
Combination Therapies Increase the Efficacy of Melanoma
Treatment with Reduced Side Effects
Haoming Zhang
1
, Jiayi Zong
2
, Jingyi Guo
3
, Lawrence Ma
4
, Yijia Chen
5
and Zhaojun Qiu
6,*
1
College of Animal Sciences and Technology, Zhongkai University of Agriculture and Engineering, Guangzhou,
Guangdong 510025, China
2
Jinling High School, Nanjing, Jiangsu 210029, China
3
Beijing National Day School, Beijing 100039, China
4
Ridge High School, Basking Ridge, New Jersey 07920, U.S.A.
5
School of Life Science, Fudan University, Shanghai 200433, China
6
Shanghai Pinghe School, Shanghai 201206, China
lawrencema0512@gmail.com, yijiachen18@fudan.edu.cn,
*
qiuzhaojun@shphschool.com
Keywords: Immune Checkpoint Inhibitor, Kinase Inhibitor, Oncolytic Virus, Combinational Therapy.
Abstract:
T Immunotherapy is found to have a promising effect on cancer treatment. It initiates activation of the immune
response to fight against cancer. Previous studies have demonstrated that immune checkpoint inhibitors (ICIs),
kinase inhibitors, and oncolytic viruses (OV) are possible cancer immunotherapies. Immune checkpoint
inhibitors targeting programmed cell death-1 (PD-1) and programmed death-ligand 1 (PD-L1) have achieved
great success in cancer immunotherapy. We hypothesized that utilizing anti-PD-1 antibodies, mitogen-
activated protein kinase (MAPK) inhibitors, and oncolytic virus (T-VEC) could boost the efficacy of
traditional PD-1 therapy towards melanoma, while the side effects can be alleviated with the supplement of
anti-cytokine antibodies and anti-inflammatory drugs. A series of experiments were designed to be conducted
in melanoma murine models. Expected outcomes of this combination therapy include enhanced tumor
regression, extended survival, and mitigated side effects. The success of this study could bring up a new
strategy for melanoma therapies.
1 INTRODUCTION
1.1 Melanoma
Melanoma is a major type of skin cancer that arises
from genetic mutations in melanocytes, the pigment-
producing cell, which can be found throughout the
skin, eye, inner ear, and leptomeninges. Although it
only takes up a small percentage of all malignant skin
cancer, it is the most aggressive and deadliest type.
As shown in Figure 1, Once it becomes metastatic
(move to other organs from where it originated), the
prognosis is very poor (Domingues, 2018), needing
better treatments to be studied and applied to.
1.2 Theraputical Mechanism in the
Proposal
As shown in Figure 2, T cells, recognizing peptide
antigen with the aid of cell surface major
histocompatibility complex (MHC) molecules, have
two broad classes with very different functions,
named by their expression of CD4 or CD8 co-
receptor: CD4+ T cells detect the antigen in MHC
class II molecules and act as the headmaster in the
adaptive immune system by producing cytokine,
chemokine, and pro-inflammatory responses. While
CD8+ T cells detect antigen with MHC class I
molecules and carry out direct toxicity to kill infected
or cancerous cells (Darvin, 2018).
104
Zhang, H., Zong, J., Guo, J., Ma, L., Chen, Y. and Qiu, Z.
Combination Therapies Increase the Efficacy of Melanoma Treatment with Reduced Side Effects.
DOI: 10.5220/0012014600003633
In Proceedings of the 4th International Conference on Biotechnology and Biomedicine (ICBB 2022), pages 104-115
ISBN: 978-989-758-637-8
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
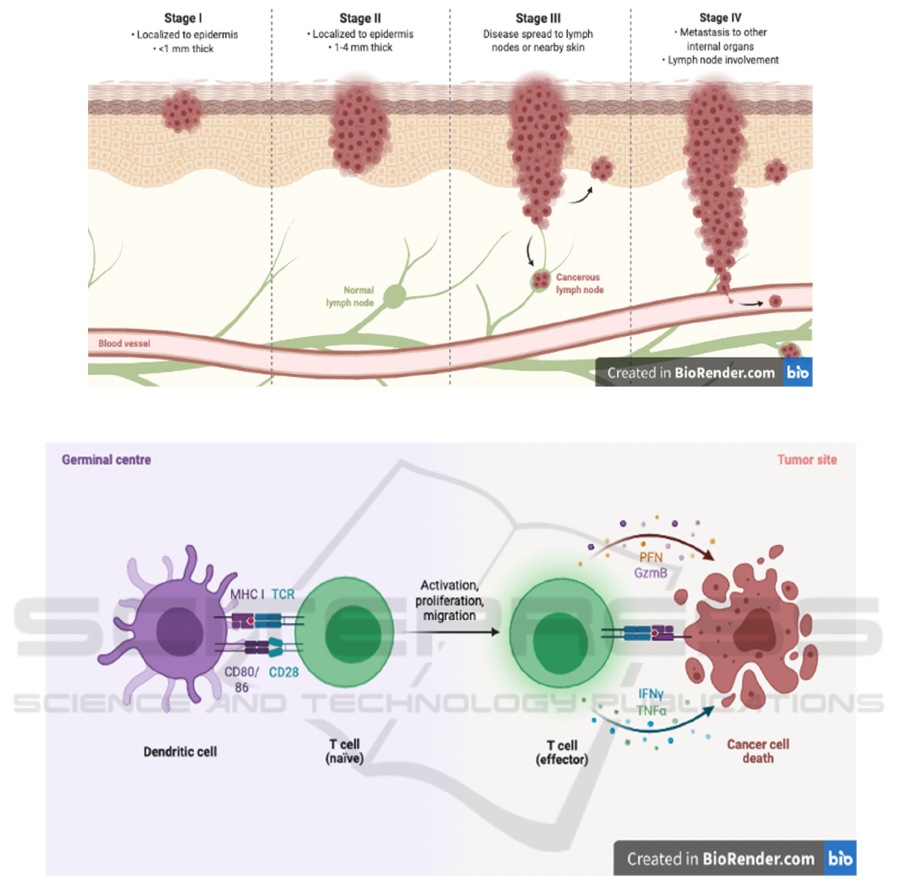
Figure 1: The four stages of melanoma (Biorender. 2021).
Figure 2: CD8+ T cell activation by Dendritic cell and effects on cancer cell (Biorender. 2021).
