Production and Application of Lactic Acid: A Review
Zike Zhou
*
Shanghai Qibaodwight High School, Shanghai, 201101, China
Keywords: Lactic Acid, Fermentation, Bacteria, Preservative, Renewable Plastic.
Abstract: In organic chemistry and food science, lactic acid is one of the most industrially widely used
hydroxycarboxylic acids. This work focuses on the production method, fundamental properties, the reason
for lactic functioning as a preservative, and various applications of lactic acid in different areas. The common
pathways to produce lactic acid are microorganism fermentation and chemical synthesis. Furthermore, the
acidity due to hydrogen bonding and the opposite effect of the hydroxyl group makes lactic acid a good
preservative. In addition, Poly Lactic Acid (PLA) originates from lactic acid and is another promising
application from industrial uses to household usage, such as food takeaway containers.
1 INTRODUCTION
Lactic acid also expressed as 2-hydroxypropanoic
acid or milk acid has molecular formula of
CH
3
CH(OH)COOH and C
3
H
6
O
3
. Its molar mass is
90.078g mol
-1
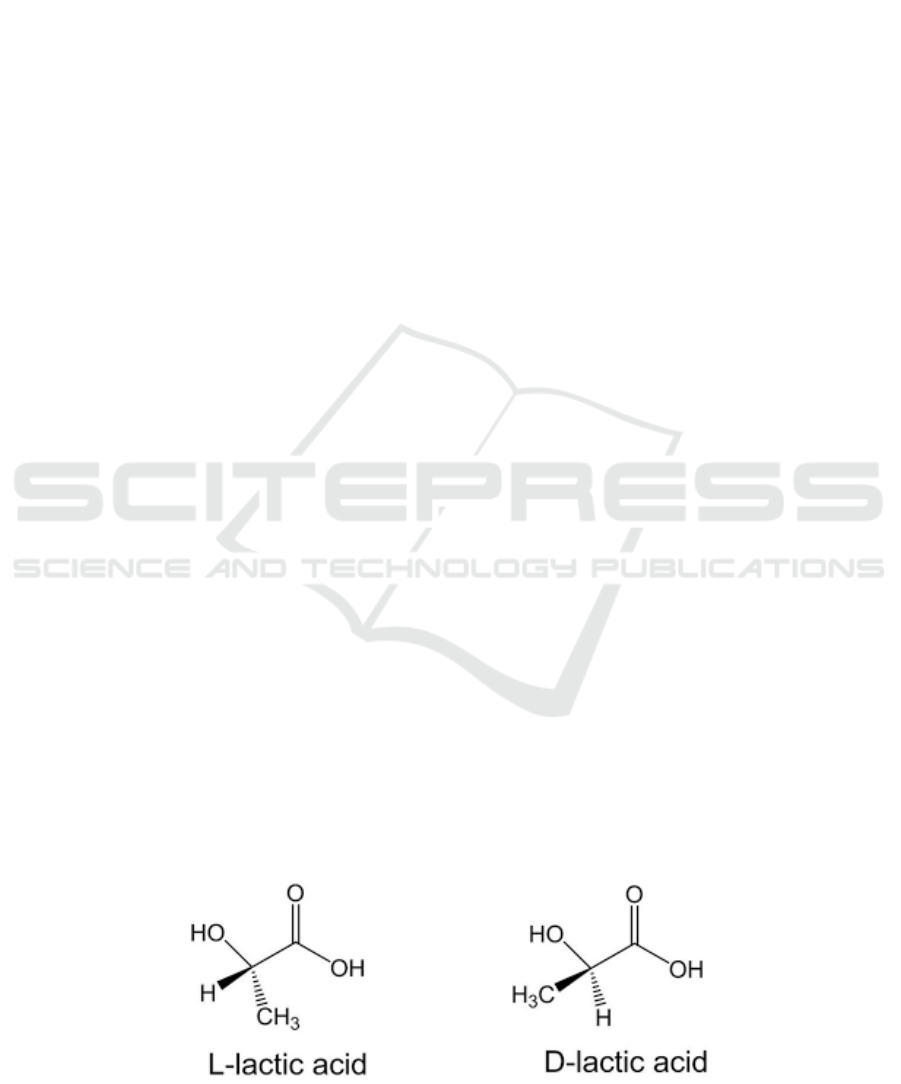
. It exists in two enantiomeric forms:
L(+)-lactic acid or D(-)-lactic acid. The structures are
shown in Figure 1.
