
The Influences of Antioxidants on the Stability of Coix Seed Oil
Liposomes Under Ultraviolet Irradiation
Yin Wang, Songbo Ma, Meilan Yuan
*
, Li Zhao and Chunqing Bai
*
College of Life Science, Jiangxi Science and Technology Normal University, Nanchang, China
* Correspondence Author: 1020140969@jxstnu.edu.cn
Keywords:
Liposomes, Coix Seed Oil, Antioxidant.
Abstract: In this research, coix seed oil (CSO) liposomes containing antioxidants were prepared by ethanol injection
method. The influence of types and concentrations of antioxidants on the physicochemical stability of
CSO liposomes irradiated by ultraviolet (UV) light was investigated in terms of particle size distribution
and malondialdehyde (MDA) production. The results showed that lipid peroxidation and liposomal
particle size change were induced by UV irradiation. The tert-butylhydroquinone (TBHQ) and
dibutyl-hydroxytoluene (BHT) exhibited better resistance on size change and peroxidation induced.
However, β- carotene exerted good anti oxidative activity at low concentration; the antioxidant effect was
weaken and even promoted oxidation at higher concentration. Although the antioxidant effect of
α-tocopherol was enhanced as the concentration increased, its influence on liposomal size varied and
dependent largely on exposed time. In addition, the lower MDA value of CSO liposomes than that of
control indicates the oil could supply anti oxidative activity against the peroxidation of liposomal
membrane. This research would supply good foundation for prolonging the shelf of CSO liposomes.
1 INTRODUCTION
Coix seed is a traditional herbal planted in many
Asian countries, such as Indian, China, and so on,
where the seed could be consumed as medicine and
food (Bai, 2019). According to literatures, the oily
abstracts named as coix seed oil was the main active
ingredients in the seed. Numerous experiments have
proved that the oil exhibited excellent antitumor,
anti-inflammation and analgesia activities (Zhu,
2017). However, the drawbacks of water
water-insolubility, low accessibility, combined with
poor oxidative stability significantly confined the
wide utilization of CSO. Thus, strategies that could
solve the above problems are needed to be thought
out.
To deal with these drawbacks of CSO, various
delivery systems (microcapsule, microsphere,
microemulsion, and liposomes) have been
developed by many researchers (
Nakhaei, 2021;
Chen, 2022)
. During the past several years, we also
carried out relative researches, and liposomes were
used to encapsulate CSO. The delivery system was
proved to be an efficient carrier that could supply
good protection for CSO from adverse environments
and promote controlled release of CSO in
gastrointestinal tract (Bai, 2019). However,
phospholipids, as the main membrane materials for
liposomes, contain certain amount of unsaturated
fatty acids. That means the bilayer is sensitive to
external environment (oxygen, lights, and so on) and
easily to be oxidized and decomposed, producing
harmful chemicals, including lysophospholipids etc.
Introducing antioxidant into liposomes was a useful
way to delay the degradation (
Rafaela, 2021;
Walker, 2017; Palmina, 2021)
. Usually, the
efficiency of the antioxidant activity was largely
dependent on the propertied of liposomes
themselves as well as the types and concentrations
of antioxidant used. However, nothing was known
about how to effectively protect liposomes
containing CSO.
In this sense, four common used antioxidants
(TBHQ, BHT, β-carotene, and α-tocopherol) were
chosen and encapsulated together with CSO to delay
the oxidant of CSO liposomes. The physical and
chemical stability of the liposomes during UV-light
exposure was monitored and determined in details.
This research would supply certain foundation for
the development of stable CSO liposomes.
52
Wang, Y., Ma, S., Yuan, M., Zhao, L. and Bai, C.
The Influences of Antioxidants on the Stability of Coix Seed Oil Liposomes Under Ultraviolet Irradiation.
DOI: 10.5220/0012001400003625
In Proceedings of the 1st International Conference on Food Science and Biotechnology (FSB 2022), pages 52-58
ISBN: 978-989-758-638-5
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

2 MATERIALS AND METHODS
2.1 Materials
Egg yolk phospholipids and cholesterol were bought
from Shanghai Lanji Technology Development Co.,
Ltd (Shanghai, China). Coix seed oil was purchased
from Hecheng Sanxian Biotechnologies Co., Ltd.
