
Chronic Toxicity of Silver Nanoparticles to Tigriopus Japonicus
Xinwei Wang
*
and Jiaying Zhao
Key Laboratory of Marine Environment and Ecology, Ministry of Education (Ocean University of China),
Qingdao, P. R. China
Keywords: Silver Nanoparticles, Chronic Toxicity.
Abstract: Silver nanoparticles (Ag NPs) have been widely used because of their excellent bactericidal properties, and
with them comes their massive discharge, which may pose potential risks to marine ecology and the
environment, but relatively few studies have been conducted on the chronic toxicity of Ag NPs to marine
organisms. Here, the marine copepod Tigriopus japonicus was used to investigate the effects of Ag NPs on
the survival, development, and reproduction under two generations. The results showed that Ag NPs
significantly increased the mortality of T. japonicus at 0.2 mg/L. In the F
0
generation, 0.3 mg/L Ag NPs
significantly prolonged the developmental time of T. japonicus, while in the F
1
generation, it was significantly
prolonged at only 0.1 mg/L. And Ag NPs significantly inhibited the hatching number of T. japonicus at F
0
generation while it was alleviated at F
1
generation.
1 INTRODUCTION
Silver nanoparticles (Ag NPs) are widely used in a
variety of consumer products, including textiles, care
products, and food packaging, due to their
physicochemical properties, especially their excellent
bactericidal effect. It was estimated that the global
production of Ag NPs was 500 tons/year. and more
than 60 tons of Ag NPs were released into the water
environment each year (Handy, 2012). Several
studies have shown that Ag NPs can have acute toxic
effects on a variety of organisms, including oxidative
stress, genotoxicity, and behavioral effects, but their
possible chronic toxicity to organisms was less well
studied.
As a key link in the marine food web, zooplankton
plays an important role in the process of material
cycling and energy transfer and influences the
transport of pollutants (Batel, 2016). Tigriopus
japonicus belongs to Arthropoda, Crustacea,
Harpacticoida, is a common species in the estuaries
of the western Pacific Ocean, with short generation
time, strong reproduction, and easy cultivation,
widely used in the detection of microplastics, heavy
metals, organic matter, and other pollutants toxicity
(Juan, 2020). It has been classified as a standard
organism for toxicity testing by OECD.
In this paper, we investigated the effects of Ag
NPs on the growth, development, and reproduction of
T. japonicus under two generations. The chronic
toxicity data of Ag NPs were supplemented to
provide information and methods for assessing the
effects of Ag NPs on marine invertebrates and to
provide a reference for understanding the
reproductive toxicity of Ag NPs.
2 MATERIAL AND METHODS
2.1 Ag NPs and Organisms
Silver nanoparticles (PVP - Ag NPs, ﹤100nm) were
obtained from Sigma-Aldrich (Germany). Tigriopus
japonicus and natural seawater were taken from the
sea near Qingdao, China, and have been continuously
cultured in the laboratory for many generations. The
culture conditions were: 32‰ of filtered and
sterilized natural seawater as culture medium, 2100 lx
of light, 12 h: 12 h of light to dark ratio, and 24℃.
During the culture period, the algae were fed with a
1:1 mixture of Phaeodactylum tricornutum and
Isochrysis galbana every 3 days at a feeding density
of 1×10
6
cells/mL.
2.2 Experimental Methods
Before the formal experiment, the acute toxicity of
Ag NPs to T. japonicus was tested, and the 96 h - LC
50
12
Wang, X. and Zhao, J.
Chronic Toxicity of Silver Nanoparticles to Tigriopus Japonicus.