L-lactic acid can be used for
metabolizing by the human body or animals, so it's
more beneficial for organisms, while the D-lactic acid
cannot be metabolized, and a large proportion of it is
excreted (Dashmeet, 2018).
Lactic acid was first found by C.W.Scheele in sour
milk in 1780, and Fermi successfully extracted lactic
acid by fermentation in 1881, which was then used in
the industrial synthesizing of lactic acid. Lactic acid
exists in different fermented foods like yoghurt and
butter. By 2012, the demand for lactic acid was over
259,000 metric tons per year (Castillo, 2013). The
vast demand is attributed to several functions of lactic
acid.
Lactic acid is widely used as a preservative to
prevent food spoilage by inhibiting the growth of
putrefying bacteria. When it is changed into
potassium or sodium lactate, the shelf life of fish and
meat can be extended by its addition. It also serves as
an acidulant to add savoury flavour to pickled
vegetables, beverages, and baked products. As a pH
regulator, lactic acid produces chocolates and sweets
to achieve the correct pH value.
The textile industry works as a mordant (fixative)
to dye clothes. In addition, lactic acid can be
converted to ethanol, propylene glycol, and acrylic
polymers. In the pharmaceutical industry, lactic acid
works as an electrolyte in implants, pills, and dialysis.
In the cosmetic industry, lactic acid has the functions
of brightening skin and helping remove the brown
spots on the skin. The role of moisturizer due to its
retaining water capacity also makes lactic acid a
popular ingredient in hygiene and aesthetic products
(Krishna, 2019).
Figure 1: The structures of D(-)-lactic acid and L(+)-lactic acid (Dashmeet, 2018).
Zhou, Z.
Production and Application of Lactic Acid: A Review.
DOI: 10.5220/0012003800003625
In Proceedings of the 1st International Conference on Food Science and Biotechnology (FSB 2022), pages 143-148
ISBN: 978-989-758-638-5
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
143
For industrial production, there are several pathways
to obtain lactic acid. The two most popular ones are
fermentation and chemical synthesis (Krishna, 2019).
2 FERMENTATION FOR LACTIC
ACID
Fermentation is a relatively fast and cheap way to
lead one of the enantiomers of lactic acid with high
yields. However, like any other fermentation process,
its yield and efficiency are determined by starting raw
materials, nutrients in the medium and the
microorganisms used, such as bacteria type
(Krishna,
2019).
There are two main patterns of lactic acid bacteria
used in fermentation. The first one is
heterofermentative; these organisms produce several
byproducts and are, therefore, unsuitable for
industrial processes. The second one is
homofermentative. Those organisms only produce
fewer byproducts, but the large yield of lactic acid is
used in commercial production (Dashmeet, 2018).
Table 1 is a summary table of the fermentation
bacteria and their respective isomer result,
fermentation pattern and necessary raw materials.