(Guangzhou, China). TBHQ, BHT, α-tocopherol,
β-carotene, and 2, 2 -azobis(2-methypropionamidin)
dihydrochloride (AAPH) were kindly supplied by
Sigma-Aldrich (Shanghai, China). All other
chemicals used were of analytical grade.
2.2 Preparation of Liposomes
Liposomes were prepared by ethanol injection
method as described previously by us with slight
modification (
Bai, 2019)
. Briefly, weighted amounts
of antioxidants were dissolved in CSO, and diluted
with the oil to obtain a serial concentration of
antioxidants/CSO solution. The mixture, egg yolk
lecithin and cholesterol were all added into ethanol
and mixed thoroughly until all the agents were
solute. The mixture was then dropped into to a
phosphate buffer solution maintained at 45℃ under
stirring. After 20 mins, the sample was subjected to
rotary evaporation and then sonication treatment.
The obtained samples were stored in refrigerator at
4 ℃ until further use. The nothing loaded liposomes
(control) and CSO loaded liposomes were also
prepared by the same procedure for comparison.
2.3 Particle Size Distribution
The particle size of all liposomal formations were
analyzed on a zeta sizer instrument (Nano ZS90,
Malvern Instruments, Malvern, UK)
2.4 Measurement of Lipid Oxidation
The extent of lipid oxidation in liposomes was
determined by an assay (thiobarbituric acid reactive
substances, TBARS) as described by Walker et al.,
2017 (
Walker, 2017)
. Briefly, 1 mL of liposomes
was added into 5 ml of TBA working solution,
mixed thoroughly and then heated in a water bath at
75℃ for 15 min. This mixture was cooled to room
temperature, and then centrifuged at 2500 rpm for
5min. The absorbance of supernatant at 532 nm was
recorded on a UV-visible spectrophotometer. The
TBARS content was calculated and expressed as ng
MDA equivalent per mg phospholipids.
2.5 UV-light Exposure Stability
In order to accelerate the instability induced by
UV-light irradiation, APPH was added into all
liposomes systems (Pires, 2019). Freshly prepared
liposomes added with APPH (0.05 mol/L) were
placed in closed quartz cells and irradiated with a
254 nm UVC germicide lamp (Philips TUV PL-S
5W/2P 1CT) at a radiance of 1.9W/m2. After
exposure for 15, 30, 45, 60, 90, 120 mins, samples
were withdrawn. The instability caused by UV
radiation was recorded by the determining size
distribution and MDA values.
2.6 Statistical Analysis
All determinations were repeated triplicate, and
presented as means ± standard deviations (SD). The
results were analyzed statistically for significance
(p≤ 0.05) using SPSS 18.0 software.
3 RESULTS & DISCUSSION
3.1 The Irradiation Stability of
Liposomes at 0.002% Antioxidant
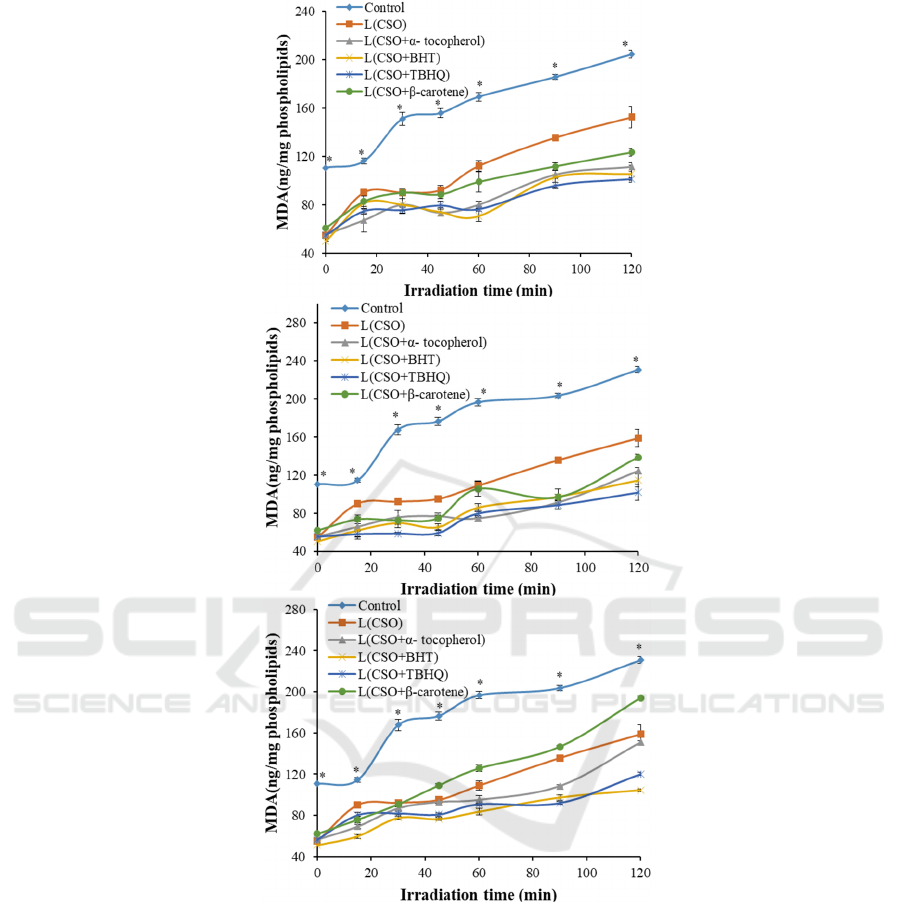
Fig. 1A shows changes of the peroxidation product
