virus has infected many people in
various countries.
Vaccines are biological products containing
antigens that, if given to humans, will trigger the
formation of antibodies and cause active immunity in
certain diseases. One of them is the Sinovac vaccine
and the AstraZeneca vaccine. Sinovac is an
inactivated whole virus developed by Life Science,
while AstraZeneca is a vaccine containing the gene
encoding the full-length S Protein and is one of
the vaccines developed by the University of
Oxford(Kezia and Ramatillah 2022) (Araminda and
Ramatillah 2022).
2 MATERIALS AND METHODS
This research was conducted with a quantitative
approach using a prospective cross-sectional design
study. The data collection technique was carried out
using a survey method using google forms distributed
offline and online to motorcycle taxi drivers who had
been vaccinated with complete doses of Sinovac and
AstraZeneca vaccines with a convenience sampling
method. This research was conducted in the period
August-October. The inclusion criteria were Online
drivers over 18 years old who had received Sinovac
244
Manurung, L. and Ramatillah, D.
Evaluation of Exposure to COVID-19 From Participants in the Complete Dose of Sinovac and AstraZeneca Vaccinations for Online Drivers.
DOI: 10.5220/0011979300003582
In Proceedings of the 3rd International Seminar and Call for Paper (ISCP) UTA â
˘
A
´
Z45 Jakarta (ISCP UTA’45 Jakarta 2022), pages 244-249
ISBN: 978-989-758-654-5; ISSN: 2828-853X
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
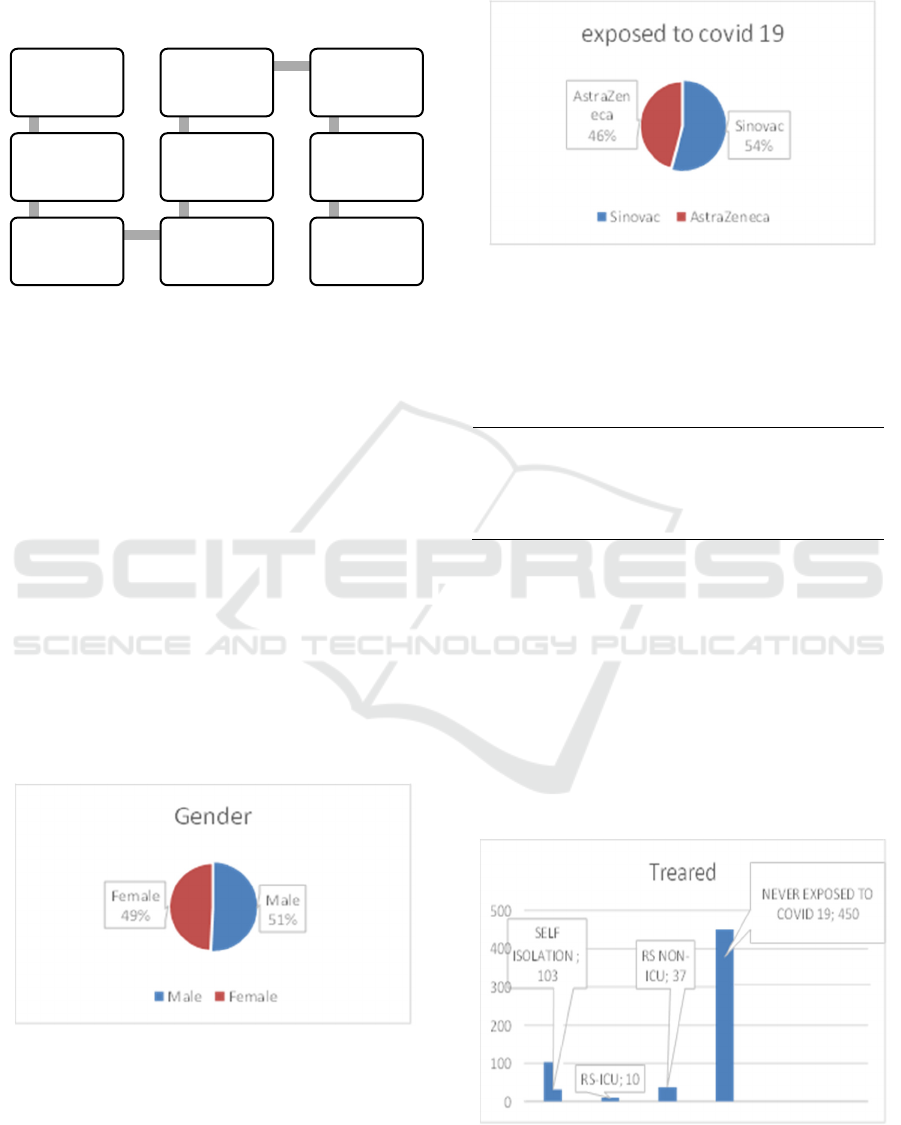
and AstraZeneca vaccines and were willing to be
respondents in this study. The number of respondents
in this study was 600 respondents (300 Sinovac and
300 AstraZeneca).
Figure 1: Research Framework.
2.1 Ethical Approval
As seen in fig. 1 this research was approved by the 17
august 1945 university Jakarta ethics committee with
reference numbers:
No.50/KEPK-UTA45JKT/EC/EXP/07/2022.
3 RESULT AND DISCUSSION
3.1 Results
The number of respondents from this study was 600
respondents who had received two (2) doses of the
Sinovac vaccine and AstraZeneca vaccine and were
included in the inclusion criteria. Respondents in this
study received questionnaires through social media
such as Facebook, WhatsApp, and Instagram.
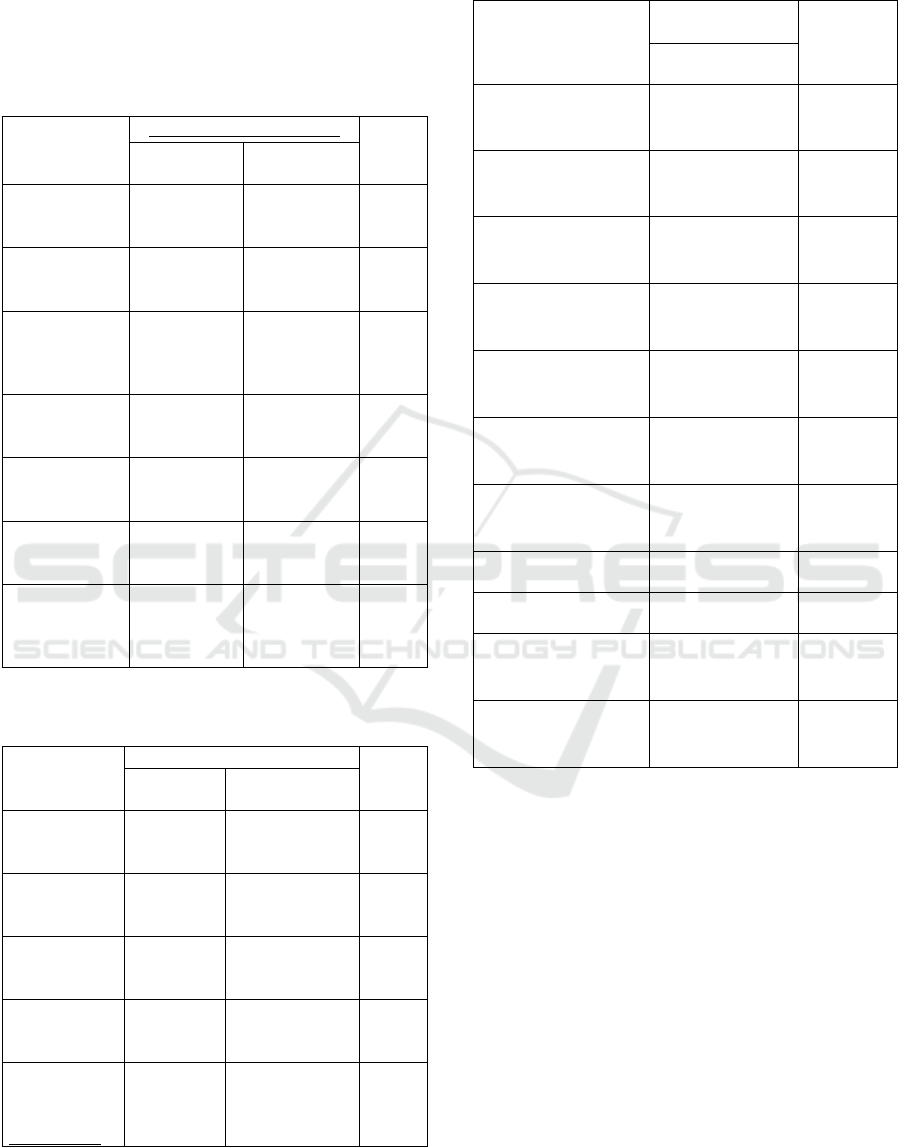
Figure 2: Participants based on gender.