Generally, the interaction between multiple
checkpoints and regular stimulatory signals regulates
T cells in an effective but not autoreactive fashion
(Sharpe, 2018). But tumor cells can abnormally
utilize this signal system in two ways – reduced
stimulatory signals or overexpressed checkpoint
signals. Immune checkpoint inhibitors (ICIs) can
target the overexpressed checkpoint signals that allow
tumor cells to evade immunosurveillance, and
function by releasing the natural breaks on immune
activation and enhancing the T-cell immune ability to
eliminate tumor cells (Darvin, 2018).
The proposal mainly focuses on the druggable
inhibitors of the T cell PD-1 (Programmed cell death-
1) pathway and its complementary ligand (PD-L1).
As shown in Figure 3, PD-1 is a transmembrane
inhibitory receptor, and signals through its pathway
are mainly responsible for controlling initial T cell
activation as well as the effecting function of the cell.
On the other hand, PD-L1 is located on cancer cells
binds to the PD-1 inhibitor, and it tricks the T cell
from functioning (Sharpe, 2018). Antibodies have
been engineered to specifically target either PD-1 or
PD-L1, the former takes off the brakes from T cells,
and the latter prevents cancer cells from "hiding". It
is also noteworthy that their feasibility has been
further proved by structural analysis (Lee, 2016).
Combination Therapies Increase the Efficacy of Melanoma Treatment with Reduced Side Effects
105
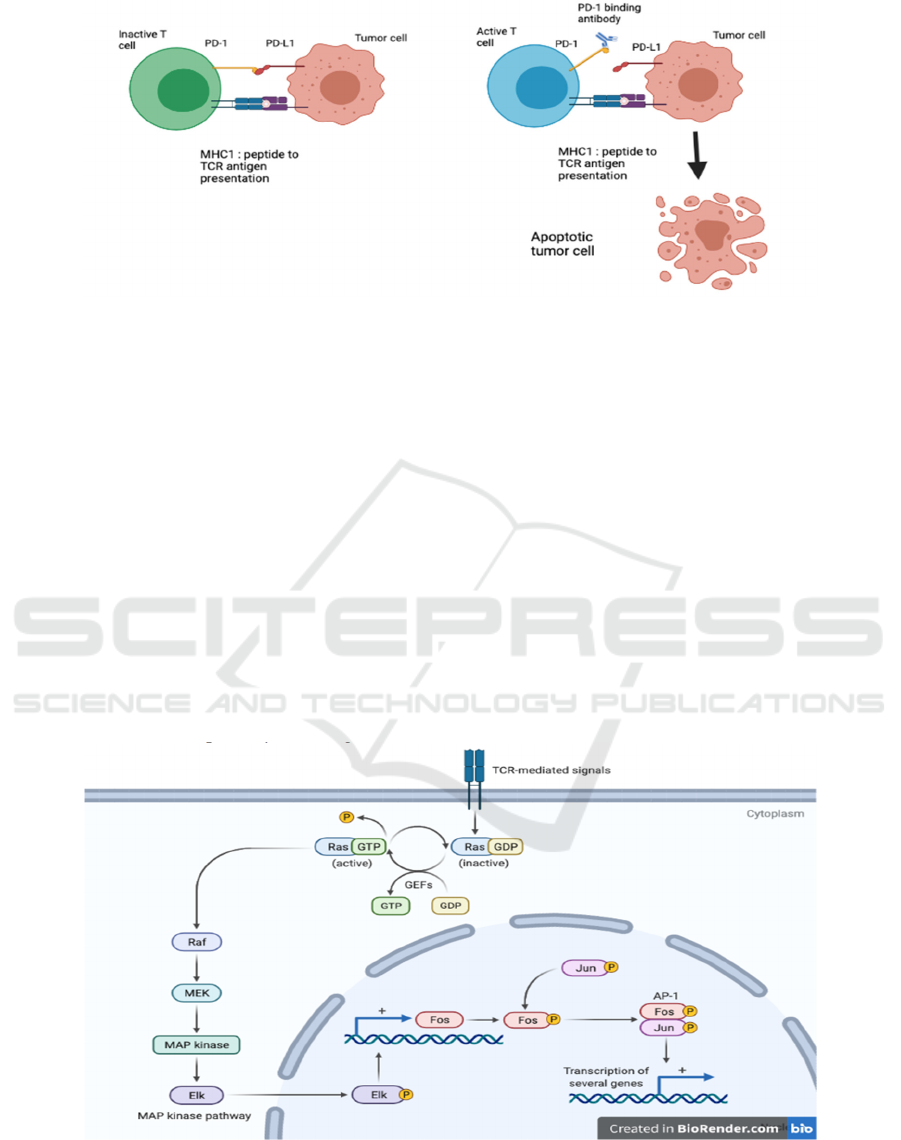
Figure 3: PD-1-PD-L1 interaction and its inhibition strategy.
Left: The schematic model of PD-1-PD-L1
interaction in a tumor cell. The binding suppresses the
activation of T cell.Right: The antibody (ICI) binds to
PD-1, therefore, inhibits the interaction which leads
to T cell activation and induces apoptotic death of
tumor cell.
Tumors can evolve to evade both innate and
adaptive arms of the immune system, thereby
rendering ICI therapy ineffective (Pfirschke, 2016;
Mueller, 2015). A subset of patients receiving
immune-checkpoint inhibitor therapy develop
unconventional response patterns (termed 'pseudo-
progression') that can be misinterpreted as disease
progression (Nishino, 2017). The MAPK pathway
provides significant therapeutic targets due to its
aberrant activation in cancer and strong interference
with complex molecular pathways, leading to wider
use of MAPK inhibitors in melanoma, lung cancer,
colorectal cancer, and other types (Smith, 2014;
Germann, (2017). Permanent activation of RAS
protein caused by mutations accounts for a very high
proportion of all human cancers through activating
downstream signaling pathways, including the
MAPK family (the downstream of RAS GTPase,
represented by RAF and its variant), then the MEK
family (MAP kinase-ERK kinase), and
ERK1/2(Extracellular signal-regulated kinases),
specifically shown in Figure 4. Activated Therapies
that target RAS-activating pathways or RAS effector
pathways could be combined with these direct RAS
inhibitors, immune checkpoint inhibitors, or T cell-
targeting approaches to treat RAS-mutant tumors
(Moore, 2020; Braicu, 2019).
Figure 4: The more detailed and specific schematic demonstration of the RAS/MAPK pathway (Biorender 2021).
Yet some MAPK pathway effectors (e.g., p38α)
play a dual role, with suppression in one cancer but
inflammation in another cancer, making preclinical
studies more considerable (Grossi, 2014). In
ICBB 2022 - International Conference on Biotechnology and Biomedicine
106
melanoma, MAPK inhibitors are mainly resisted by
macrophage-derived TNF-α (Smith, 2014), lowering
down the efficacy and implying the possibility of
adverse effects. To further strengthen the
development of MAPK inhibition, complementary
combination therapies can be added to raise the
efficacy.