in liposomes during irradiation. Obviously, the
MDA values of the control (nothing was loaded)
increased as the exposure time increased, indicating
oxidation of the liposomal bilayer occurred.
Meanwhile, the values of CSO loaded liposomes
were generally lower than that of the control
(p<0.05), suggesting the embedding CSO could
somewhat delay the oxidative degradation of the
membrane. This may be accounted to the antioxidant
activity of un-entrapped CSO. According to
literatures, the content of unsaturated fatty acid in
CSO was higher than 70% (Xiao, 2019). The
protective role of CSO may be originated from the
oxidization itself. In addition, the peroxidation
produced in liposomes containing antioxidants was
lower than that in CSO liposomes. What's more, the
MDA values of them was in the order of
THBQ>BHT>α-Tocopherol>β-carotene. The higher
antioxidant effect of β-carotene may be explained by
its conjugated polyenes structure, which could help
to remove free radicals and quench singlet oxygen,
as a result, inhibiting the decomposition of primary
oxidation products to secondary oxidation products
(
Walker,
2017). The result was consistent with our
previous report that the co-loaded CSO and β-
carotene exhibited synergistic antioxidant (Bai, 2019).
The Influences of Antioxidants on the Stability of Coix Seed Oil Liposomes Under Ultraviolet Irradiation
53
A
B
C
*indicates the value of the Control is significantly different from that of all the other liposomes at each time point (p<0.05).
Figure 1: The effects of antioxidant on oxidation stability of CSO liposomes during UV irradiation. A (0.002%), B (0.01%),
C (0.02%).
Figure 2 shows that particle size distribution of all
samples generally shifted to higher values after UV
irradiation. What’s more, the longer the exposure
time the larger the particle was. The results were in
consistence with previous reports (
Palmina, 2021;
Pires, 2019). Pires et al. (Pires, 2019) found that UV
irradiation affects the phosphate and carbonyl
groups of 1, 2-dipalmitoyl-sn-glycero-3-
[phospho-rac-(1-glycerol) in liposome. The enlarged
particle size may be due to the oxidation of
phospholipids induced by UV irradiation, which
might change the emulsifying property of
phospholipids, and disturbing the integrity and
stability of liposomes in the end. Meanwhile, more
encapsulated CSO may have leaked out from
liposomes and absorbed on the surface of bilayers as
the result of the changed integrity, that the increased
viscosity may promote particle aggregation (Sabet,
2021). In addition, the particle size changes for
liposomes containing CSO+BHT was the smallest,
indicating incorporating BHT into liposomes could
supply better shield against UV radiation.