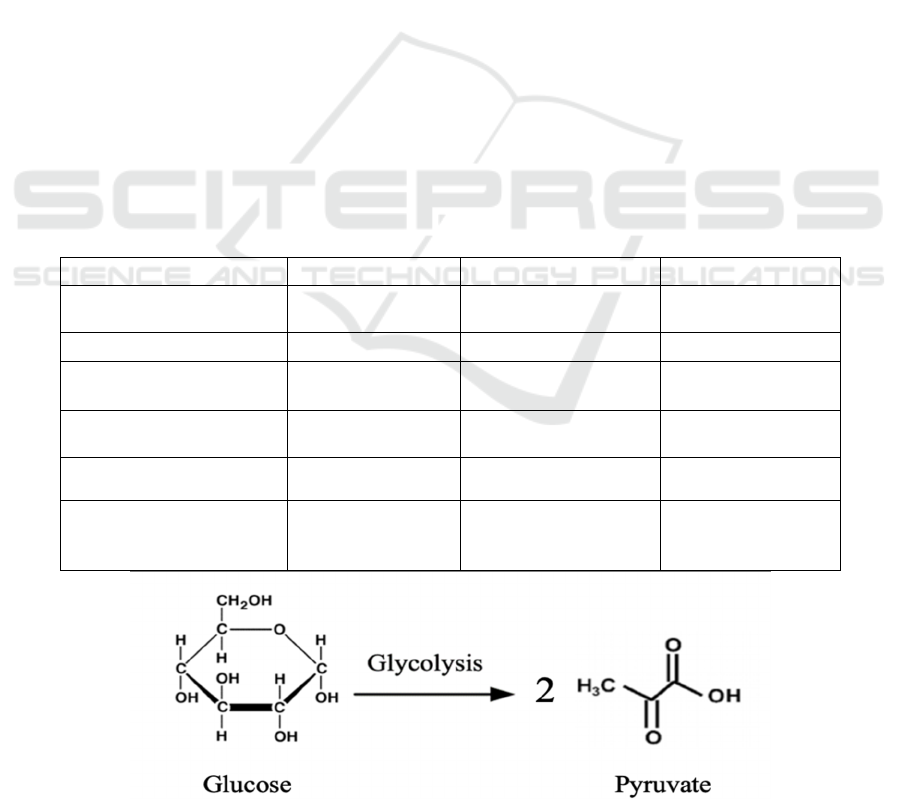
The first kind of fermentation: homofermentative
fermentation produces more than 85% lactic acid
from glucose (Boontawan, 2010). One mole of
glucose can be converted to two moles of lactic acid.
The reaction process is shown by the diagram below.
Step 1.
Figure 2 shows the first step of homofermentative
fermentation. In step 2 shown by Figure 3, pyruvate
is reduced from the aldehyde or ketone oxidation
level to the alcohol oxidation level. The NADH, also
known as dihydropyridine, loses one proton and
becomes positively charged as NAD
+
.
Figure 4 shows the second kind of fermentation:
heterofermentative fermentation produces only 50%
lactic acid and large quantity of ethanol, and carbon
dioxide. With the help of bacteria, 1 mole of glucose
can be converted to 1 mole of lactic acid, 1 mole of
ethanol, and 1 mole of carbon dioxide (Boontawan,
2010).
3 INDUSTRIAL WAY OF
SYNTHESIZING LACTIC ACID
(DRAW DETAILED SCHEME)
The industrial process for commercial production is
Table 1: Characteristics of selected bacteria and molds of interest in lactic acid production (Dashmeet, 2018).
Microorganism Lactic acid isomer Fermentation pattern Raw material
Bacteria:
Lactobacillus amylophilus
L (-) Homofermentative Starch
L. amylovorus DL Homofermentative Starch
L. casei subsp. Rhamnosus
(L. delbueckii NRRL B-445)
L (+) Facultative
heterofermentative
Glucose, sucrose
(molasses)
L. delbueckii subsp.
bulgaricus
D (-) Homofermentative Cheese whey and
permeate (Lactose)
L. helviticus DL Homofermentative Cheese whey and
permeate (Lactose)
Molds:
Rhizopus arrhizus
R. oryzae
L (+)
L (+)
Homofermentative
Homofermentative
Glucose, starch
Glucose, starch
Figure 2: First step of homofermentative fermentation.