DOI: 10.5220/0012011900003633
In Proceedings of the 4th International Conference on Biotechnology and Biomedicine (ICBB 2022), pages 12-16
ISBN: 978-989-758-637-8
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
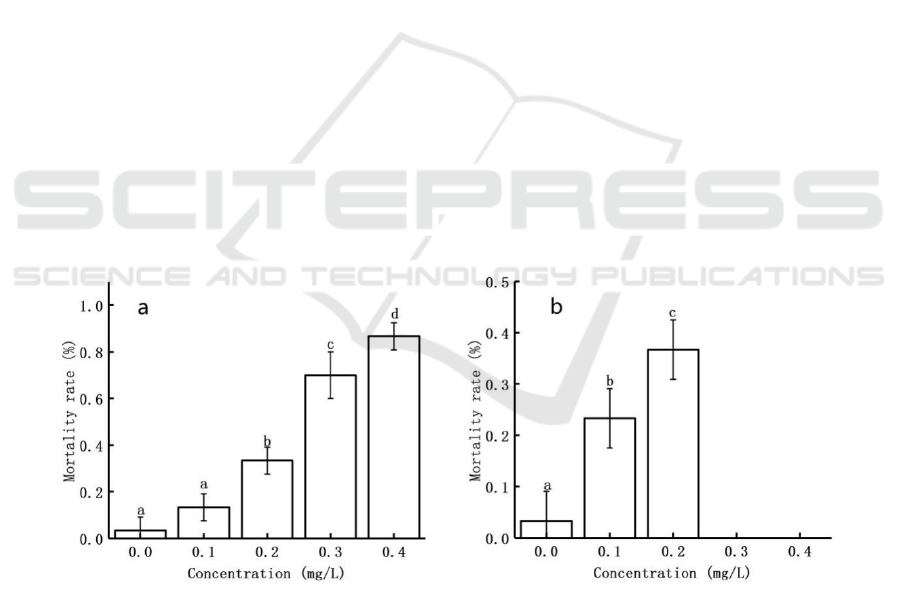
was 0.892 mg/L (0.778 - 1.079 mg/L). Before starting
the experiment, T. japonicus females with eggs were
transferred to 6-well culture plates, one per well, and
fed with bait. The nauplius with hatching time <24h
were randomly selected for the experiment. Four
concentration groups (0.1, 0.2, 0.3, 0.4 mg/L) and
control groups were set up, with three parallels in
each group. The nauplius (F
0
) were transferred to 12-
well plates with 10 per well, and the culture
conditions were as above. After 12 h of exposure,
baits were fed and the experimental solution was
changed after 24 h. The development of T. japonicus
(nauplius - copepodite - adult) and the number of dead
individuals were observed and recorded, and the dead
individuals were removed. For the nauplius stage, 5
mL of experimental solution was added to each
group, and 10 mL was added to the copepodite and
adult stage. After the females of T. japonicus held
eggs, the females were transferred to 12-well culture,
one in each well, and the same concentration of Ag
NPs was added. Similarly, fed after 12 h of exposure
and changed the solution after 24 h. Females were
observed for reproduction, and the number of eggs
carried, incubations, and hatching were recorded for
10 d. At the peak of reproduction in each group of
females, nauplius hatchlings with an incubation time
<24 h were randomly selected for the second
generation (F
1
) experiment. The experimental
methods were the same as those for F
0
.
2.3 Statistical Analysis
The experimental data were analyzed by one-way
ANOVA and LSD multiple comparison analysis
using SPSS 16.0 to compare the significance of
differences between concentrations, with P ﹤0.05
indicating a significant difference.
3 RESULTS
3.1 Effects of Ag NPs on the Growth
and Development of T. Japonicus
The mortality rate of T. japonicus F
0
and F
1
generations after 21 days of exposure to Ag NPs was
shown in Fig. 1. The mortality rate in the control
group was 3.33%, and 13.4%, 33.33%, 70%, and
86.7% for each concentration of the F
0
generation,
while 23.3% and 36.7% for the F
1
generation,
respectively. The results showed that the mortality
rate of T. japonicus increased gradually with the
increase of Ag NPs concentration. Compared with F
0
,
the mortality rate increased in all groups in F
1
. It can
be seen that Ag NPs had a greater effect on the
survival of T. japonicus at higher concentrations.