FSB 2022 - The International Conference on Food Science and Biotechnology
54
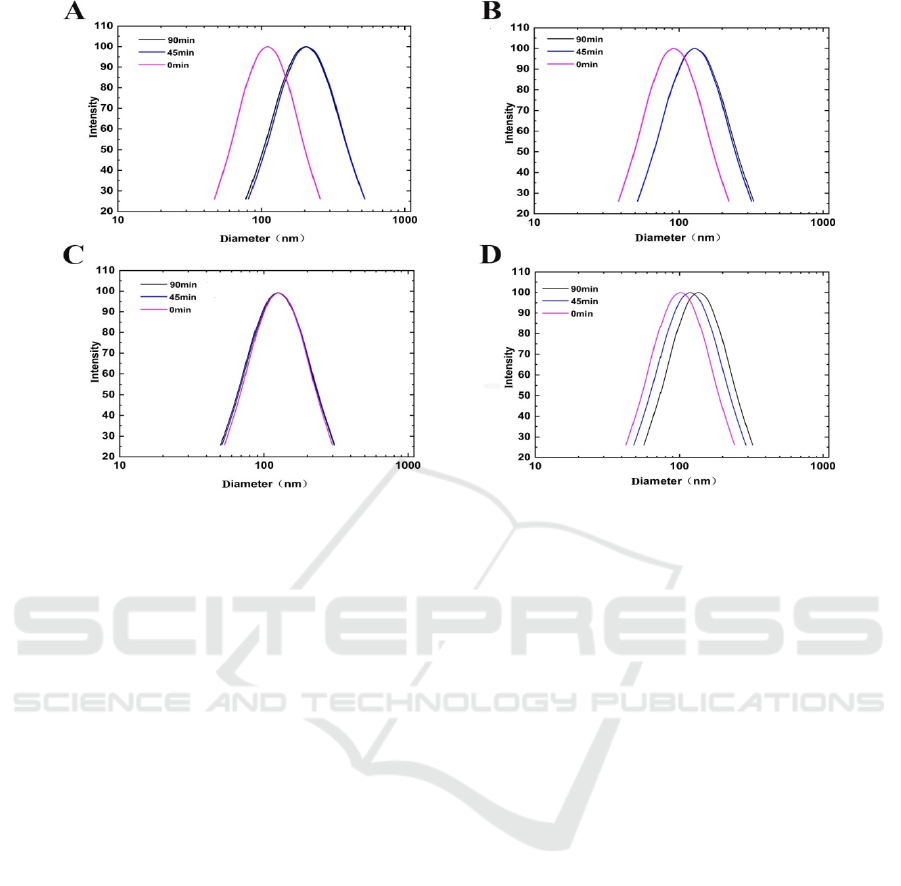
Figure 2: The effects of 0.002% antioxidant on the particle size distribution of liposomes during irradiation. A:
L(CSO+β-carotene), B: L(CSO+TBHQ), C: L(CSO+BHT), D: L (CSO+α- tocopherol).
3.2 The Irradiation Stability of
Liposomes at 0.01% Antioxidant
Similarly, the MDA value were all increased as the
function of time, and the values were in the order of
control>CSO liposomes>CSO + antioxidant
liposomes at fixed irradiation time when 0.01%
antioxidant was embedded (Fig. 1B). It indicates
that all types of antioxidant exhibited certain
inhibition effect on the degradation of liposomes
(Temprana, 2011). In addition, comparing with Fig.
1A, the MDA values increased more slowly,
indicating fewer lipids were decomposed and the
anti-oxidative activity of antioxidant were increased
when the concentration of antioxidant increased
(Feng, 2018). However, nearly no significant
difference was detected among the liposomes loaded
with antioxidant.
