
How HPV Vaccines with Various Compositions Work for Humans
Sichu Chen
1,†
and Chen Cheng
2,*,†
1
University of California – Santa Barbara, Santa Barbara, California 93106, U.S.A.
2
Coe College – Cedar Rapids, Iowa 528400, U.S.A.
Keywords:
HPV, Vaccines, Immunotherapy.
Abstract: Human papillomavirus (HPV) is the main cause of genital infection, which further causes cervical cancer and
other HPV-related diseases. These diseases severely affect the physical and psychosexuals of both males and
females. Cancer vaccines have contributed significantly to cancer prevention or therapy these years, especially
for cervical cancer. In this review, the topics of what HPV is and how the innate immune system fights against
it have been introduced. Furthermore, the mechanisms of three prophylactic HPV vaccines- Gardasil®,
Cervarix® and Gardasil® 9- that contain various virus-like particles (VLP) have also been discussed. In
addition, this paper discusses and compares the composition of each of these HPV vaccines and how effective
they are in prevention of cervarix cancer, how the body reacts to immunization of these vaccines. Lastly, the
achievements and the future of the vaccination programmes are investigated.
1 INTRODUCTION
Over 570,000 women are affected by cervical cancer,
and about 311,000 women pass on each year
worldwide, with almost 90% of them living in low
and middle- low-income countries (Okuhara et al.,
2021). The International Agency for Research on
Cancer has accepted a direct causative association
between HPV and genital infections, such as in the
cervix, penis, vulva, but the severity of the correlation
varies. As a result, HPV is widely regarded as the
most important oncogenic virus affecting humans
and the leading cause of uterine cervical cancer. The
direct relationship between the prevalence of HPV
and women is all around depicted, with rates peaking
in younger women and then steadily declining. HPV
prevalence in males is higher than in women overall
and remains stable over time among different age
groups. In certain male populations, like who have
sex with men,especially those with HIV infection, the
HPV prevalence can be exceedingly high (over 70
percent) and is commonly linked to anal carcinoma
(Mariani et al., 2017).
In recent years, cancer immunotherapy has been
utilizied more frequently, with cancer vaccines
emerging as a novel approach to cancer treatment.
†
These authors contributed equally
The development of therapeutic vaccines capable of
activating cytotoxic T cells or generating antibodies,
so initiating an immunological response, has been
aided by a better understanding of how cancer cells
avoid detection and are targeted by the immune
system. In clinical trials, cancer vaccines are
commonly combined with other forms of therapy,
such as surgery and chemotherapy, to improve
efficacy. Cell immunizations (cancer or safe cell),
protein/peptide antibodies, and hereditary (DNA,
RNA, and viral) immunization are a few of the major
categories of clinical studies (Mannan et al. 2016).
Almost all high-resource nations have been building
HPV vaccination programs since 2007. The Global
Alliance for Vaccines and Immunization (GAVI) is
coordinating and sponsoring several pilot projects in
low- and middle-income countries. Cervical cancer
incidence and death rates are higher in disadvantaged
areas where immunization efforts are almost non-
existent (Mariani et al., 2017).
This review mainly focuses on the mechanisms
and clinical trials of the three prophylactic HPV
vaccines, including their compositions. There is also
including the effect of cancer vaccines on males and
females of all ages.
156
Chen, S. and Cheng, C.
How HPV Vaccines with Various Compositions Work for Humans.