FSB 2022 - The International Conference on Food Science and Biotechnology
144
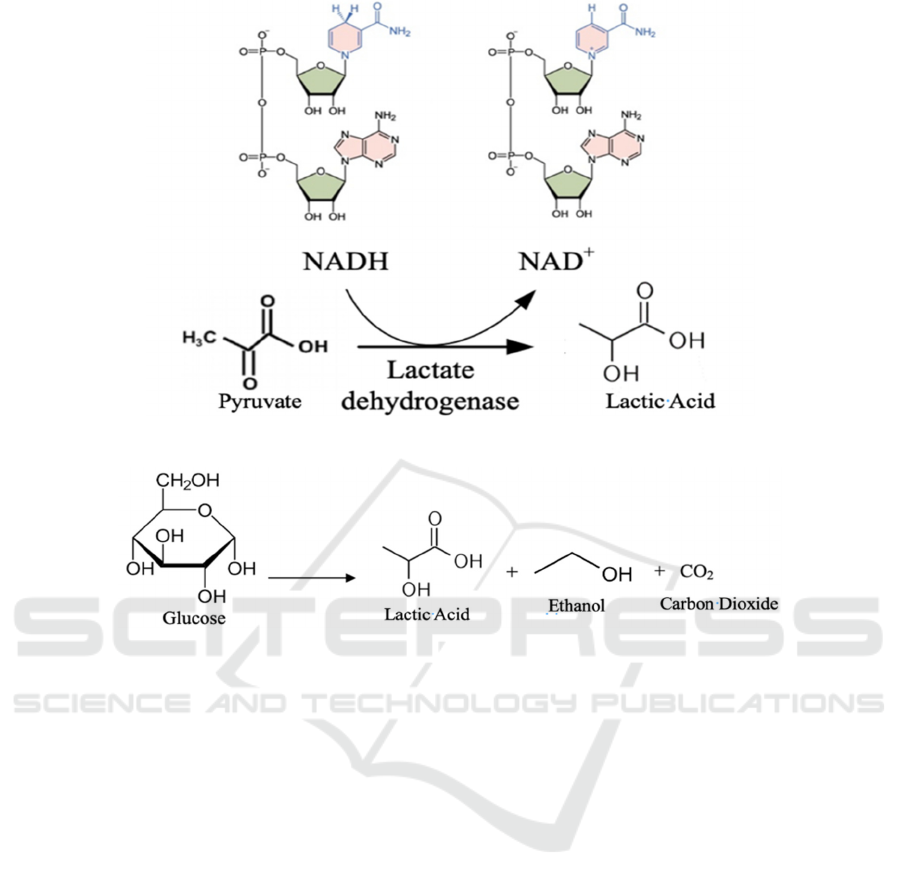
Figure 3: Second step of homofermentative fermentation.
Figure 4: Heterofermentative fermentation.
also based on chemical synthesis. The starting
material is lactonitrile produced by acetaldehyde and
hydrogen cyanide. The reaction should be conducted
in liquid phase under high atmospheric pressures. The
crude lactonitrile is then purified by distillation. After
that, concentrated HCl or H
2
SO
4
is used to hydrolyze
the lactic acid to produce ammonium salt and lactic
acid. As methyl lactate is easier to purify through
distillation, the product lactic acid is then esterified
with methanol. After distilling, methyl lactate is
hydrolyzed by water under an acid catalyst to produce
purer lactic acid and methanol. Unlike the
fermentation process, the chemical synthesis method
would produce a mixture of DL-lactic acid. The
process is presented in Figure 6 and Figure 7.
(Boontawan, 2010).
4 LACTIC ACID AS A
PRESERVATIVE
The growth of bacteria and its releasing of mycotoxin
in food are main factors causing food spoilage and
food poisoning. Lactic acid can permeate into the
membrane of the bacteria, reducing the intracellular
pH, to kill the food spoilage bacteria such as
Enterobacteriaceae and Pseudomonadaceae.
(Nasrollahzadeh, 2022)
It is more acidic than another organic acid in
households: acetic acid. Lactic acid has pKa value of
3.86, due to the existence of hydrogen bonding and
the polar effect of hydroxyl group.
In Figure 7, there are two attraction forces from O
to the H in the bottom right. Shown in the right part
of the diagram, H is relatively more electronegative
than O, so the O atom will make the H atom more
positive. The proton boxed is therefore more acidic.
In addition, hydroxyl OH is an electronegative
functional group, so it will further pull the electrons
far away from the middle H as lactic acid has two
hydroxyl group, which results in the polar effect.