Figure 3 shows that the particle size distribution
of the liposomes added with BHT or TBHQ
exhibited slightly shifts during irradiation, while that
of ones enclosed with α-tocopherol or β-carotene
was more complex. This suggests that BHT and
TBHQ at higher concentration could supply good
protection against the size change induced by
UV-light. It could be found that, the size of CSO+α-
tocopherol co-loaded liposomes became larger after
exposure for 45 mins, whereas, the size changed to
much smaller after 90 mins’ irradiation. According
to literatures, there are continuous movement and
collision among liposomal particles (Brilliantov,
2007). What’s more, the leakage of embedded
materials and state transition of bilayer membranes
co-existed during irradiation and affected the
collision (Pires, 2019). When the irradiated time was
short, α-tocopherol could supply efficient protection
for phospholipids, resulting in low extent of
oxidation. At this stage, the increase in particle size
caused by the collision may be dominantly
influenced by the leakage of embedded materials,
which enhanced the surface viscosity of liposomes
and increased the chances adhering with each other.
However, a large part of unsaturated fatty acids
might have undergone peroxidation during
long-term irradiation since the limited ant oxidative
activity of α-tocopherol. As a result, the melting
point of phospholipid bilayer membrane was
increased and transferred to gel phase, leading to
increased rigidity with decreased deformability for
liposomal membrane, which in turn inhibited the
leakage of CSO.
3.3 The Irradiation Stability of
Lipidosomes at 0.02% Antioxidants
Fig. 1C showed that the general trends of all
samples at 0.02% antioxidants were increased as
increasing the irradiation time. What’s more, the
The Influences of Antioxidants on the Stability of Coix Seed Oil Liposomes Under Ultraviolet Irradiation
55
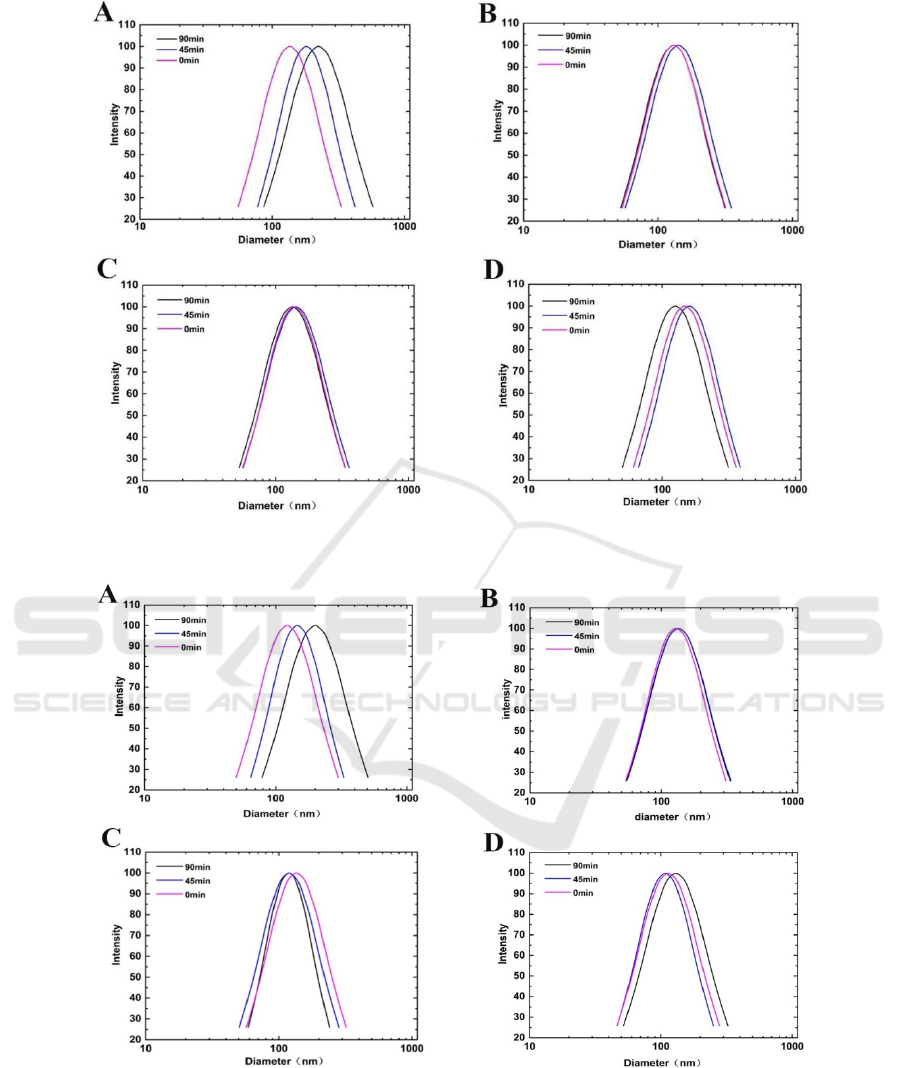
Figure 3: The effects of 0.01% antioxidant on the particle size distribution of liposomes during irradiation. A:
L(CSO+β-carotene), B: L(CSO+TBHQ), C: L(CSO+BHT), D: L (CSO+α- tocopherol).