DOI: 10.5220/0012015200003633
In Proceedings of the 4th International Conference on Biotechnology and Biomedicine (ICBB 2022), pages 156-162
ISBN: 978-989-758-637-8
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
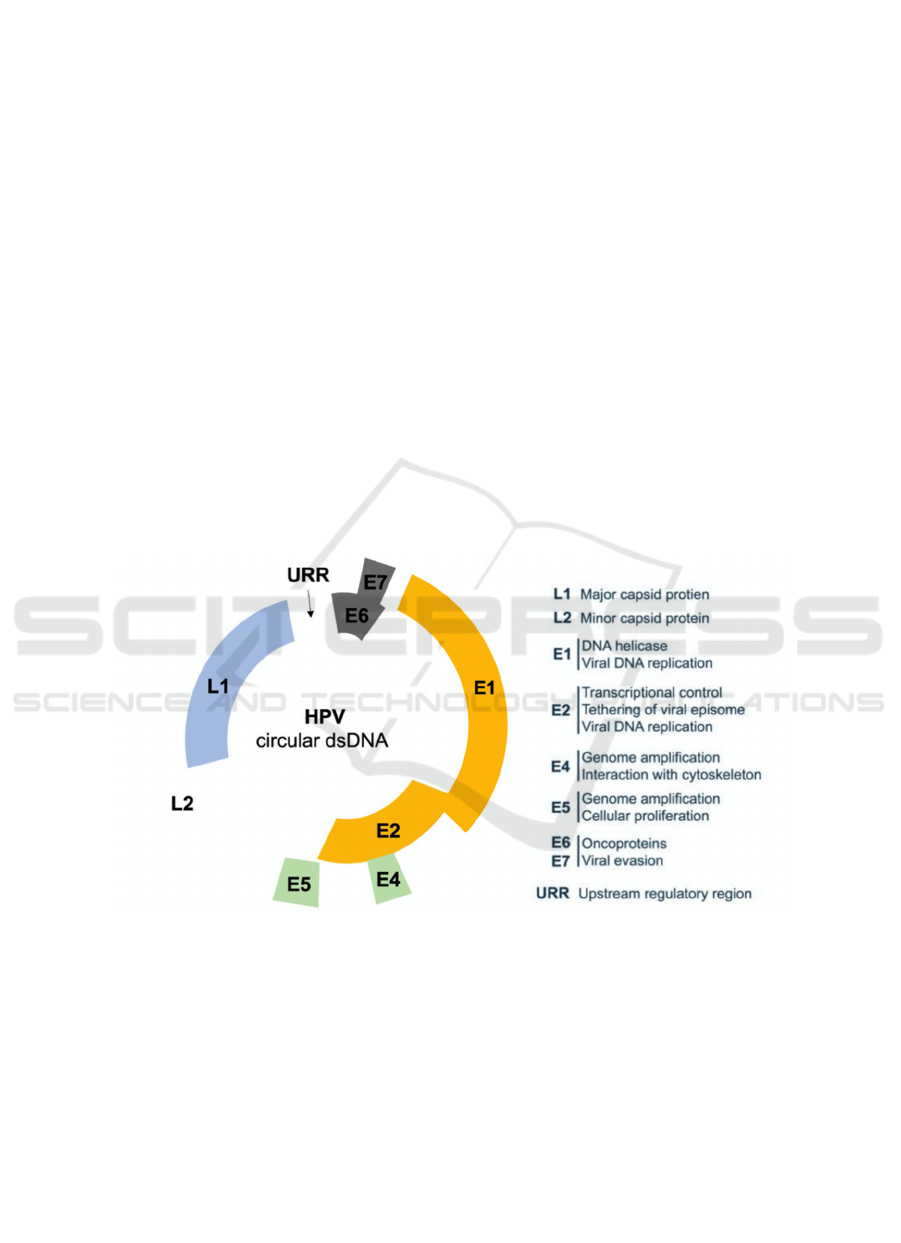
2 THE DISCOVERY AND
DEVELOPMENT OF HPV
HPV are double-stranded circular tiny DNA viruses
that infect epithelial tissues such as the basal
epidermis (skin or mucosal) cells and the upper
respiratory and anogenital tract epithelial linings
(Figure 1). HPVs are classified into low-risk and
high-risk types based on their ability to cause
malignant transformation. According to the
international agreement, "high-risk" genotypes such
as 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and
66 have been associated to cervical cancer and other
mucosal anogenital malignancies. Low-risk
infections can cause benign or low-grade cervical
tissue changes and genital warts, which are growths
on the cervix, vagina, vulva, or anus in women and
the penis, scrotum, or anus in men (Cutts et al., 2007).
HPV16 and 18 are the two most frequent cancer-
causing DNA subtypes, accounting for around 79%
of carcinomas in North America and 68% in Africa,
respectively, whereas HPV 6 and 11 lead to 90% of
recurrent respiratory papillomatosis (RRP). These
papillomaviruses are the only known viral infections
that start spontaneously by interacting with the cell
surface when they assault. From then, HPV requires
a simple 24 hours to thoroughly acclimatize the
nuclear DNA of the basal cell with its own. As a
result, the infectious genetic material is transcribed
and translated, enabling viral proteins to be
synthesized that interact with the infection. The
genome encodes six early regulatory proteins (E1,
E2, E4, E5, E6, and E7), as well as two late structural
proteins (L1 and L2). The early genes code for viral
DNA replication, transcription, and oncogenic
transformation proteins, whereas the late genes code
for virus capsid proteins. The late viral genes L1 and
L2 are translated when the infected basal cells go up
and separate, accordingly provoking the vegetative
phase of the virus life cycle, which is marked by
substantial genome expansion (Ferreira et al., 2020).