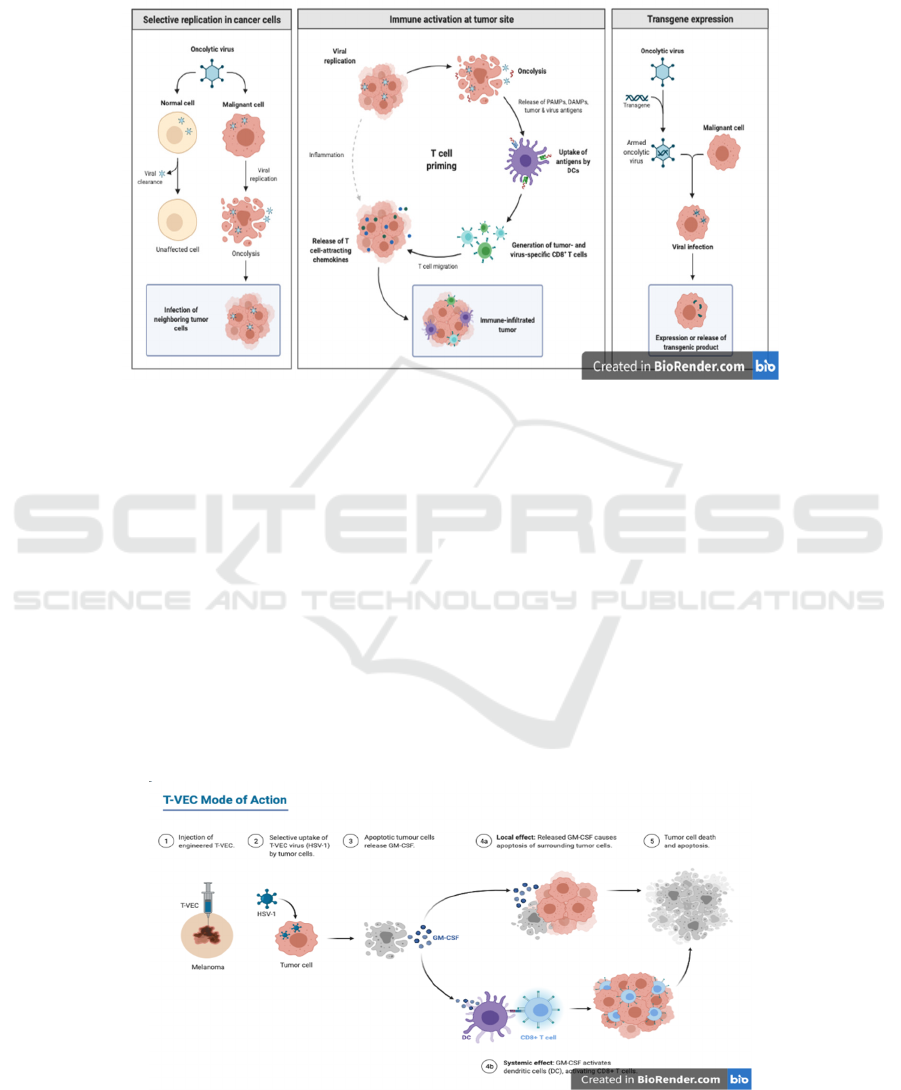
Figure 5: The mechanism of oncolytic virus against tumors (Biorender 2021).
Viruses can be used in attacking tumor cells (Bai,
2019). The left panel illustrates the oncolytic viral
response to normal cell vs malignant cell. The middle
panel illustrates a general mechanism of the oncolytic
virus with T cell activation at the tumor site. The right
panel illustrates transgene expression in oncolytic
virus.
Oncolytic viruses (OVs) are treated as natural or
engineered viruses that replicate specifically in
cancer cells and kill them. The mechanism of their kill
process is shown in Figure 5. They are harmless to
normal organisms (Fukuhara, 2016) and able to be
delivered both systemically and locoregionally. Thus,
they can act at the primary or metastatic tumor sites
(Twumasi-Boateng, 2018).
According to Figure 6, T-VEC is a type of OV for
melanoma that consists of a double-mutated oncolytic
herpes simplex virus type 1 (HSV-1) armed with
granulocyte-macrophage colony-stimulating factor
(GM-CSF) (Biorender 2021). It was approved
officially as the first oncolytic virus drug by the US
Food and Drug Administration (FDA) on 2015,
October 27th (Pol, 2016).
Though T-VEC is designed to be injected locally
into the tumor, systemic effects are often induced,
leaving a body free of cancer, but with flu-like
symptoms. Besides, due to the potential for viruses to
attack healthy cells, the risk of infection still exists
(Marelli, 2018).
Figure 6: The mechanism of T-VEC oncolytic virus to aid T cell against tumor cell (Biorender 2021).
Combination Therapies Increase the Efficacy of Melanoma Treatment with Reduced Side Effects
107

2 FORMULATION OF
HYPOTHESIS
2.1 Combination of Different
Orthogonal Therapeutic
Approaches
For an optimistic therapeutic effect to be reached, a
combinational therapy consisting of checkpoint
inhibitors (PD-1, PD-L1), as well as oncolytic viruses
(T-VEC) may be adopted. Studies have previously
demonstrated the improved efficacy combined
therapies are likely to yield, details of which will be
introduced later as this article progresses. Recently,
therapies of OV combined with ICI have been
uncovered. The oncolytic virus can secrete PD-L1-
secreting-OVs. It systemically cleaves and inhibits
PD-L1 on tumor cells and immune cells, initiating
tumor neoantigen-specific T cell responses. Tumor-
specific oncolytic immunotherapies for cancer
treatment have been made available to patients
(Wang, 2020).
Furthermore, a triple combination of OV, ICI, and
kinase inhibitors is proposed and compared with a
dual combination of OV and ICI or kinase inhibitors
and ICI in mice models. The triple combination is
consisting of a phosphatidylinositol 3-kinase (PI3K)
inhibitor, a PD-L1 inhibitor, and VSVΔ51, which is
an engineered OV strain from vesicular stomatitis
virus (VSV), commonly is used as a therapy for
PTEN-deficient glioblastoma. The result shows that
dual combination enhances immune response
slightly, while triple combination therapy increases
the treatment efficacy significantly. Tumor regression
is induced accompanied by complete tumor
eradication in most mice that are treated with triple
combination therapy. Long-term antitumor immune
memory is established in these mice (Xing, 2021).