Based on pictures 2 out of 600 respondents, 49%
(295 respondents) were women and 51% (305
respondents) were a man. Based on research in
Sumatra Selatan 440 respondents have completed the
questionnaire, 53,4% of respondents are female and
46,6% of respondents are male. (Argista 2021).
Figure 3: Participants exposed to Covid 19.
Based on pictures 3 out of 600 respondents, 25%
(150 respondents) have been exposed to covid 19.
Table 1: Correlation between type of vaccine and exposure
to Covid 19.
Kind of vaccine Exposed to covid 19
/percentage %
P value
Sinovac 81/24,3%
AstraZeneca 69/20,3
Total p-value
0,352
Fisher test, #Chi-square test
Table 1, it is explained drivers online who were
exposed to covid 19 and those who were most
exposed from drivers online who received the
Sinovac vaccine (24,3%). AstraZeneca vaccine
efficacy based on the full-length encoding of the
SARSCov-2 viral spike protein RDB will result in
better protection. (Ghosh 2021) While in the Sinovac
vaccine, the risk of exposure to Covid 19 was reduced
by 65,3% compared to those who did not receive the
Covid 19 vaccine.(Marwan 2021).
Figure 4: Treated.
research (approval
of research and
ethical approve)
qustionnaire
assesment of
questionnaire by the
experts
pilot sudy (using 30
respordents)
received filled up of
questionnaire
validity and reability
test
questionnaire
deployment using
social media
data interpertation
and reporting
covclusion
Evaluation of Exposure to COVID-19 From Participants in the Complete Dose of Sinovac and AstraZeneca Vaccinations for Online Drivers
245
It can be seen in table 4 that Covid 19 patients that
there are 103 patients doing self-isolation, 10 being
treated at the ICU hospitals, and 37 being treated at
non-ICU hospitals.
Table 2: Correlation between type of vaccine and side
effects and efficacy of the vaccine 1
st
.
Variables
Frequency/Percentage (%)
p-value
Sinovac = 300 AstraZeneca
= 300
Side Effects of
fever after the
1
st
vaccination
90/30 131/43.6 0.001
Pain in the 1
st
vaccination
injection area
189/33 202/67.3 0.304
Side effects of
coughing after
the 1
st
vaccination
16/5.3 31/10.3 0.032
Side effects of
the flu after 1
st
vaccination
28/9.3 38/12.6 0.240
Feel nausea
after the 1
st
vaccination
23/7.6 40/13.3 0.032
Feeling dizzy
after the 1
st
vaccination
73/24.3 109/36.3 0.002
Cholesterol
levels increase
after the 1
st
vaccination
0 4/1.3 0.124
Table 3: Correlation between type of vaccine and side effect
and efficacy of the vaccine 2
nd
.
Variable
Percenta
g
e/fre
q
uenc
y
(
%
)
P-value
Sinovac=
300
AstraZeneca=
300
Side Effects of
fever after the
2
nd
vaccination
59/19.6 112/37.3 0.000
Side effects of
the flu after 2
nd
vaccination
16/5.3 36/12 0.005
Feeling dizzy
after the 2
nd
vaccination
53/17.6 78/26 0.017
Loss of loss
and taste after
2
nd
vaccination
13/4.3 29/9.6 0.015
Experienced
diarrhea after
2
nd
vaccination
7/2.3 12/4 0.351
Table 4: Correlation between age and side effects of the
vaccine after 6 months.