Figure 4: The effects of 0.02% antioxidant on the particle size distribution of liposomes during irradiation. A:
L(CSO+β-carotene), B: L(CSO+TBHQ), C: L(CSO+BHT), D: L (CSO+α- tocopherol).
MDA value was lower than that with less
antioxidant, except for the one with β-carotene.
Obviously, the MDA value of liposomes containing
CSO+ β-carotene was higher than that containing
less β-carotene after irradiation for the same time.
What’s more, some values were even higher than
that of CSO liposomes. This suggests that
β-carotene might exhibit good antioxidative at
FSB 2022 - The International Conference on Food Science and Biotechnology
56

limited concentration, whereas would promote
oxidation when excess certain concentration.
Scoccianti et al. reported tocopherols could help
against lipid peroxidation induced by 1 mM Cr(III),
but generated oxidative stress at the highest
concentration (Scoccianti, 2016).
Figure 4A shows that there are significant
changes in particle size distribution of L
(CSO+β-carotene) during irradiation. And the mean
particle size was in the order of 90 mins treated > 45
mins treated > freshly prepared, indicating
irradiation induced polymerization of the liposome.
As to L (CSO+α- tocopherol), the mean particle size
of 90 mins treated was smallest, while 45 mins
treated samples were the largest. On the contrary, the
changes in L (CSO+TBHQ), L (CSO+BHT) were
not so significant, indicating that TBHQ and BHT
had a strong irradiation stabilization effect at this
concentration.
4 CONCLUSIONS
In this research, the influence of TBHQ, BHT, α-
Tocopherol and β-carotene on the physiochemical
stability of CSO liposomes during UV irradiation
were investigated. The results showed that CSO
could exert certain protection for liposomal bilayer
from oxidation. The ant oxidative efficiency of
antioxidant was largely dependent on the type,
concentrations, and exposure time. β -carotene could
supply good shield at low concentration, whereas
promote oxidation when the concentration increased
to 0.2%. On the contrary, TBHQ and BHT exhibited
good irradiation stabilization effect, and nearly no
particle size changes were detected for all
concentrations. Although the oxidation of CSO
liposomes could be inhibited by α- tocopherol; its
particle size stabilization function was limited.
However, whether these antioxidants influence the
leakage of CSO and the structural integrity of
liposomal bilayer need further investigation.
ACKNOWLEDGMENTS
This research was funded by the Jiangxi Provincial
Department of Education Project (Grant number
GJJ201114), National Natural Science Funds of
China (Grant number 31560465), and Open fund
project of Jiangxi Aquatic Product Processing and
Safety Control Engineering Research Center (Grant
number KFJJ2101, KFJJ2102).
REFERENCES
Bai, C., Zhen, J., Zhao, L., Chen, L., Xiong, H.,
McClements, D.J. (2019) Development of oral
delivery systems with enhanced antioxidant and
anticancer activity: Coix seed oil and β Carotene
co-loaded liposomes. J Agri food Chem. 67: 406–
414. DOI:10.1021/acs.jafc.8b04879.
Brilliantov, N.V., Albers, N., Spahn, F. (2007) Collision
dynamics of granular particles with adhesion. Physical
Review E. DOI:10.1103/PhysRevE.76.051302.
Chen, C., Sun-Waterhouse, D., Zhao, J., Zhang, Y.,
Waterhouse, G.I.N., Lin L., Zhao, M., Sun W.