Production and Application of Lactic Acid: A Review
145
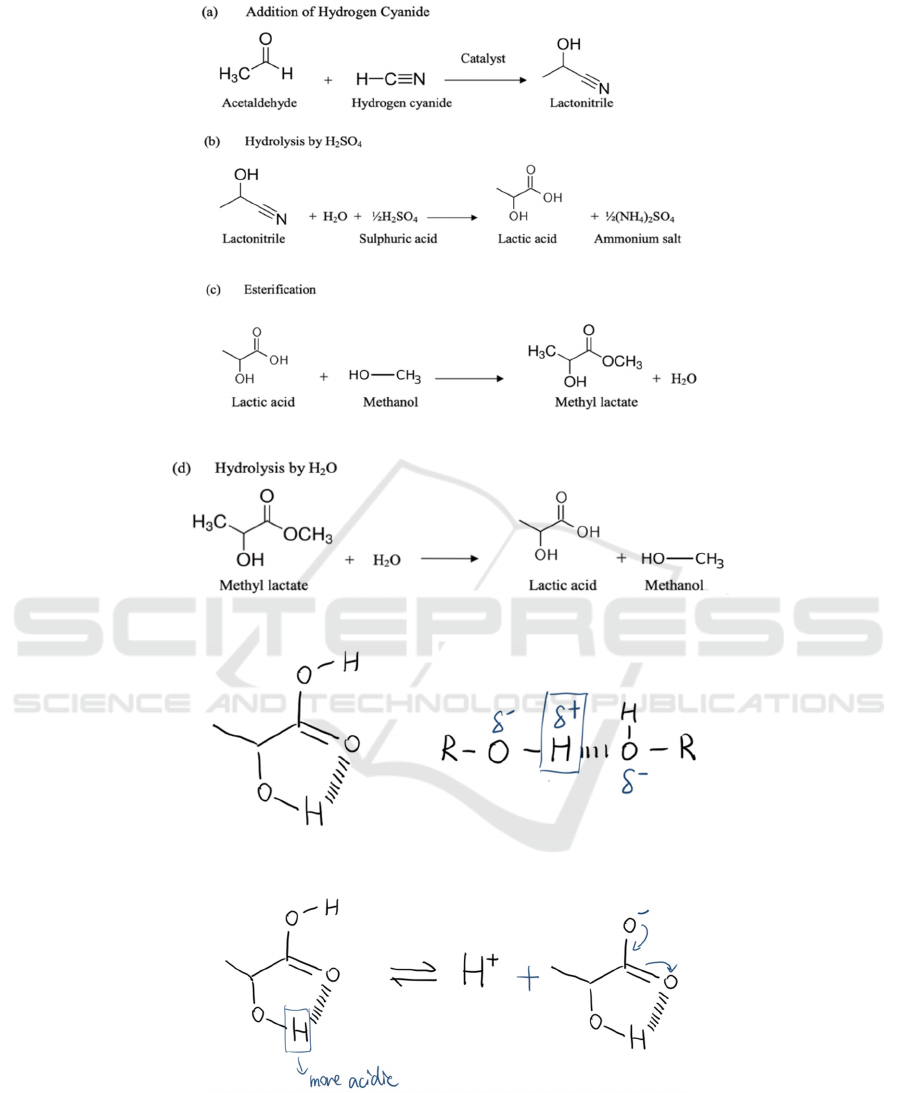
Figure 5: Step (a)(b) and(c) of chemical synthesis.
Figure 6: Step(d) of chemical synthesis
Figure 7: Lactic acid with hydrogen bonding.
Figure 8: Formula showing resonance form of lactic acid.
Figure 8 illustrates the resonance form when the
lactic acid loses a proton. The negative charge can
either stay on the top O atom or come down as a
double bond and push the electron to the left O atom.
The two resonance forms also help the negative
charge to spread over the conjugate base of lactic
acid, making it more stable. Therefore, it further
lowers the pKa of lactic acid.
As the growth of bacteria is inhibited by the acidic
environment, lactic acid functions as a good inhibitor.
FSB 2022 - The International Conference on Food Science and Biotechnology
146

Along with its flavouring function and little smell,
lactic acid outstands among a series of organic acids
and becomes a popular food preservative
(Nasrollahzadeh, 2022).
5 OTHER APPLICATIONS OF
LACTIC ACID
Lactic acid can serve as a starting material and
involves in numerous reactions. Table 2 is a summary
table of different kinds of reactions.
The most common reaction involving lactic acid
is to produce lactide, which can be further
transformed into poly lactic acid (PLA). PLA is a
biodegradable plastic and is applied in daily life as
food packages, containers, trash bags, protective
clothing, etc. The full application is shown by Table
3 (Södergård, 2002).
Table 2: Reactions and chemical produced involving lactic
acid (Krishna, 2019).