2.2 Management of Side Effects
ICIs’ ability to stimulate the immune system has
further contributed to them being appreciated as a
desirable treatment for cancer (Simonaggio, 2019).
However, they also lead to toxicities and several kinds
of inflammation in different organs. Frequently
affected organs include the skin, and organs in the
digestive, and endocrine systems (Durrechou, 2020).
New research has found that the mechanism of action
of ICIs reveals a new toxicity profile called immune-
related adverse events (irAEs) (Richmond, 2008).
Recurrences (both same types and different types)
of irAE after recovery is another difficult problem
(Martins, 2019). Currently, the commonly used PD-
1/PD-L1 monoclonal antibody has the mechanism of
removing immunosuppression and activating T cell
function. T cells are also found in normal tissue.
While it is killing tumor tissue the side effects may
occur at the same time.
The co-stimulation of T-lymphocyte leads to
inflammatory cytokines. When the body meets
inflammation, the immune system will secrete many
inflammatory cytokines, which regulate lots of
aspects of cell growth and differentiation and play a
key role in the coordination of immune defenses
against invading. They are potentially immunogenic
which could generate the anti-cytokine
autoantibodies (aCA) (Meager, 2014). A significant
portion of the anti-inflammatory cells express PD-L1
by inducing target cells, and then T lymphocytes kill
the target cells.
With low irAE grade, ICI was discontinued
follow-up, and steroids were used for grade 2 or
higher adverse events. The core principles of
management included continuing treatment with ICI,
early detection and adequate control of irAEs may
contribute to improved patient prognosis (Matsuoka,
2020). High-risk patients receiving ICIs should be
regularly monitored for associated complications by
a professional multidisciplinary team, preferably
using a personalized monitoring strategy (Martins,
2019).
2.3 Mini Review
The purpose of the proposal is to solve the problem
that how to increase the therapeutic effect and range
of ICI-associated therapy while decreasing its side
effects. Based on previous findings among three
kinds of therapies and other related research, we have
eventually chosen to focus on melanoma and formed
a hypothesis: utilizing the combination between ICI
(PD-1 and PD-L1 antibodies), MAPK inhibitor, and
oncolytic virus (Talimogene laherparepvec) can
effectively increase the therapeutic effect of
melanoma treatment, while the side effects can be
reduced by anti-cytokine antibodies and anti-
inflammatory drugs.
Through the design of experiments in mice
models and the estimated results, we expect to shed
light on the triple combination immunotherapy in
melanoma treatment and provide our views on ideal
therapies with high efficacy and low adverse effects.
ICBB 2022 - International Conference on Biotechnology and Biomedicine
108
3
EXPERIMENTAL
APPROACHES
In order to test the hypothesis, there will be two major
experiments in this study – one is to test the effect of
combinational therapy, one is to test the reduction in
side effects with anti-cytokine antibodies and anti-
inflammatory drugs.
3.1 Experimental Object
3.1.1 A Brief Introduction to Xenograft
Tumor on Humanized Mice
In order to examine a patient’s tumor’s response to a
certain therapy, research must examine with human
tumor instead of mouse generated tumor (Richmond,
2008). To do so human tumor xenografts can be
implanted subcutaneously into immunosuppressed
mice, so the mice acquire human tumor cells to
establish tumor microenvironment and propagation
inside the mice. This is a critical model for various
studies on cancer interaction and behavior with the
cardiovascular and immune systems and response to
the various drugs (Martins, 2019).
3.1.2 Processes of Mouse Model Generation
Hetero transplantation of human cancer cells or tumor
biopsies into immunodeficient rodents as patient
xenograft models) (PDX)constituted the major
preclinical screen for the development of novel
cancer. the models have identified clinically
efficacious agents and make effort in pharmaceutical
industry therapeutics (Morton, 2016). In order to
make mouse models, several processes are needed.
SCID is mice with defective combinations of T, B,
and NK cells. First, the il2R γ KO mice were
backcrossed after the deletion of the IL-2 receptor γ
chain gene, which is a common domain of cytokine
receptors. Then, NOD/SCID−γ−/−c mice are
irradiated (sublethal, 1 Gy, whole-body irradiation)
(Gonzalez, 2013), followed by CD34+ stem cells
from the same cord blood donor each with a unique
UCB donor was performed. In all cases, recipient
mice were evaluated for human hematolymphoid
engraftment at 12 to 16 weeks post-injection. For
new-boring mice, human hematolymphoid cells
(HSC) engraftment of newborn mice by intracardiac
(IC) injection can be better (Brehm, 2010).
Meanwhile, subcutaneous implantation of autologous
human thymic tissue (1–2 mm3 fragments) could also
work (Shankar, 2020). Followed by the establishment
of in vitro human cell lines to be propagated
subcutaneously, reconstituting solid tumors (Russell,
2018).
3.1.3 Advantages and Disadvantages of the
Processed Mouse Models
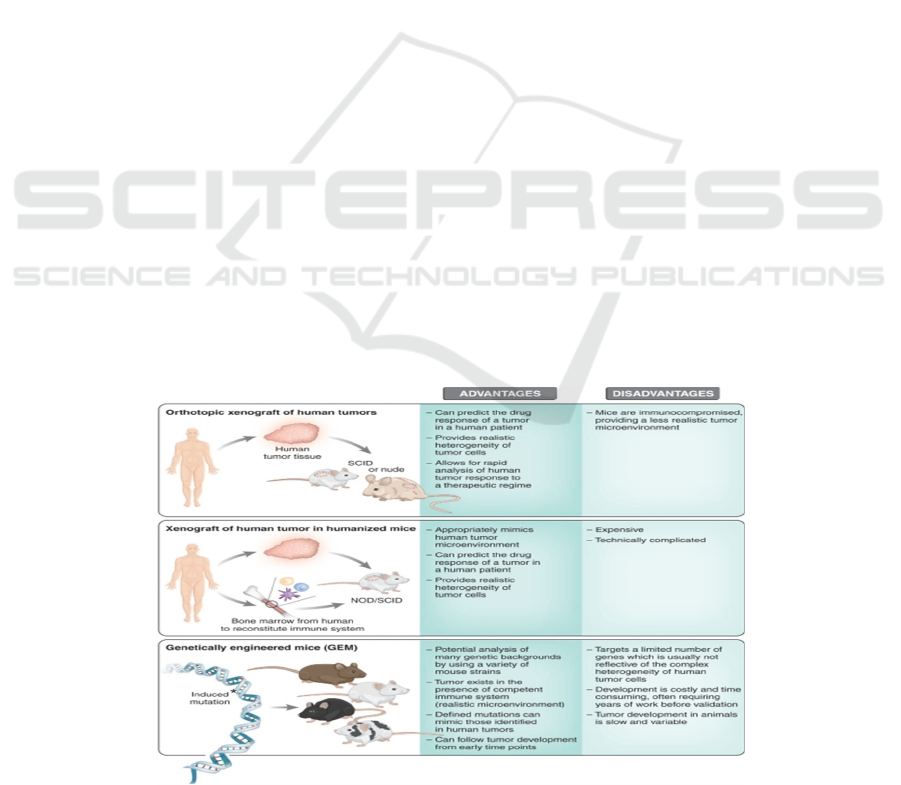
As shown in Figure 7, the feature complexity of
genetic and epigenetic abnormalities that only present
inhuman compared to mice. The results can be
obtained relatively rapidly (a few weeks), and
multiple therapies can be tested from a single tumor
biopsy. Although the humanized mice can partially
reconstruct the human immune system, the process to
generate humanized mice is tedious and costly (Gong,
2018).