When the cell arrives at the peripheral layer of the
epithelium, the recently generated viral DNA is
exemplified to shape new virions, which are
delivered, and the existence cycle is rehashed
(Thomas T. L. 2016).
Figure 1: Illustration of HPV circular dsDNA (Ferreira et al., 2020).
The HPV vaccine is a crucial strategy for
preventing cervical cancer, primarily when used with
a healthy sexual way of life and appropriate
contraception. Scientists started to examine the
possible role of HPV in cervical cancer in the range
of 1974 and 1976. In 1976, Meisels and Fortin found
the presence of Koilocytes in cervical smears, which
demonstrated the presence of papillomavirus disease.
In 1983 and 1984, the scientists secluded cervical
cancer-related HPV type, HPV16, and HPV18, from
cancer biopsies of the cervix, separately. In 1987, de
Villiers et al. led the first epidemiological
investigation of HPV infections. The first virus-like
particles (VLPs), which are collected from
recombinant HPV capsid proteins and are non-
irresistible because of the absence of viral DNA were
produced in 1991. HPV was first proposed as an
important reason for cervical malignant growth
improvement in 1999. Harro et al. revealed the
wellbeing and immunogenicity of VLP
immunizations in people in 2001 (Figure 2) (Wang et
al., 2020). There are three authorized prophylactic
HPV vaccinations available now. In June 2006, a
quadrivalent HPV (4xHPV; Gardasil®) vaccine that
How HPV Vaccines with Various Compositions Work for Humans
157
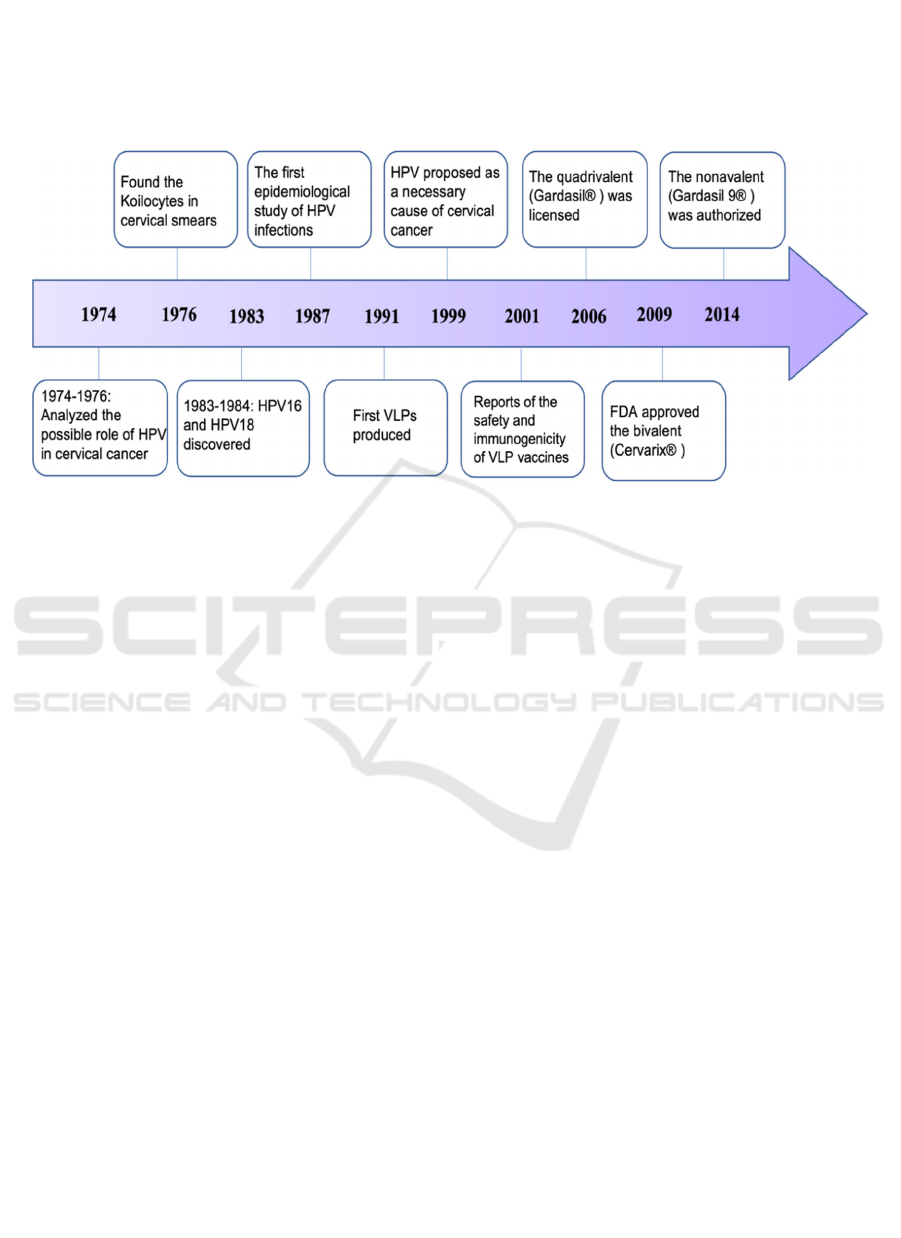
protects against HPV6, HPV11, HPV16, and HPV18
was authorized in the United States and afterward in
the European Union (September 2006). The Food and
Drug Administration (FDA) approved a bivalent
HPV (2xHPV; Cervarix®) vaccination
(GlaxoSmithKline) in October 2009, which protects
against HPV16 and HPV18. The nonavalent HPV
(9xHPV; Gardasil 9®) vaccine (Merck & Co) was
licensed in 2014 that covers five more HPV types,
which are HPV31, HPV33, HPV45, HPV52, and
HPV58 (Wang et al., 2018).
Figure 2. The research chronology for HPV and HPV vaccines (Wang et al., 2020).
Despite the fact that prophylactic or preventive
HPV vaccinations have been a huge step forward in