Reaction Chemical produced
Hydrogenation Propylene oxide
Decarboxylation Acetaldehyde
Deh
y
dration Acr
y
lic aci
d
Reduction Pro
p
anoic aci
d
Condensation 2,3-
p
entanedione
Self-esterification Dilactide
Jem and Tan use a diagram (Figure 9) to illustrate
two pathways from lactic acid to PLA. One is through
condensation, depolymerization, and ring opening
polymerization. The other one involves only
azeotropic dehydrative condensation (Jem, 2020).
Table 3: Polymers types and their applications (Jem and Tan).
Lactide Pol
y
mers A
pp
lications
L-lactide L-lactide for producing PLLA
Membranes and films for medical applications and 3D printing for
p
rosthesis
D-lactide D-lactide for
p
roducin
g
PDLA H
y
dro
g
el and
p
articles for dru
g
deliver
y
L-lactide L-lactide with PEG
Medical applications, drug vehicles, nanoparticles loaded with bioactive
compounds, treatment for cancer and infections
D-lactide D-lactide with PEG Biochemical device and packaging
L-lactide
L-lactide with poly (trimethylene
carbonate)
Biodegradable elastomeric scaffold for vascular engineering
L-lactide L-lactide with PCL
Absorbable suture medical application due to good tensile properties
Packa
g
in
g
a
pp
lication thanks to tunable barrier
p
ro
p
erties
L-lactide/
D-lactide
Lactide with lignin Bio-based composite materials
L-lactide/
DL-lactide
L-lactide with 𝜀-caprolactone and
h
y
drox
y
a
p
atite
Composite materials for bone reconstruction
L-lactide L-lactide with hydroxyapatite Composite scaffolds for bone tissue engineering
L-lactide
L-lactide, glycolide, butyl
succinate/citrate
Bioabsorbable block copolymers for tissue engineering
L-lactide L-lactide with PGA Smart
p
ol
y
mer used as dru
g
deliver
y
device
Figure 9: Reactions of converting lactic acid into PLA.
Production and Application of Lactic Acid: A Review
147

6 CONCLUSION
Lactic acid is widely used in the food, textile,
pharmaceutical, and cosmetic industry as a
preservative, flavouring agent, pH regulator and
moisturizer. It can be obtained through chemical
synthesis or biological fermentation as a functional
organic compound. The selection of a certain
enantiomer of lactic acid is possible in fermentation
by choosing different types of bacteria. Lactic acid
can also be a starting material to produce PLA, a
promising bio-degradable plastic waiting for further
study.
REFERENCES
Boontawan, P 2010, ‘Development of Lactic Acid
Production Process from Cassava By Using Lactic Acid
Bacteria’, Doctor Thesis, Suranaree University of
Technology, pp. 1–204.
Castillo M, FA, Balciunas, EM, Salgado, JM, Domínguez
González, JM, Converti, A & Oliveira, RP de S 2013,
‘Lactic acid properties, applications and production: A
review’, Trends in Food Science & Technology, vol.
30, no. 1, pp. 70–83.
Dashmeet 2018, Lactic Acid: Structure, Biosynthesis,
Fermentation Process and Uses in Food | Industries |
Biotechnology, BioTechnology Notes.
Jem, KJ & Tan, B 2020, ‘The development and challenges
of poly (lactic acid) and poly (glycolic acid)’, Advanced
Industrial and Engineering Polymer Research, vol. 3,
no. 2.
Krishna, BS, K.V, NS, Gantala, S, Tarun, B & Gopinadh,
R 2019, ‘Industrial production of lactic acid and its
applications’, International Journal of Biotech
Research, vol. 1, no. 1, pp. 42–54.
Nasrollahzadeh, A, Mokhtari, S, Khomeiri, M & Saris, PEJ
2022, ‘Antifungal Preservation of Food by Lactic Acid
Bacteria’, Foods, vol. 11, no. 3, p. 395.
Södergård, A & Stolt, M 2002, ‘Properties of lactic acid
based polymers and their correlation with
composition’, Progress in Polymer Science, vol. 27, no.
6, pp. 1123–1163.
FSB 2022 - The International Conference on Food Science and Biotechnology
148