Figure 7: The advantages and disadvantages between three common types of mouse models in cancer research (Russell, 2018).
Combination Therapies Increase the Efficacy of Melanoma Treatment with Reduced Side Effects
109
3.1.4 Control Factors
All the humanized mice are expected to have same
age and gender. They are treated with same
environmental condition (25 Celsius, same water and
food). All the Xenograft tumor is taking from the
same human patient. The xenograft human bone
marrow is derived from HLA compatible healthy
participant. Every kind of drug are produced in same
company in similar date.
3.2 Experiment for Combinational
Therapy
In this experiment, we will test out the efficacy of ICI
and ICI combination therapy by utilizing different
combinations of treatment on controlled mice models.
3.2.1 Positive and Negative Control
The positive control in our experiment is humanized
mice without tumor xenograft to test out the natural
rate of death and normal symptoms as a comparison
to the experimental groups. The negative control is
tumor xenograft humanized mice without any
treatment in order to test out the effect of advanced
melanoma on the mice without any outer interference.
3.2.2 General Protocol
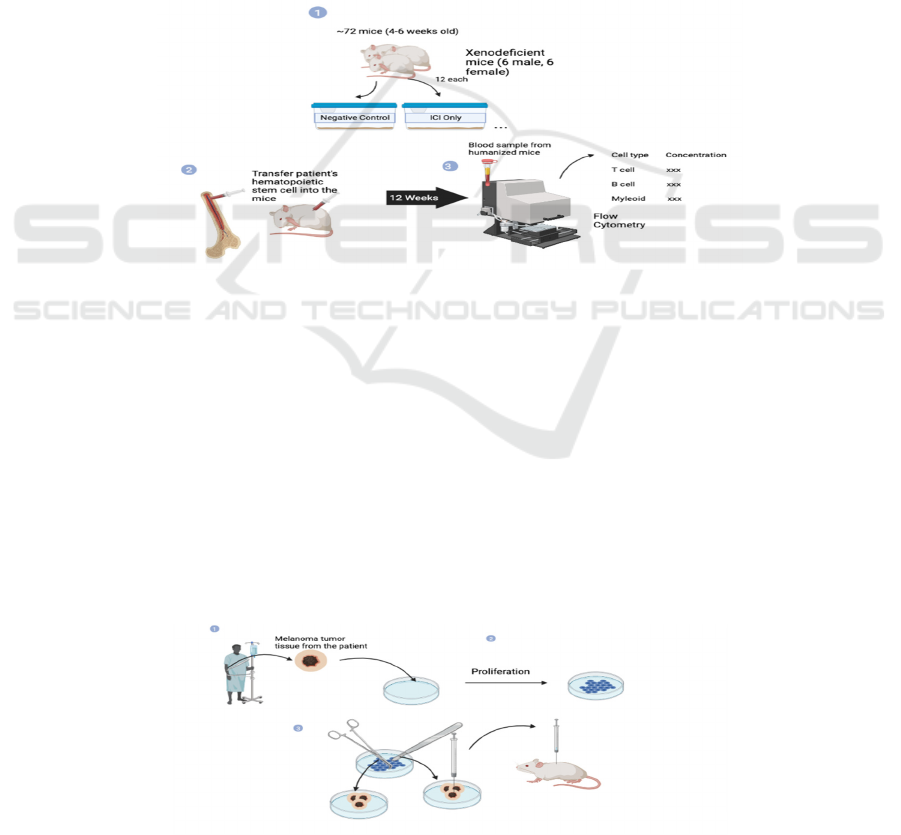
Step 1 – humanized mice cultivation (see Figure 8)
Figure 8: The schematic diagram about the first step in the experiment protocol.
Prepare 72 humanized mice (4-6 all male) and
stratified into six sub-groups evenly (n=12) by
randomization. Inject healthy individual’s (HLA
matched with tumor xenografted patient)
hematopoietic stem cell into the mice and wait at least
12 weeks to let the immune system become mature.
After 12 weeks, examine blood sample from 2-3 mice
in each group by flow cytometry to estimate amount
of immunological cell in the mice. If it reaches the
expected standard, then start Step 2.
Step 2 - Prepare and culture xenograft advanced
melanoma tumor from the human patient (see Figure
9).
First, extract tumor tissue sample from an
advanced melanoma patient, who is HLA-matched
with the hematopoietic stem cell donor. Then
cultivate it under ideal environment until reach
enough amount for transplantation. Mince the
proliferated tumor tissue into equal pieces that are
sufficient to cause melanoma in the mice in a short
amount of time while not lethal immediately. Last,
Inject the equivalent minced tissue into the
humanized mice by subcutaneous injection.
Figure 9: The second step of the experiment is where cultures and transfer of human melanoma tumors are performed.