Figure 1 21 - day mortality of T. japonicus F
0
(a) and F
1
(b) generations exposed to Ag NPs. The data of 0.3 mg/L and 0.4
mg/L in (b) were missing because T. japonicus in F
0
did not incubate enough nauplius to continue the experiment. Values are
shown as mean ± S.D., different letters: P ﹤0.05.
Effect of Ag NPs on the time from nauplius to
copepodite (N-C) and from nauplius to adults (N-A)
in the F
0
and F
1
generations of T. japonicus are shown
in Fig. 2. In the F
0
generation, the duration of N-C and
N-A in the control was 4.67 and 12.33 days,
respectively, and 5.33, 5.67, 6.33, and 7.67 days for
each concentration of N-C; and 12.33, 13.33, 14.67,
and 16.33 days for N-A, respectively. In the F
1
generation, the duration of N-C and N-A in the
control group was 4.67 and 12.67 days, respectively.
Chronic Toxicity of Silver Nanoparticles to Tigriopus Japonicus
13
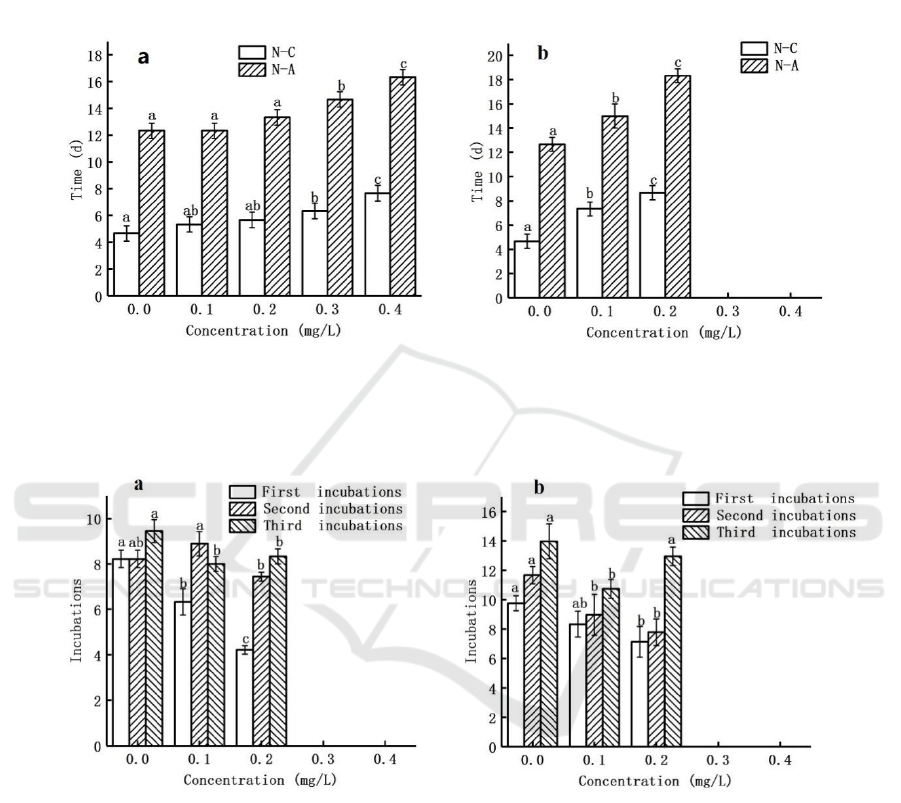
The duration of N-C and N-A for each concentration
group was 7.33 days, 8.67 days, and 15 days, 18.33
days, respectively. The results showed that the N-C,
N-A of both T. japonicus F
0
and F
1
increased with the
increase of Ag NPs concentration, and the growth
time of F
1
was longer than F
0
. There was no
significant difference in N-C and N-A of F
0
generation compared to the control at concentrations
of 0.1 mg/L and 0.2 mg/L.