preventing HPV infections and diseases, there is such
an unresolved HPV-related infection problem
throughout the world that there are no therapeutic
HPV vaccines approved for use in humans.
Furthermore, the majority of nations having national
HPV inoculation programs are high or upper-middle-
income countries. There is also concern that
traditional expression techniques may result in more
expensive products, making them unavailable to be
utilized in the low-income countries with the most
significant rates. Thus, medications for managing,
controlling and eliminating existing HPV infections
and related diseases are earnestly needed.
3 NATURE IMMUNE RESPONSE
Cell-mediated immunity (CMI) is an effective
immune response to the genital HPV infections. The
innate immune system detects the damage via local
antigen-presenting cells (APCs), then the pro-
inflammatory cytokines and chemokines move to
part of the lymph nodes to uphold the viral antigen
processing. Activated APCs can stimulate CD4+ T
cells to either help activation of CD8+ T cells.
Effector T cells get response to the early proteins,
mainly E2, E6 and E7, which can attack the virus-
infected cells. Helper T cells that recognize L1 major
capid protein help induce neutralizing antibodies
(nAbs), thereby preventing virus spread and host
reinfection. However, the expressions of E6 and E7
proteins are depressed with HPV persistence. This
may arise from methylation of the E2 promoter but is
usually associated with viral integration that can
drive lesion growth.
4 VLP IMMUNOGENICITY
Virus-like particles (VLPs) are a more effective
technique to develop highly immunogenic
vaccinations. They combine safety, ease of
production, and the presense of both high-density B
cell epitopes and intracellular T cell epitopes to
produce potent humoral and cellular immune
responses. VLPs are similar to native HPV particles.
The VLPs are fully non-infectious and non-
oncogenic due to the lack of viral genes, forming a
structure that resembles the outer shell of the HPV
virus. They activate antibody synthesis in response to
infection and contain conformational epitopes that
contribute in the development of neutralizing
antibodies by limiting target cell uptake (Stanley et
al., 2010) (Dadar et al., 2018).