Variable
Frequency/
p
ercentage (%)
P value
Age n:600, mean
:24.27
Have been exposed to
covid-19 1-3 months
after vaccination
56/33.6 0.507
Have been exposed to
c o v i d - 1 9 4 - 6 m o n t h s
after vaccination
48/28.8 0.315
Current menstrual 1-3
months after
vaccination
215/1,29 0.009
Current menstrual 4-6
months after
vaccination
210/1.26 0.396
Feel easily tired 1-3
months after
vaccination
88/52.8 0.002
Feel easily tired 4-6
months after
vaccination
44/26.4 0.830
Feel pain in arm 1-3
months after
vaccination
67/40.2 0.129
Bleeding 1-3 months
afte
r
vaccination
2/1.2 0.194
Bleeding 4-6 months
afte
r
vaccination
2/1.2 0.194
Experience heart
disorder 4-6 months
after vaccination
4/1.3 0.29
Cholesterol levels
increase 1-3 months
after vaccination
2/0.6 0.469
*Man-Whitney test, #Kruskal Wallis test
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
246
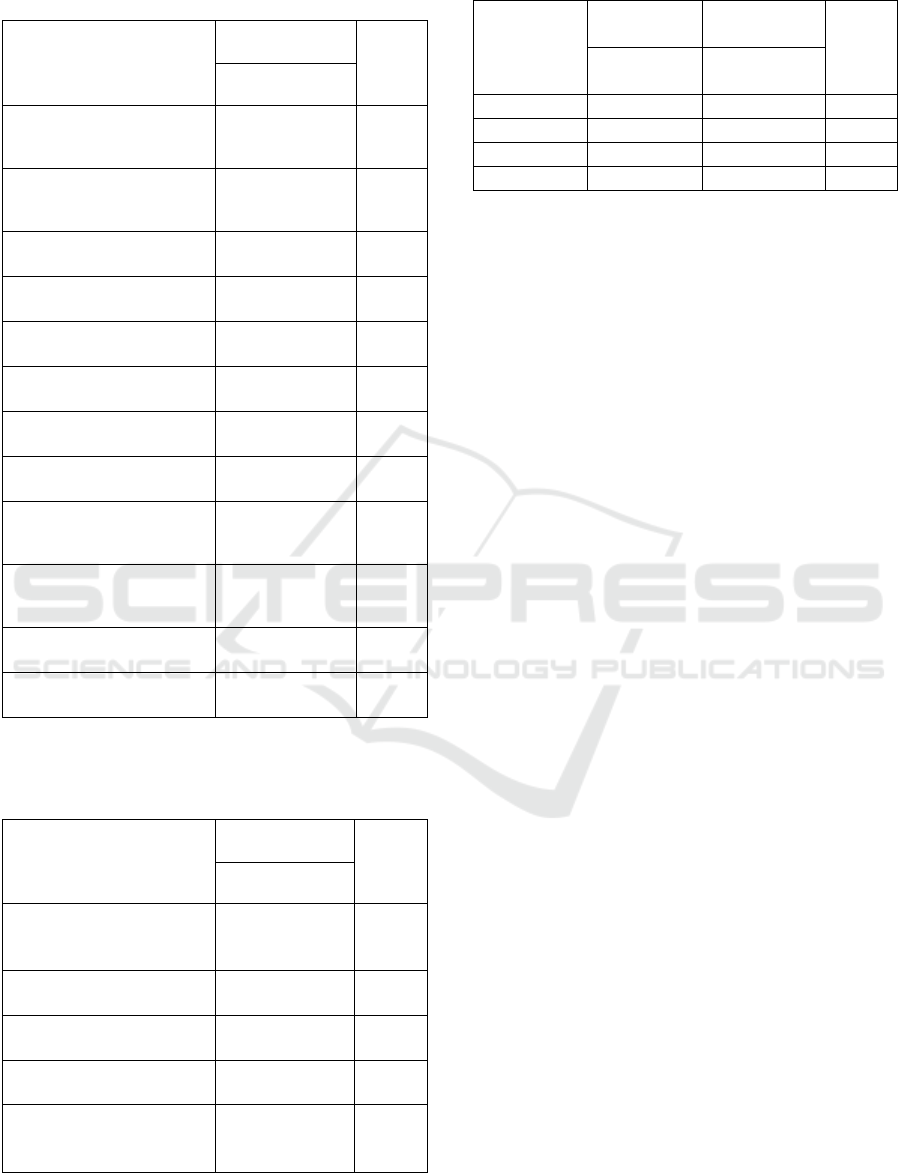
Table 5: Correlation between Gender and side effects of the
vaccine after 6 months.