Figure 2 Effect of Ag NPs on the time from nauplius to copepodite (N-C) and from nauplius to adults (N-A) in the F
0
(a) and
F
1
(b) generations of T. japonicus. The data of 0.3 mg/L and 0.4 mg/L in (b) were missing because T. japonicus in F
0
did not
incubate enough nauplius to continue the experiment. Values are shown as mean ± S.D., different letters: P ﹤0.05.
Figure 3 Effect of Ag NPs on the number of three hatchings in T. japonicus females F
0
(a) and F
1
(b) generations. The data
of 0.3 mg/L and 0.4 mg/L were missing because the mortality rate was too high for the hatchings to be known. Values are
shown as mean ± S.D., different letters: P ﹤0.05.
3.2 Effects of Ag NPs on the
Reproduction of T. japonicus
The effect of Ag NPs on the number of three
hatchings of T. japonicus females for 10 days was
shown in Fig. 3. In F
0
generation, the total number of
hatchings in 10 days of the control group was 25.89,
and 22.00 and 14.46 at 0.1 mg/L and 0.2 mg/L,
respectively; in the F
1
generation, the total number of
hatchings in 10 days of the control group was 29.40,
and 24.18 and 20.28 at 0.1 mg/L and 0.2 mg/L,
respectively. The results showed that the hatching
number of T. japonicus gradually decreased with the
increase of concentration and the number of hatchings
increased with the number of incubations at the same
concentration in both F
0
and F
1
generations.
ICBB 2022 - International Conference on Biotechnology and Biomedicine
14

4 DISCUSSION
In the ocean, the survival, growth, and reproduction
of T. japonicus were affected by a variety of factors.
For example, seawater acidification and various
pollutants (heavy metals, organic matter,
microplastics, etc.). It has been shown that 4-
methylbenzylidene camphor (4-MBC) (Hong, 2021),
Ni (Mohammed, 2010), the microplastics
polyethylene (PE), and polyamide-nylon 6 (PA 6)
(Yu, 2020) all reduced T. japonicus survival in a
dose-dependent manner, and that nauplius was more
sensitive to pollutants than copepodite and adults,
similar to Ag NPs. In addition to its effect on survival,
the impact on growth and reproduction was also a
major concern. Hong et al. (2021) noted that 4-MBC
reduced the developmental time of T. japonicus at the
N-C stage and the number of female hatchings
decreased with increasing concentration. For the
incubation number, 4-MBC barely affected the F
0
generation, but at high concentrations (5 and 10
μg/L), the F
1
and F
2
generations were significantly
inhibited, and inhibition was relieved at the F
3
generation (Chen, 2018). This differs from Ag NPs,
which in this study significantly prolonged the
developmental time of T. japonicus although they
inhibited the hatching number of females. Of course,
some pollutants did not adversely affect the survival
of T. japonicus but inhibit reproduction. For example,
dibutyl phthalate (DBP) did not have a lethal effect
on T. japonicus at the concentrations tested, but
prolonged incubation time and inhibited hatching
numbers (Li, 2020). Most contaminants reduced the
hatching number while prolonging the development
of T. japonicus. It has been shown that when T.
japonicus were exposed to oil-contaminated
sediments, the growth rate of nauplius decreased,
developmental time increased significantly, and the
number of egg-bearing females decreased and
hatching was significantly reduced (Won, 2018).
Seawater contaminated with various metals
(containing Cr, Zn, Ni, As, etc.) can significantly
inhibit the survival and reproduction rate of T.
japonicus. And ZnO nanoparticles completely
inhibited the reproduction of T. japonicus at 0.5 mg/L
(Jeong, 2019). Similar to the metal contaminants
mentioned above, Ag NPs had adverse effects on the
growth, development and reproduction of T.
japonicus.