Antigens from HPV's capsid can trigger a range
of type-specific antibodies, the majority of which can
bind to the local virion, but not all of them can
eradicate the infection by preventing uptake by the
ICBB 2022 - International Conference on Biotechnology and Biomedicine
158
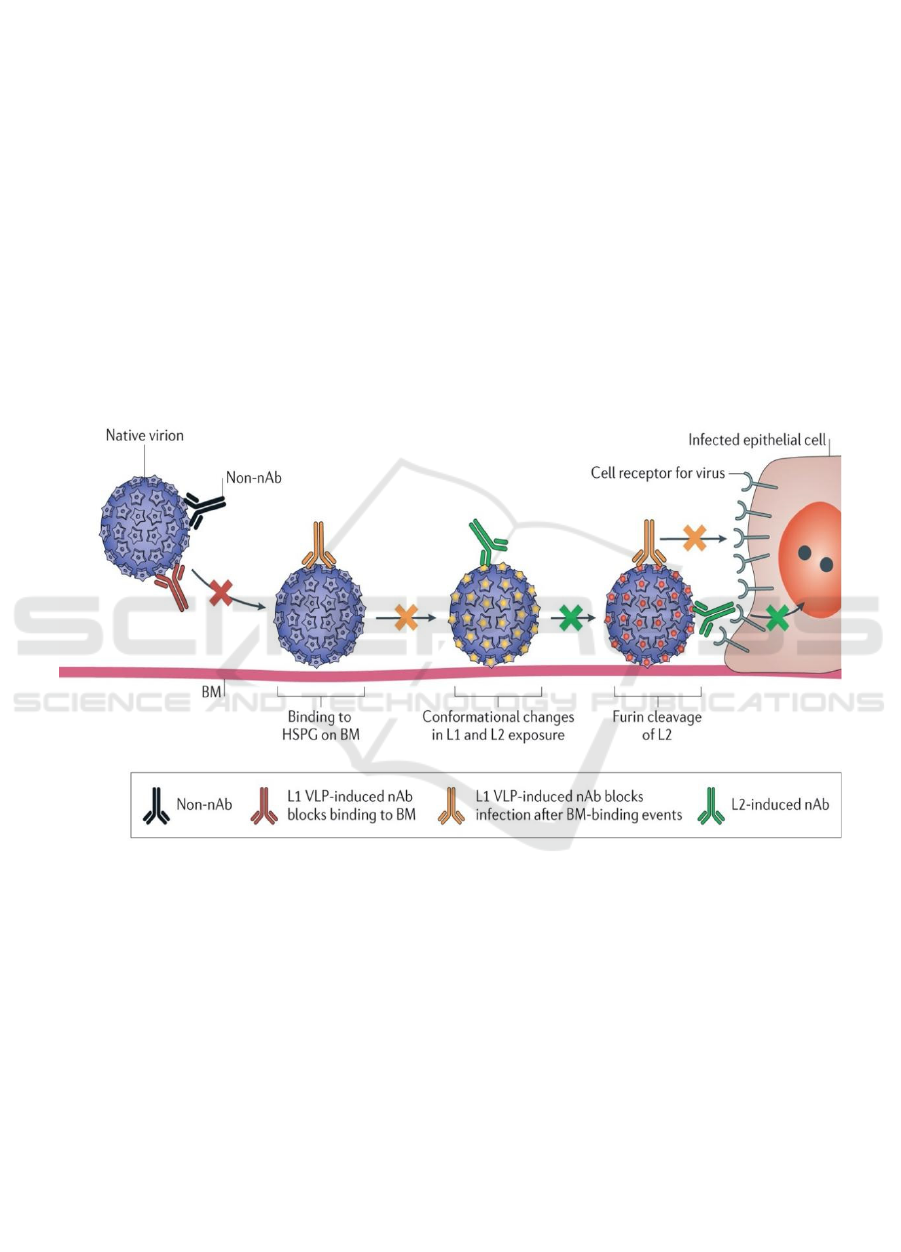
target cell. The non-neutralizing antibodies (non-
nAbs) are unable to directly affect the infectivity of
the virus. The nAbs generated by L1 VLP can,
however, prevent the binding to heparin sulfate
proteoglycans (HSPGs) on the basement membrane
(BM), but they cannot stop the infection completely.
After a conformational shift in the capsid and
cleavage of L2 by extracellular furin, the nAbs
generated by L2 vaccination can neutralize the virion
and deliver L2 defensive epitopes (Figure 3) (Roden
et al., 2018)
The quadrivalent vaccination comprises a
combination of four different VPLs obtained from the
major capsid protein L1 of HPV 6, 11, 16, and 18.
The nonavalent vaccine contains nine VPLs of
HPV6, 11, 16, 18, 31, 33, 45, 52, and 58. These
type specific L1 VLPs are synthesized by
recombinant expression of major capsid antigen L1
in the Saccharomyces cerevisiae (S. cerevisiae) yeast
with the amorphous aluminum hydroxyphosphate
sulfate (AAHS) adjuvant. In females and males aged
9 to 26 years, the quadrivalent HPV vaccination
protects against genital warts, precancerous or
dysplastic lesions, and cervical cancer. The
nonavalent HPV vaccine is prescribed to avoid
genital warts, precancerous lesions, cervical, vulvar,
vaginal, and anal cancer in females and males aged 9
to 45 years. The bivalent vaccine has been authorized
to prevent cervical and anogenital infection against
HPV16 and 18. It is produced in the baculovirus
expression vector system with the AS04 adjuvant
containing aluminum hydroxide and 3-deacylated
monophosphoryl lipid A (MPL) (Wang et al., 2020).