Variable
Frequency
/
p
ercenta
g
e
(
%
)
P value
Gender n:600,
mean :1.49
Have been exposed to
covid-19 1-3 months after
vaccination
56/33.6 0.309
Have been exposed to
covid-19 4-6 months after
vaccination
48/28.8 0.003
Current menstrual 1-3
months after vaccination
215/1,29 0.000
Current menstrual 4-6
months after vaccination
210/1.26 0.000
Feel easily tired 1-3
months after accination
88/52.8 0.000
Feel easily tired 4-6
months after accination
44/26.4 0.051
Feel pain in arm 1-3
months after vaccination
67/40.2 0.120
Feel pain in arm 4-6
months after vaccination
24/14.4 0.185
Experience heart disorder
1-3 months after
vaccination
4/1.3 0.332
Experience heart disorder
4-6 months after
vaccination
4/1.3 0.331
Cholesterol levels increase
1-3 months after vaccination
2/0.6 0.163
Cholesterol levels increase
4-6 months after vaccination
2/0.6 0.163
*Mann – Whitney test, #Kruskal Wallis test
Table 6: Correlation between body mass index and side
effects of the vaccine after 6 months.
Variable
Frequency
/percentage (%)
P value
BMI n:600,
mean:22.3
Have been exposed to
covid-19 1-3 months after
vaccination
56/33.6 0.305
Current menstrual 4-6
months after vaccination
210/1.26 0.324
Feel easily tired 1-3
months after vaccination
88/52.8 0.116
Feel easily tired 4-6
months after vaccination
44/26.4 0.356
Cholesterol levels
increase 1-3 months after
vaccination
2/0.6 0.033
*Man-Whitney test, #Kruskal Wallis test
Table 7: Correlation kind of vaccine and comorbid.
Variable
Percentage/
frequenc
y
P-
value
Sinovac =
300
AstraZeneca
= 300
Cholesterol 7/3.1 2/0,6 0.176
Gou
t
12/4 1/0.3 0.003
Asthma 10/3.3 5/1.6 0.296
Rheumatic 4/1.3 1/0.3 0.373
3.2 Discussion
Correlation between type of vaccine and side effects
and efficacy of the vaccine 1
st
. There is a significance
between the type of vaccine and the side effects felt
by the patient after receiving dose 1 where the result
showed that the AstraZeneca vaccine had more side
effects than Sinovac. It is known from a total of 600
respondents who received the AstraZeneca vaccine,
that 43,6% felt the side effect of fever, 67,3% feel the
effect of pain at the injection site, 10,3% feel the
effect of coughing, 12,6% feel the effect of flu, 13,3%
feel the effect of nausea, 36,3% feel the effect of
dizzy, 1,3% feel the effect of the cholesterol level
increase. The Indian Ministry of Health Secretary
stated that common side effects of the AstraZeneca
vaccine will disappear within 24 hours. According to
the AstraZeneca company, the prophylactic use of
Acetaminophen can reduce some symptoms.(Ghiasi
et al. 2021). In a study conducted in England
explained that the AstraZeneca vaccine achieved 75%
effectiveness from 35 days after the first dose.
‘(Bernal et al. 2021) but in each country in
determining the criteria for signs and symptoms of
COVID-19 referring to the provisions of WHO
(Hidayani 2020).
Correlation between type of vaccine and side
effect and efficacy of the vaccine 2
nd
. Table 3 explains
the efficacy after being vaccinated with dose 2,
AstraZeneca vaccine has a higher effect as in table 2,
which is also compared to the Sinovac vaccine with
an average p-value below 0.5. development and
antibody levels increase significantly with each dose,
in line with real-world data obtained from the UK
which showed that the second dose increased
protection against SARS- CoV-2 infection from 65%
with dose 1 to 70% with dose 2 among recipients
(Chau et al. 2022).