5 CONCLUSIONS
Ag NPs inhibited the survival and development of T.
japonicus at all stages, and the inhibition increased
with increasing concentration and generations. In
contrast, the inhibition of hatching numbers
decreased with increasing generations. In future
studies, the chronic toxicity of Ag NPs should
continue to be investigated and attention the effects
on T. japonicus reproduction under multiple
generations.
ACKNOWLEDGMENTS
This work was supported by the National Natural
Science Foundation of China (Grant No. 41276104),
and Public Science and Technology Research Funds
Projects of Ocean (Grant No. 201505034-2).
REFERENCES
Batel, A., Linti, F., Scherer, M., Erdinger, L. & Braunbeck,
T. 2016. Transfer of benzo[a]pyrene from microplastics
to Artemia nauplii and further to zebrafish via a trophic
food web experiment: CYP1A induction and visual
tracking of persistent organic pollutants. Environmental
Toxicology and Chemistry, 35, 1656-1666.
Chen, L., Li, X., Hong, H. & Shi, D. 2018.
Multigenerational effects of 4-methylbenzylidene
camphor (4-MBC) on the survival, development and
reproduction of the marine copepod Tigriopus
japonicus. Aquatic Toxicology, 194, 94-102.
Handy, R. D., Cornelis, G., Fernandes, T., Tsyusko, O.,
Decho, A., Sabo-Attwood, T., Metcalfe, C., Steevens,
J. A., Klaine, S. J., Koelmans, A. A. & Horne, N. 2012.
Ecotoxicity test methods for engineered nanomaterials:
practical experiences and recommendations from the
bench. Environmental Toxicology and Chemistry, 31,
15-31.
Hong, H., Wang, J. & Shi, D. 2021. Effects of salinity on
the chronic toxicity of 4-methylbenzylidene camphor
(4-MBC) in the marine copepod Tigriopus japonicus.
Aquatic Toxicology, 232, 105742.
Jeong, C. B., Kang, H. M., Lee, M. C., Byeon, E., Park, H.
G. & Lee, J. S. 2019. Effects of polluted seawater on
oxidative stress, mortality, and reproductive parameters
in the marine rotifer Brachionus koreanus and the
marine copepod Tigriopus japonicus. Aquatic
Toxicology, 208, 39-46.
Juan, Y., Yuan, T. J., Rui, X., Yu, Z. Z., Peng, Y. G., Dan,
W. X., Guang, L. J. & Rong, C. 2020. Effects of
microplastics exposure on ingestion, fecundity,
development, and dimethylsulfide production in
Chronic Toxicity of Silver Nanoparticles to Tigriopus Japonicus
15

Tigriopus japonicus (Harpacticoida, copepod).
Environmental Pollution, 267.
Li, Z., Zhou, H., Liu, Y., Zhan, J., Li, W., Yang, K. & Yi,
X. 2020. Acute and chronic combined effect of
polystyrene microplastics and dibutyl phthalate on the
marine copepod Tigriopus japonicus. Chemosphere,
261, 127711.
Mohammed, E. H., Wang, G. & Jiang, J. 2010. The effects
of nickel on the reproductive ability of three different
marine copepods. Ecotoxicology, 19, 911-916.
Won, E. J., Lee, Y., Gang, Y., Kim, M. S., Kim, C. J., Kim,
H. E., Lee, K. W., Chung, C. S., Kim, K., Lee, J. S. &
Shin, K. H. 2018. Chronic adverse effects of oil
dispersed sediments on growth, hatching, and
reproduction of benthic copepods: Indirect exposure for
long-term tests. Marine Environmental Research, 137,
225-233.
Yu, J., Tian, J. Y., Xu, R., Zhang, Z. Y., Yang, G. P., Wang,
X. D., Lai, J. G. & Chen, R. 2020. Effects of
microplastics exposure on ingestion, fecundity,
development, and dimethylsulfide production in
Tigriopus japonicus (Harpacticoida, copepod).
Environmental Pollution, 267, 115429.
ICBB 2022 - International Conference on Biotechnology and Biomedicine
16