Figure 3: Antibody-mediated protection (Roden et al., 2018).
5 CLINICAL TRIALS
Several clinical investigations have been conducted
to demostrate the advantages of the vaccine. A total
of 14,000 girls aged 16 to 26 years old took part in a
Gardasil 9 HPV vaccination phase III clinical
research study. This study showed the significant
differences between the result of Gardasil that only 1
out of 6,016 females who received three doses of
Gardasil 9 acquired illnesses caused by HPV types
31, 33, 45, 52, and 58, contrasted with 30 out of 6,017
women who received three dosages of Gardasil.
Epidemiological research in Europe attempted to
quantify the advantages of the nonavalent
vaccination over the quadrivalent vaccine. According
to the findings, the nonavalent vaccination can
prevent 19% more cervical cancers than the
quadrivalent vaccine, and vaccine coverage for
precancerous lesions of the vagina, vulva, cervix, and
anus is expected to be 75% (Manini et al., 2018). The
result of a long-term bivalent HPV vaccine study that
involved 7466 women aged 18-25 years was
characterized histopathologically affirmed CIN2+ or
cervical intraepithelial neoplasia grade 3 or more
terrible related with HPV 16/18 cervical
contamination distinguished at colposcopy reference.
These vaccines have components that are
manufactured through biochemical synthesis. These
How HPV Vaccines with Various Compositions Work for Humans
159

components are part of the epitope of L1. Gardasil 9
is found to contain more virus-like particles that are
double the amount of the antigens and higher than a
double portion of aluminum hydroxide that is found
in Gardasil. The amount of concentration of virus-
like-particles in Gardasil 9 makes it more efficient in
protection against and treatment of HPV 16 and HPV
18 since it can effectively introduce inferior
responses of the antibodies as compared to Gardasil.
In addition, Cervarix contains antigens of a lower
concentration when compared with these other two
vaccine composition that is highly integrated and of
greater enhancement. These look similar to receptors
that inhibit antigens to stimulate and make an
improvement in the human immune responses and as
a result, long-lasting action of response by antibodies
is built up.
Table 1: Vaccine composition of a 0.5 ml dose of the HPV vaccine (Hildesheim et al., 2020).
Gardasil Gardasil9 Cervarix
Onco
g
enic
p
rotein subunit
HPV 16 41 60 21
HPV 18 22 41 20
HPV 31 22
HPV 33 20
HPV 45 23
HPV 52 23
HPV 58 23
Verrucouis
p
rotein subunit
HPV 6 22 30
HPV 11 40 41
Sodium Chloride 9.55 9.55 4.4
Sodium
b
orate 34 34
Sodium dih
y
dro
g
en
p
hos
p
hate dih
y
drate 0.625
Amorphous aluminum hydroxyphosphate sulfate 224 224
3-O-Desac
y
l-4′-mono
p
hos
p
hor
y
l li
p
i
d
(
MPL
)
A adsorbe
d
on 50
Aluminu
m
hydroxide 500
The trials that were conducted on women of ages
between 16 and 26 years and the findings showed for
the ones under 16 years, as shown by the standard of
WHO, this lack of superiority is acceptable and
especially at the endpoint. The individuals who were
over 26 years of age showed that the persistent of this
infection can be protected against for utmost 6
months given some specific vaccine and subjected to
a certain certified treatment and attention required
was found fit and accepted to be used in preventing
abnormal growth of cells in the female cervical areas.
The ability of the vaccine to protect against the
disease occurring in the vagina gives it the approval
to be used in the treatment and defined as16/18 HPV
and 2/3 HPV intraepithelial neoplasia.
There is not a clearly defined bare maximum level
regarding protection that antibody titers offer against
the cancer-causing and the disease itself in response
to these studies of how cervical cancer vaccines
define the endpoint of these VLPs. However, since
this HPV-causing cancer is found to be one of the
most dangerous and its treatment is limited, and is
also a long-term disease as shown by the studies. As
a result, they are used in the estimation of the
appropriate amount of time it would take to offer
protection to individuals of a certain age. Therefore,
there timing of a vaccine is very important and very
necessary since it determines the effect on the
antibody titers.