ICBB 2022 - International Conference on Biotechnology and Biomedicine
110
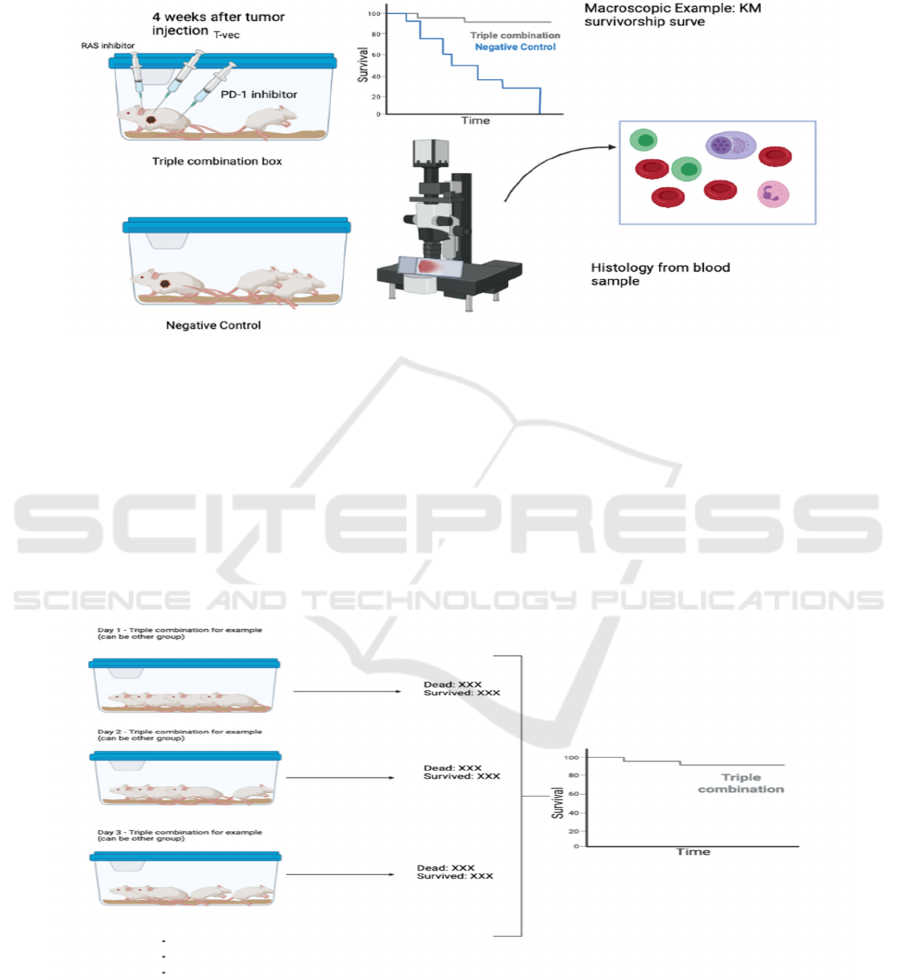
Step 3 - Inject the corresponding treatment to
different humanized Mice groups (see Figure 10)
Wait 4 weeks after tumor injection in order to let
the tumor spread in the mice. Then, inject
corresponding treatment intravenously to each group
of mice.
Figure 10: The lab protocol demonstrates the experiment phase where injection of treatments and observations are taking
place.
Step 4 - Observation and record
Observe the mice for at least 2 months. The
purpose and method to acquire data will be presented
in the following section.
3.2.3 Technology and Index for Result
Measurement
Both macroscopic observation and data (number of
mice dead and clinical symptoms) and microscopic
and data (T cell density and activation status) are
necessary to support studies about combination
therapy. In macroscopic observation, the survival
curve and clinical statistics can be obtained (see
Figure 11). Kaplan Meier (KM-Curve) is a great
estimation of how the model survives during long-
time observation.
Figure 11: The figure shows how the Kaplan Meier graph is obtained by counting the number of survived and dead mice
models in each group every day.
3.2.4
Anticipated Results
Survival rates of mice, average tumor size and T cell
density are main index being expected to be
measured, to evaluate the efficacy of each treatment
through macro and micro approaches.
Negative control (no treatment):
Combination Therapies Increase the Efficacy of Melanoma Treatment with Reduced Side Effects
111
Negative control group is expected to have
Poorest Kaplan-Meier (K-M) curve that has the
steepest slope (which means many mice dead in a
short amount of time). It also expected to have largest
average tumor size and most tumors with least
number of lymphocytes and myeloid cells. Also, it is
expected to have least amount of fluorescence (least
amount of activated T cell against tumor cell)
ICI (PD-1) only:
With only ICI treatment only, the mice expected
to have a better and flatter K-M curve compared to
negative control. The tumor size and density should
have smaller average tumor size and reduced number
of tumor (despite pseudo-progression). It should have
more active (higher fluorescence density)
lymphocytes compared to negative control.
ICI with MAPK (RAS inhibitor):
As expected, double treatment should result in
better K-M curve, smaller average tumor size and
density, and more and active lymphocytes compare to
ICI treatment only and negative control.
ICI with the oncolytic virus (T-VEC):
The result obtained with ICI and T-VEC double
treatment expected to similar with ICI and MAPK
double treatment without significance differences
(p>0.05).
Triple combination (ICI + T-VEC + kinase
inhibitors):
The triple combination will be expected to have
best K-M curve, least tumor size and density, and
most numerous and active lymphocytes (with tumor).
Positive control (no tumor): normal lifespan
without injection
The K-M curve should follow natural rate of
death, and with no tumor observed. Also, there should
be normal number of lymphocytes and normal
activation status since no infection or cancer takes
place.
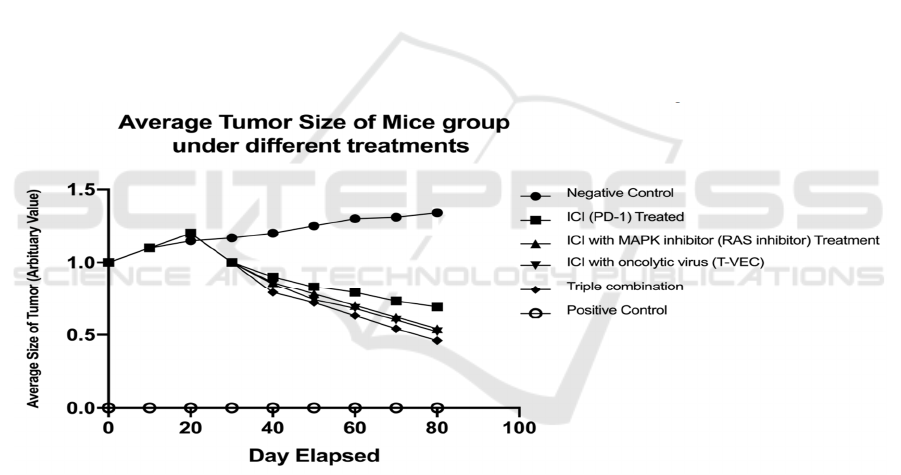
ICI treatment can initially increase tumor size,
then suppress the tumor size at later date, as expected
with pseudo-progression (see Figure 12). The
negative control group is a mice model that grafted
with a tumor but does not receive any kind of
treatment. The positive control group is a mice model
that is normal (no tumor graft).
Figure 12: The average tumor size of each experimental group.
3.3 The Experiment of Management of
Side Effects
3.3.1 Positive and Negative Control
The positive control in this experiment is healthy
mice without xenograft tumors. The negative control
is tumor xenograft humanized mice with the triple
combination but does not have any medicine to
manage side effects.