Correlation between age and side effects of the
vaccine after 6 months. The data in table 4 explains
that age affects the side effects of vaccines because as
a person gets older, the antibodies decrease and vice
versa. At a young age, someone has strong antibodies,
Evaluation of Exposure to COVID-19 From Participants in the Complete Dose of Sinovac and AstraZeneca Vaccinations for Online Drivers
247

but some countries include a priority age criterion for
receiving the Covid19 vaccine. (Voysey et al. 2021).
A study by Muller et al, found that there was a lower
frequency of neutralizing antibodies in the older
population after vaccination compared to the younger
population (Xiong et al. 2021).
Correlation between Gender and side effects of
the vaccine after 6 months. Table 5 by gender shows
that most respondents are male but in table 4 gender
has nothing to do with Covid 19 vaccine. A study
conducted in Malaysia used the chi-square test to
investigate the possible relationship between gender
and perception in receiving accurate and adequate
vaccine-related information.(Elnaem et al. 2021) As
for gender, the Covid 19 vaccination was similar
between males and females. However significantly
higher in males than females (Xiong et al. 2021).
Correlation between body mass index and side
effects of the vaccine after 6 months. In table 6 it is
explained that a BMI below 25 has a risk of the effects
of the covid 19 vaccine. BMI (kg/m²) was taken from
the general practice medical records, and we used the
last measured BMI before study entry for everyone
(Piernas et al. 2022). Because the p-value shows
below 0,5. Based on a study conducted in Spain said
that most of the side effects experienced were
significantly higher in those who were not overweight
compared to those who were overweight. (Iguacel et
al. 2021). Negligence or underrepresentation of
participants with higher weights can result in poorer
outcomes of vaccine coverage for people with higher
body weights and contribute to greater health
inequities (Campbell et al. 2021).
Correlation kind of vaccine and comorbid. It can
be seen in table 7 that the frequency of comorbid from
driver online respondents shows that only 42
respondents (6%) have a comorbid history and 94%
have no comorbid history. Vaccines are only given to
healthy people. But as many as 6% of respondents
have a history of comorbid. Because respondents who
have a history of comorbid are usually more prone to
having a good immune system, someone who has a
history of comorbid diseases can still take part in the
Covid 19 vaccination (Yulyani et al. 2022).
4 CONCLUSION
This study found that the efficacy of the Sinovac and
AstraZeneca vaccines was almost the same for online
drivers in Indonesia. Because it can be seen from the
number of patients exposed to Covid 19 between
Sinovac vaccine recipients and AstraZeneca vaccines
inhibit the same. But the side effects of the
AstraZeneca vaccine are higher than the Sinovac
vaccine. From the table above, it can also be seen that
age and BMI can affect the efficacy of vaccination.
for gender, the comparison is only slightly for the
effect of vaccination.
REFERENCES
Araminda, Gena Nafta, and Diana Laila Ramatillah. 2022.
“Evaluation Comparison Between Astrazeneca and
Moderna Vaccine’S Side Effects and Efficacy Among
Indonesia Society Based on Sociodemography.”
International Journal of Applied Pharmaceutics
14(Special Issue 2): 37–43.
Argista, Zisi Lioni. 2021. Jurnal Keperawatan Persepsi
Masyarakat Terhadap Vaksin Covid-19 Di Sumatera
Selatan.
Bernal, J. L. et al. 2021. “Effectiveness of the Pfizer-
BioNTech and Oxford-AstraZeneca Vaccines on
Covid-19 Related Symptoms, Hospital Admissions,
and Mortality in Older Adults in England: Test
Negative Case-Control Study.” The BMJ 373.
Blum, Bianca, and Bernhard K. J. Neumärker. 2021.
“Lessons from Globalization and the COVID-19
Pandemic for Economic, Environmental and Social
Policy.” World 2(2): 308–33.
Campbell, Jessica et al. 2021. “Equity in Vaccine Trials for
Higher Weight People? A Rapid Review of Weight-
Related Inclusion and Exclusion Criteria for COVID-
19 Clinical Trials.” Vaccines 9(12): 1–11.
Chau, Nguyen Van Vinh et al. 2022. “Immunogenicity of
Oxford-AstraZeneca COVID-19 Vaccine in
Vietnamese Health-Care Workers.” American Journal
of Tropical Medicine and Hygiene 106(2): 556–61.