Giving three doses of the vaccine tends to be
considered a complete dose especially in women who
are between the age of 12 and 15 years. The
seropositivity that is realized in the response to the
titers of anti- HPV 16 is seen to be high after
vaccination for Cervarix and Gardasil vaccines
(Kumar et al., 2011). The long-term protection that is
expected for the specific vaccine is most of the time
is affected by the decrease in titers, especially the
Gardasil vaccine. Cervarix has higher retention for
seropositivity when compared with Gardasil and
therefore Cervarix shows an ability to bind serum and
response of the antibody for HPV 16 and HPV 18
than Gardasil. Both Gardasil and Gardasil 9 have
shown similar anti-A HPV 16 and 18 responses. In
terms of geometric mean numbers, Cervarix tends to
be superior when put in comparison with Gardasil.
When Gardasil 9 is administered in three doses, it
shows a similar reduction in geometric mean number
and an equal loss of seropositivity when taken for
protection against HPV 18 like Gardasil. However, in
22 months after giving the vaccine, a reduction of the
observable titers. In 1.4 years of vaccination, over
ICBB 2022 - International Conference on Biotechnology and Biomedicine
160

12% of female individuals showed no presence of
detectable titers while 255 of women could show the
absence of observable titers after 3 years of
vaccination and 35% women after 5 years.
After 24 months of administering the Gardasil 9
vaccine, 15% of women shoed to have lost the
detectable titers for HPV 45. The loss of these
antibody titers for HPV 45 is considerably larger than
the lowest percentage loss shown by the same
vaccine for HPV 16 type and shows direct protection.
The intervals that the doses of the vaccines are taken
are also termed to be very important and help in the
determination of the GMT.
Two doses of Gardasil 9 and Cervarix were given
at an interval of six months thereafter followed by a
booster for the adolescents between 9 and 10 years of
age showed that hat Gardasil maintained a lower
amount of HPV 16 and 18 titers than for the dose of
Cervarix in one month of vaccination.
Three doses of Gardasil when given to pre-
adolescent girls tend to show an absence of
antibodies for different HPV types different from
those administered with Cervarix. A random test was
conducted for three doses of Gardasil 9 that showed
a higher number of HPV titers which was higher than
those administered with Gardasil 9 (Arbyn et al.,
2018). Both Gardasil and Cervarix have similar
antibody titers when administered at an interval of six
months and only two doses given. Women who are
above the age of 25 have inferior antibodies unlike
those who are below 18 years.
Vaccines need to be examined and their safety
determined and create an assurance that they will
provide the maximum protection to the recipients
against the different types of HPVs (Pennella et al.,
2020). Administering the most efficient of these
vaccines to women enhances protection against pre-
cancer infections. Surveillance that was carried out
by the Vaccine Safety Datalink depicted that these
three vaccines are safe for use by the recipients and
offer the most effective protection against these types
of HPV infections. Before any vaccine is licensed and
is accepted to be used, its safety is evaluated. In am
examination to determine how safe these vaccines
are, a similarity in the safety image of Gardasil and
Gardasil 9 which was the earlier version of Gardasil
9. However, the latter was found out to show more
side effects than Gardasil which however disappear
away after some time.
Through the program for vaccination, the
government has attained a significant decrease in the
occurrence of the various HPV causing disease types
that lead to the development of cervical cancer. An
approximately 50% in the go down of appearance of
abnormalities to teenage girls whose age is below 18
years. Through this, society is able to create
assurance to both men and women that vaccination is
important to their health and help them get away from
this dangerous disease. This also helps them to avoid
the expensive treatment of these long-term diseases
by just prevention them by taking a vaccination.
Since cancer is one of the fatal and dangerous and is
becoming a threat in society today by the number of
deaths it contributes to. Due to this, society today
together with the government has enabled individuals
to receive special attention and experienced medical
care by enabling the introduction and inputting the
required equipment for treatment and screening of
cancer cells to help determine early detection and
early treatment and later these have led to the
achievement of a healthy society (Kumar et al.,
2011).
6 FUTURE AND GOALS
The vaccination programs today are working in hands
with the various respective governments to give more
quality care to the individuals who are at a high risk
of contracting the cancer disease. It, therefore, aims
to achieve an educated society with people who are
aware of the danger of these diseases and are aware
of the importance of seeking early checkups and
treatment. Through this, a healthy society and the
number of deaths from this disease is achievable
7 CONCLUSION
In conclusion, cancer vaccines have been realized to
be a very important aspect in both the health sector
and even in the society at large. This is because they
enabled the finding of the solution to one of the
threats to individuals in the world today. Scientists
and research centers through the World Health
Organisation put these vaccines into place to help in
the fight to eradicate this deadly disease. Though the
cure has not yet been found, protection through these
vaccines saves a great deal. Even though these
vaccines bring different side effects to the body and
tend to react with different types of bodies of diverse
ages. More research enables determine which is the
most effective vaccine to administer to what age and
gender will work with their bodies without causing
diverse effects and abnormalities.