3.3.2 Anticipated Results
In the ideal state, the same number of the humanized
mice in the four groups have both symptoms cause by
ICIs. Here we use DCR (disease control rate) to
describe the anti-irAE efficacy through each
treatment. Overall survival rate is expected to be
measured to characterize disease control and is
demonstrated in Figure 13.
ICBB 2022 - International Conference on Biotechnology and Biomedicine
112
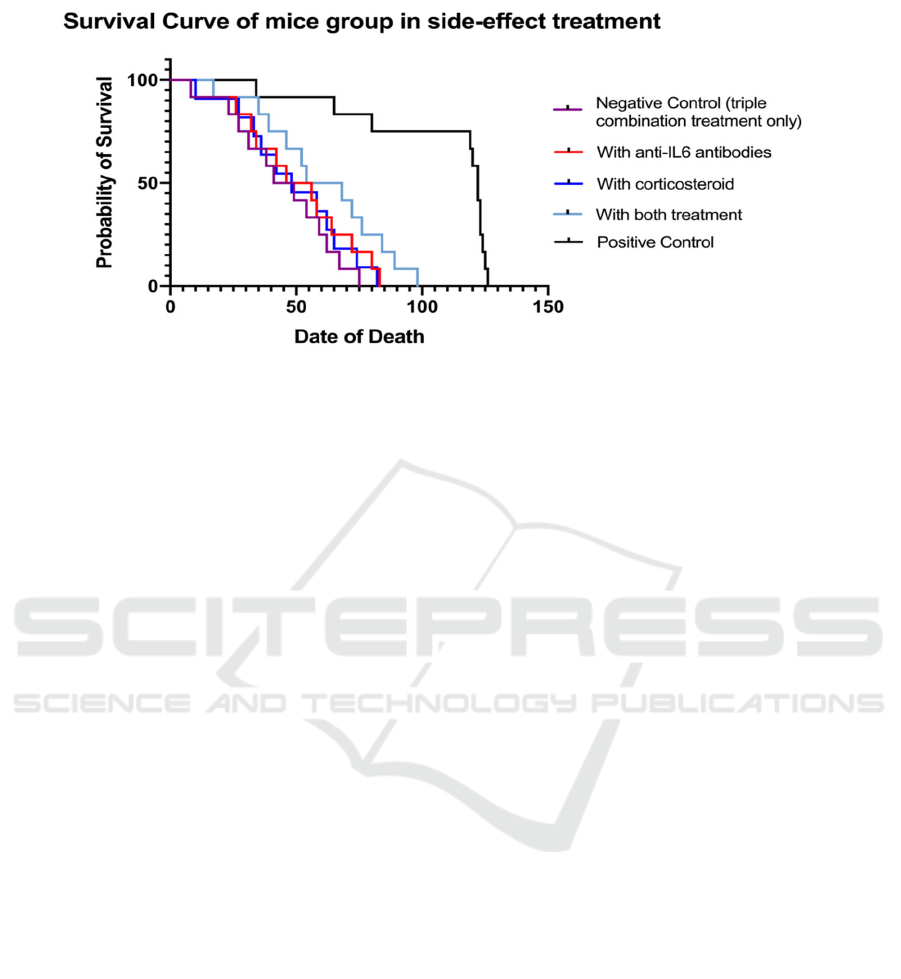
Figure 13: The overall survival (OS) curve of experimental groups. OSR stands for Overall Survival Rate.
The negative control is a mice model with
melanoma xenograft tumor that only treated with
combinational therapy (without any anti-side effect
treatment). The positive control is normal mice with
a natural rate of death. The triple combination with
anti-IL6 antibodies performs worse than
corticosteroids because anti-IL6 antibodies reduce the
therapeutic effect of the triple combination.
4 DISCUSSION
4.1 Advantages and Limitations for
Melanoma
Combination with a variety of drugs can be induced
by a different mechanism and thus increase antitumor
immune response, leading to improvements in the
overall curative effect. Despite the humanized mice
more closely mimicking diverse populations
reconstruct the human immune system, discrepancies
still exist. Triple therapy might be incompatible with
anti-cytokine inhibitors such as by reducing or even
abolishing the effects.
4.2 Possibilities of Triple Combination
Therapy in Other Cancers
As hundreds of clinical trials have been conducted on
malignancies that have a relatively dramatic response
to PD-1 and PD-L1 blockade, nine cancer types have
been approved for the PD-1/PD-L1 treatment (Gong,
2018). Among them, MAPK inhibitors and oncolytic
viruses were seen significant activity in non-small
cell lung cancer (NSCLC), Renal cell carcinoma
(RCC), and Hepatocellular carcinoma (HCC) in
previous studies (Baines, 2011) Therefore, we can
presume the possibilities of such a triple therapeutic
modality applying for these kinds of cancers. Xing F
et al have made efforts on the combination of anti-
PD-1 treatment, kinase inhibitor (PI3K), and OV and
testified that its efficacy was synergistically and
safely restored in PTEN-deficient GBM models
(Matsuoka, 2020). Under the support that ICIs, kinase
inhibitors (MAPK), and OV each have positive
responses in these cancer types, meanwhile, dual
combinations among these therapies have enhanced
the effects, we can similarly expect higher
effectiveness of therapeutic responses by utilizing our
triple combination therapy. Facing the challenge of
irAEs caused by ICI therapies, we launched our
solutions that using inflammatory cytokines
inhibitors or corticosteroids collaboratively to reduce
the irAE grade. For cancers of NSCLC, RCC, and
HCC, the same levels of irAEs compared with
melanoma were detected (Shankar, 2020); Baines,
2011; Ornstein, 2017). After triple combination
therapy is used to raise efficacy, anti-irAE strategies
(anti-cytokine and corticosteroids) may also be able
to be applied.
ACKNOWLEDGMENT
All authors contributed equally to this work and
should be considered co-first authors.
REFERENCES
Bai, Y., et al. (2019). "Updates to the antitumor mechanism
of oncolytic virus." Thoracic cancer 10(5): 1031-1035.
Combination Therapies Increase the Efficacy of Melanoma Treatment with Reduced Side Effects
113

Baines, A. T., et al. (2011). "Inhibition of Ras for cancer
treatment: the search continues." Future medicinal
chemistry 3(14): 1787-1808.
Braicu, C., et al. (2019). "A comprehensive review on
MAPK: a promising therapeutic target in cancer."
Cancers 11(10): 1618.
Brehm, M. A., et al. (2010). "Parameters for establishing
humanized mouse models to study human immunity:
analysis of human hematopoietic stem cell engraftment
in three immunodeficient strains of mice bearing the
IL2rgamma(null) mutation." Clin Immunol 135(1): 84-
98.