Elnaem, Mohamed Hassan et al. 2021. “Covid-19
Vaccination Attitudes, Perceptions, and Side Effect
Experiences in Malaysia: Do Age, Gender, and Vaccine
Type Matter?” Vaccines 9(10): 1–15.
Ghiasi, Nasrin et al. 2021. “Efficacy and Side Effects of
Sputnik V, Sinopharm and AstraZeneca Vaccines to
Stop COVID-19; a Review and Discussion.”
Immunopathologia Persa 7(2): e31–e31.
Ghosh, PrasantaKumar. 2021. “Generation of Efficacy
Data on 60 Years and Older Population Using SARS-
CoV-2 Vaccines.” MGM Journal of Medical Sciences
8(3): 289.
Hidayani, Wuri Ratna. 2020. “Faktor Faktor Risiko Yang
Berhubungan Dengan COVID 19 : Literature Review.”
Jurnal Untuk Masyarakat Sehat (JUKMAS) 4(2): 120–
34.
Iguacel, Isabel et al. 2021. “Association between Covid-19
Vaccine Side Effects and Body Mass Index in Spain.”
Vaccines 9(11): 1–12.
Kezia, Valerie, and Diana Laila Ramatillah. 2022.
“Intensive Monitroing of Sinovac Vaccine for Safety
and Efficacy Among Indonesian Population.”
International Journal of Applied Pharmaceutics
14(Special issue 2): 44–48.
ISCP UTA’45 Jakarta 2022 - International Seminar and Call for Paper Universitas 17 Agustus 1945 Jakarta
248

Marwan. 2021. “Peran Vaksin Penanganan Pandemi
COVID19.” Fakultas Kedokteran Universitas
Mulawarman - RSU A. W. Sjahranie Samarinda
1(covid).
Ndwandwe, Duduzile, and Charles S. Wiysonge. 2021.
“COVID-19 Vaccines.” Current Opinion in
Immunology 71(Figure 1): 111–16.
Piernas, Carmen et al. 2022. “Associations of BMI with
COVID-19 Vaccine Uptake , Vaccine Effectiveness ,
and Risk of Severe COVID-19 Outcomes after
Vaccination in England : A Population-Based Cohort
Study.” THE LANCET Diabetes & Endocrinology
10(8): 571–80.
Susilo, Adityo et al. 2020. “Coronavirus Disease 2019:
Tinjauan Literatur Terkini.” Jurnal Penyakit Dalam
Indonesia 7(1): 45.
Sutardi, Azzahrotul Qona’Ah Ibnatus, and Diana Laila
Ramatillah. 2022. “Evaluation Comparison Between
Sinovac and Pfizer Vaccine Among Indonesian
Children and Teenager Under 18 Years Old.”
International Journal of Applied Pharmaceutics
14(Special issue 2): 22–30.
Voysey, Merryn et al. 2021. “Safety and Efficacy of the
ChAdOx1 NCoV-19 Vaccine (AZD1222) against
SARS-CoV-2: An Interim Analysis of Four
Randomised Controlled Trials in Brazil, South Africa,
and the UK.” The Lancet 397(10269): 99–111.
Xiong, Xiaomo et al. 2021. “Age and Gender Disparities in
Adverse Events Following COVID-19 Vaccination:
Real-World Evidence Based on Big Data for Risk
Management.” Frontiers in Medicine 8(July): 1–5.
Yulyani, Vera, Neno Fitriyani Hasbie, Achmad Farich, and
Amelia Valentine. 2022. “Hubungan Status Demografi,
Komorbid Dengan KIPI Post Vaksin COVID-19 Pada
Tenaga Kesehatan.” Jurnal Ilmiah Kesehatan Sandi
Husada 11: 153–60.
Zhang, Haibo et al. 2020. “Angiotensin-Converting
Enzyme 2 (ACE2) as a SARS-CoV-2 Receptor:
Molecular Mechanisms and Potential Therapeutic
Target.” Intensive Care Medicine 46(4): 586–90.
Evaluation of Exposure to COVID-19 From Participants in the Complete Dose of Sinovac and AstraZeneca Vaccinations for Online Drivers
249