How HPV Vaccines with Various Compositions Work for Humans
161

REFERENCES
Arbyn, M., & Xu, L. (2018). Efficacy and safety of
prophylactic HPV vaccines. A Cochrane review of
randomized trials. Expert review of vaccines, 17(12),
1085–1091.
Cutts, F. T., Franceschi, S., Goldie, S., Castellsague, X., de
Sanjose, S., Garnett, G., Edmunds, W. J., Claeys, P.,
Goldenthal, K. L., Harper, D. M., & Markowitz, L.
(2007). Human papillomavirus and HPV vaccines: a
review. Bulletin of the World Health Organization,
85(9), 719–726.
Dadar, M., Chakraborty, S., Dhama, K., Prasad, M.,
Khandia, R., Hassan, S., Munjal, A., Tiwari, R.,
Karthik, K., Kumar, D., Iqbal, H., & Chaicumpa, W.
(2018). Advances in Designing and Developing
Vaccines, Drugs, and Therapeutic Approaches to
Counter Human Papilloma Virus. Frontiers in
immunology, 9, 2478.
Ferreira, A. R., Ramalho, A. C., Marques, M., & Ribeiro,
D. (2020). The Interplay between Antiviral Signalling
and Carcinogenesis in Human Papillomavirus
Infections. Cancers, 12(3), 646.
Hildesheim, A., Wagner, S., Boland, J., Lowy, D. R.,
Schiller, J. T., Schiffman, M., Schussler, J., Gail, M.
H., Quint, W., Ocampo, R., Morales, J., Rodríguez, A.
C., Hu, S., Sampson, J. N., … Costa Rica Vaccine Trial
Group (2020). Efficacy of the bivalent HPV vaccine
against HPV 16/18-associated precancer: long-term
follow-up results from the Costa Rica Vaccine Trial.
The Lancet. Oncology, 21(12), 1643–1652.
Kumar, V. M., & Whynes, D. K. (2011). Explaining
variation in the uptake of HPV vaccination in England.
BMC public health, 11, 172.
Mariani, L., Preti, M., Cristoforoni, P., Stigliano, C. M., &
Perino, A. (2017). Overview of the benefits and
potential issues of the nonavalent HPV vaccine.
International journal of gynaecology and obstetrics: the
official organ of the International Federation of
Gynaecology and Obstetrics, 136(3), 258–265.
Mannan S. (2016). Cancer vaccine clinical trials.
Immunotherapy, 8(11), 1263–1264.
Manini, I., & Montomoli, E. (2018). Epidemiology and
prevention of Human Papillomavirus. Annali di igiene :
medicina preventiva e di comunita, 30(4 Supple 1), 28–
32.
Okuhara, T., Okada, H., Goto, E., & Kiuchi, T. (2021).
Readability Assessment of HPV Vaccination and
Cervical Cancer Information: A Systematic Scoping
Review. Healthcare (Basel, Switzerland), 9(10), 1246.
Pennella, R. A., Ayers, K. A., & Brandt, H. M. (2020).
Understanding How Adolescents Think about the HPV
Vaccine. Vaccines, 8(4), 693.
Roden, R., & Stern, P. L. (2018). Opportunities and
challenges for human papillomavirus vaccination in
cancer. Nature reviews. Cancer, 18(4), 240–254.
Stanley, M. HPV - immune response to infection and
vaccination. Infect Agents Cancer 5, 19 (2010).
Thomas T. L. (2016). Cancer Prevention: HPV
Vaccination. Seminars in oncology nursing, 32(3),
273–280.
Wang, R., Pan, W., Jin, L., Huang, W., Li, Y., Wu, D., Gao,
C., Ma, D., & Liao, S. (2020). Human papillomavirus
vaccine against cervical cancer: Opportunity and
challenge. Cancer letters, 471, 88–102.
Wang, X., Huang, X., & Zhang, Y. (2018). Involvement of
Human Papillomaviruses in Cervical Cancer. Frontiers
in microbiology, 9, 2896.
ICBB 2022 - International Conference on Biotechnology and Biomedicine
162