Cui, T.-m., et al. (2020). "Adverse Effects of Immune-
Checkpoint Inhibitors in Hepatocellular Carcinoma."
OncoTargets & Therapy 13.
Darvin, P., et al. (2018). "Immune checkpoint inhibitors:
recent progress and potential biomarkers."
Experimental & molecular medicine 50(12): 1-11.
Domingues, B., et al. (2018). "Melanoma treatment in
review." ImmunoTargets and therapy 7: 35.
Durrechou, Q., et al. (2020). "Management of Immune
Checkpoint Inhibitor Toxicities." Cancer Manag Res
12: 9139-9158.
Fukuhara, H., et al. (2016). "Oncolytic virus therapy: A new
era of cancer treatment at dawn." Cancer Sci 107(10):
1373-1379.
Germann, U. A., et al. (2017). "Targeting the MAPK
signaling pathway in cancer: promising preclinical
activity with the novel selective ERK1/2 inhibitor
BVD-523 (ulixertinib)." Molecular cancer therapeutics
16(11): 2351-2363.
Gonzalez, L., et al. (2013). "Humanized mice: novel model
for studying mechanisms of human immune-based
therapies." Immunol Res 57(1-3): 326-334.
Gong, J., et al. (2018). "Development of PD-1 and PD-L1
inhibitors as a form of cancer immunotherapy: a
comprehensive review of registration trials and future
considerations." Journal for immunotherapy of cancer
6(1): 1-18.
Grossi, V., et al. (2014). "p38α MAPK pathway: A key
factor in colorectal cancer therapy and
chemoresistance." World journal of gastroenterology:
WJG 20(29): 9744.
Kuzu, O. F., et al. (2015). "Current state of animal (mouse)
modeling in melanoma research." Cancer growth and
metastasis 8: CGM. S21214.
Lee, J. Y., et al. (2016). "Structural basis of checkpoint
blockade by monoclonal antibodies in cancer
immunotherapy." Nature communications 7(1): 1-10.
“Melanoma staging”, by Biorender.com (2021). Retrieved
from https://app.biorender.com/biorender-templates
Mueller, K. L. (2015). "Cancer immunology and
immunotherapy. Realizing the promise. Introduction."
Science 348(6230): 54-55.
Moore, A. R., et al. (2020). "RAS-targeted therapies: is the
undruggable drugged?" Nature Reviews Drug
Discovery 19(8): 533-552.
Marelli, G., et al. (2018). "Oncolytic viral therapy and the
immune system: a double-edged sword against cancer."
Frontiers in immunology 9: 866.
Martins, F., et al. (2019). "Adverse effects of immune-
checkpoint inhibitors: epidemiology, management and
surveillance." Nature Reviews Clinical Oncology
16(9): 563-580.
Meager, A. and M. Wadhwa (2014). "Detection of anti-
cytokine antibodies and their clinical relevance."
Expert Rev Clin Immunol 10(8): 1029-1047.
Matsuoka, H., et al. (2020). "Correlation between immune-
related adverse events and prognosis in patients with
various cancers treated with anti PD-1 antibody." BMC
Cancer 20(1): 656.
Morton, J. J., et al. (2016). "Humanized mouse xenograft
models: narrowing the tumor‚ Äìmicroenvironment
gap." Cancer research 76(21): 6153-6158.
Nishino, M., et al. (2017). "Monitoring immune-checkpoint
blockade: response evaluation and biomarker
development." Nat Rev Clin Oncol 14(11): 655-668.
Ornstein, M. C. and J. A. Garcia (2017). "Toxicity of
checkpoint inhibition in advanced RCC: A systematic
review." Kidney Cancer 1(2): 133-141.
Pfirschke, C., et al. (2016). "Immunogenic Chemotherapy
Sensitizes Tumors to Checkpoint Blockade Therapy."
Immunity 44(2): 343-354.
Pol, J., et al. (2016). "First oncolytic virus approved for
melanoma immunotherapy." Oncoimmunology 5(1):
e1115641.
Reprinted from “T cell Activation in Cancer”, by
Biorender.com (2021). Retrieved from
https://app.biorender.com/biorender-templates
Reprinted from “PI3K/Akt, RAS/MAPK, JAK/STAT
Signaling”, by Biorender.com (2021). Retrieved from
https://app.biorender.com/biorender-templates
Reprinted from “Properties of Oncolytic Virus”, by
Biorender.com (2021). Retrieved from
https://app.biorender.com/biorender-templates.
Reprinted from “T-VEC mode of action”, by
Biorender.com (2021). Retrieved from
https://app.biorender.com/biorender-templates
Richmond, A. and Y. Su (2008). Mouse xenograft models
vs GEM models for human cancer therapeutics, The
Company of Biologists Limited.
Russell, L. and K.-W. Peng (2018). "The emerging role of
oncolytic virus therapy against cancer." Chinese
clinical oncology 7(2): 16.
Sharpe, A. H. and K. E. Pauken (2018). "The diverse
functions of the PD1 inhibitory pathway." Nature
Reviews Immunology 18(3): 153-167.
Smith, M. P., et al. (2014). "The immune microenvironment
confers resistance to MAPK pathway inhibitors through
macrophage-derived TNFα." Cancer discovery
4(10): 1214-1229.
Simonaggio, A., et al. (2019). "Evaluation of
Readministration of Immune Checkpoint Inhibitors
After Immune-Related Adverse Events in Patients with
Cancer." JAMA Oncol 5(9): 1310-1317.
Shankar, B., et al. (2020). "Multisystem Immune-Related
Adverse Events Associated with Immune Checkpoint
Inhibitors for Treatment of Non–Small Cell Lung
Cancer." JAMA Oncology 6(12): 1952-1956.
ICBB 2022 - International Conference on Biotechnology and Biomedicine
114

Twumasi-Boateng, K., et al. (2018). "Oncolytic viruses as
engineering platforms for combination
immunotherapy." Nature Reviews Cancer 18(7): 419-
432.
Wang, G., et al. (2020). "An engineered oncolytic virus
expressing PD-L1 inhibitors activates tumor
neoantigen-specific T cell responses." Nature
communications 11(1): 1-14.
Xing, F., et al. (2021). "Modulating the tumor
microenvironment via oncolytic virus and PI3K
inhibition synergistically restores immune checkpoint
therapy response in PTEN-deficient glioblastoma."
Signal Transduction and Targeted Therapy 6(1): 275.
Combination Therapies Increase the Efficacy of Melanoma Treatment with Reduced Side Effects
115