(2022) Method for loading liposomes with soybean
protein isolate hydrolysate influences the
antioxidant efficiency of liposomal systems: Adding
after liposomes formation or before lipid film
hydration. Food Hydrocolloid. DOI:
10.1016/
j.foodhyd.2022.107629.
Feng, J., Cai, H., Wang, H., Li, C., Liu, S. (2018)
Improved oxidative stability of fish oil emulsion by
grafted ovalbumin-catechin conjugates. Food Chem.
241: 60–69. DOI:10.1016/j.foodchem.2017.08.055.
Nakhaei, P., Margiana, R., Bokov, D.O., Abdelbasset,
W.K., Kouhbanani, M.A.J., Varma, R.S., Marofi, F.,
Jarahian
, M., Beheshtkhoo, N. (2021) Liposomes:
structure, biomedical applications, and stability
parameters with emphasis on cholesterol. Front Bioeng
Biotechnol. DOI:10.3389/fbioe.2021.705 886.
Palmina, N.P., Sazhina, N.N., Bogdanova, N.G., Antipova,
A.S., Martirosova, E.I., Plashchina, I.G., Kasparov
,
V.V., Semenova, M.G. (2021) The
physico-chemical properties of liposomes made
from lipids of the liver and brain of mice receiving
nanolipid complexes. Biophysics, 66: 786–796.
DOI:10.1134/S0006350921050171.
Pires, F., Geraldo, V.P.N., Antunes, A., Marletta, A.,
Oliveira, O.N., Raposo, M. (2019) On the role of
epigallocatechin-3-gallate in protecting phospholipid
molecules against UV irradiation. Colloids Surf B
Biointerfaces. 173: 312
–
319. DOI:10.1016/j.colsurfb.
2018.09.065.
Rafaela, L., Marlene, C., Mariana,F., Paula, G., Fátima,
P.M. (2021) A new family of hydroxytyrosol
phenolipids for the antioxidant protection of
liposomal systems. BBA – Biomembranes.
DOI:10.1016/j.bbamem.2020.183505.
Sabet, A., Rashidinejad, A., Qazi, H.J., McGillivrayac,
D.J. (2021) An efficient small intestine-targeted
curcumin delivery system based on the
positive-negative-negative colloidal interactions. Food
Hydrocolloids. DOI:10.1016/j.foodhyd.2020.106375.
Scoccianti, V., Bucchini, A.E., Lacobucci, M., Ruiz, K.B.,
Biondi, S. (2016) Oxidative stress and antioxidant
responses to increasing concentrations of trivalent
chromium in the andean crop species chenopodium
quinoa willd. Ecotox Environ Safe. 133: 25–35.
DOI:10.1016/j.ecoenv.2016.06.036.
Temprana, C.F., Amor, M.S., Femia, A.L., Gasparri, J.,
Taira, M.C., Alonso, S.V. (2011) Ultraviolet irradiation
The Influences of Antioxidants on the Stability of Coix Seed Oil Liposomes Under Ultraviolet Irradiation
57

of diacetylenic liposomes as a strategy to improve size
stability and to alter protein binding without
cytotoxicity enhancement. J Liposome Res. 21(2):
141
–
50. DOI:10.3109/08982104.2010.492477
Walker, R. M., Gumus, C. E., Decker, E. A.,
McClements, D. J. (2017) Improvements in the
formation and stability of fish oil-in-water
nanoemulsions using carrier oils: MCT, thyme oil,
& lemon oil. J Food Eng. 211: 60–68.
DOI:10.
1016/j.jfoodeng.2017.05.004.
Xiao, X., Wang, F., Zhou, J., Luo, J., Li, J., Yi, X. (2019)
Oral delivery of coix seed oil in o/w microemulsion:
Preparation, characterization, and in vitro and in vivo
evaluation. J Drug Deliv Sci Tec. DOI:10.1016/
j.jddst.2019.101325.
Zhu, F. (2017) Coix: Chemical composition and health
effects. Trends Food Sci Technol. 61: 160–175.
DOI:10.1016/j.tifs.2016.12.003.
FSB 2022 - The International Conference on Food Science and Biotechnology
58
