
Effects of Silver Nanoparticles on DNA Damage in Gills of the
Ruditapes Philippinarum
Wenxia Liu
*
and Chenge Liu
*
College of Environmental Science and Engineering, Ocean University of China, Qingdao 266100, China
*
lcg1110@foxmail.com
Keywords: Silver Nanoparticles, Ruditapes Philippinarum, DNA Damage.
Abstract: With the rapid development of nanotechnology, a certain number of nanoparticles will inevitably be released
into the marine ecosystem. In this work, the effects of silver nanoparticles (Ag NPs) on DNA damage of
marine bivalve Ruditapes Philippinarum were evaluated. The clams were exposed to 0, 10 and 40 μg/L of Ag
NPs for 14 days respectively, and samples are performed at the 0, 3, 7, 14 days. The results showed that that
Ag NPs were considered to cause genotoxic effect on clam gills, and induced a time-dependent increase of
DNA damage. The gills are more sensitive to high Ag NPs concentration. The genotoxicity developed in a
dose- and time-dependent manner.
1 INTRODUCTION
Silver nanoparticles (Ag NPs) are the most
commonly used due to their unique physico-chemical
properties, such as electrical and thermal
conductivity, catalytic activity, and antibacterial
activity (Chernousova, 2013; McGillicuddy, 2017).
This fast expansion will inevitably drive the release
of nanoparticles into marine ecosystems. It is
estimated that the concentration of Ag NPs in water
is in the range of ng/L, and in the mg/kg range in the
soil and sediment (Blaser, 2008). Because aquatic
organisms are in constant contact with pollutants
through swallowing, gill entry, skin absorption, etc.,
they are more susceptible to the toxic effects of
nanoparticles than terrestrial organisms (Moore,
2006). A marine mesoderm study showed that Ag
NPs induced DNA damage and oxidative damage to
Scrobicularia plana (Buffet, 2014). Ag NPs not only
have antibacterial effects, but also have ROS-derived
oxidative stress, biofilm damage and DNA damage
(Zuykov, 2011). Therefore, the toxic effects and
mechanisms of Ag NPs on aquatic organisms have
attracted more and more attention to assess their
impact on the ecological environment and human
health.
As the most important component of cells, DNA
is critical in maintaining cell homeostasis and genetic
information transmission, and impact analysis of
aquatic DNA has proven to be a very suitable method
for assessing the genotoxicity of environmental
pollutants, enabling the detection of toxic effects of
low concentrations of pollutants in a variety of
species. Comet experiments have been applied in
previous studies to study levels of DNA damage in
marine and freshwater bivalves exposed to pollutants
(Dhawan, 2009). Comet experiments, also known as
single-cell gel electrophoresis assays (SCGE), are a
fast and sensitive technique that requires only a small
number of cells to provide information on DNA
damage and repair (Collins, 2008). Single-cell
samples are fused in a low melting point agarose gel
for lysis, followed by electrophoresis under alkaline
conditions, and after the end of the electrophoresis, it
can be observed under a fluorescence microscope that
the stained intact DNA fragments can only remain in
situ due to large molecular weights and are spherical
in shape, while smaller fragments of broken DNA
migrate to the positive electrode, forming a comet
shape (Canesi, 2012).
Ruditapes Philippinarum is often used as a
sentinel species in ecotoxicological studies due to
their ability to filter large volumes of water, leading
to contaminant (Faggio, 2018). Study found bivalve
in its organization (mainly the gills and digestive
gland) accumulation of metals and other pollutants,
and that direct contact between the gills and the
external environment can cause more serious damage
(Tice, 2000). The gills can be used as the most
Liu, W. and Liu, C.
Effects of Silver Nanoparticles on DNA Damage in Gills of the Ruditapes Philippinarum.
DOI: 10.5220/0012015300003633
In Proceedings of the 4th International Conference on Biotechnology and Biomedicine (ICBB 2022), pages 163-166
ISBN: 978-989-758-637-8
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
163

sensitive tissue sites for detecting genotoxicity, and
DNA damage in gill tissues can be measured by
comet experiments to be able to assess the potential
effects of Ag NPs on the genetic aspects of clams.
Therefore, the aim of this study was to expose
Ruditapes Philippinarum to different concentrations
of Ag NPs, identify the toxic effects of Ag NPs, and
assess the effect from the perspective of genotoxicity
by determining the degree of DNA damage in the
gills of clams.
2 MATERIAL AND METHODS
2.1 Preparation of Silver Nanoparticles
Ag NPs (purity>99.7%) were purchased from Sigma-
Aldrich with the particle size specified as <100 nm, a
stock solution of 50 mg/L was prepared in ultrapure
water, sonicated for 1 h and kept in constant shaking
to breakdown particles aggregates before adding to
the exposure tanks.
2.2 Laboratory Assay
The clams, Ruditapes Philippinarum were purchased
from Zhangcun Seafood Market in Qingdao and
domesticated for 3 days under laboratory conditions
(pH=8.1; Temperature=16.3±0.5 ℃; Salinity=31;
Dissolved Oxygen=8.3). The clams with no damage,
sensitive response and similar size were divided into
three groups: control and 10 μg/L or 40 μg/L of Ag
NPs. Set three parallel for each group, and each group
has a volume of 5 L seawater. During the experiment,
the clams were not fed satisfactorily and the test
water was changed every 24 h. On the 0, 3,7, 14 days
of exposure, clams (n=2) were randomly selected
from each group and dissected immediately, cut it
with scissors to make the tissue as small as possible,
add 3 mL trypsin, mix well, pipette the cell
suspension in a water bath at 37 °C to resolve the
tissue cells into a single cell suspension. Centrifuge
at 500 r·min
-1
and take the supernatant. Then
centrifuge at 1500 r·min
-1
, remove the supernatant,
and add 1 mL of PBS (0.1 mol/L) to the centrifuge
tube, a single-cell suspension can be obtained,
followed by single-cell electrophoresis experiments.
All of the above experimental steps are performed
under dark conditions to prevent UV rays from
affecting the results. Determination of clam gill cell
DNA Olive tail moment (OTM) by CASP comet
image analysis software, OTM = (Tail Center of
Gravity Position - Head Center of Gravity Position)
× tail DNA content.
2.3 Statistical Analysis
Data of DNA damage was shown as mean ± standard
of deviation. Significant differences between
exposure groups were detected using one-way
analysis of variance (ANOVA) and only P<0.05 was
accepted as significant. Then Origin 9.2 was used for
plotting.
3 TEST RESULTS AND
DISCUSSIONS
Fig. 1 shows that the control group with a normal
nucleus with a complete head and no migration of the
tail. On the 3 days, the nucleus DNA of clam gills
showed that a gradual increase in tail length and
migration in parallel with a slight decrease in head
size, indicating the beginning of DNA damage. On
the 7 days, it can be seen from these figures that more
DNA-strand breaks of clam gills, the length of the tail
gradually increased, and the fluorescence intensity of
the tail gradually increased, indicating that DNA
damage increases with the increase of dose and
duration of Ag NPs exposure. The highest doses (40
μg/L) of Ag NPs showed a statistically significant
increase in tail intensity compared with the control
group on the 14 days. The results of the genotoxic
effect of Ag NPs varies with the doses and duration
of exposure.
ICBB 2022 - International Conference on Biotechnology and Biomedicine
164
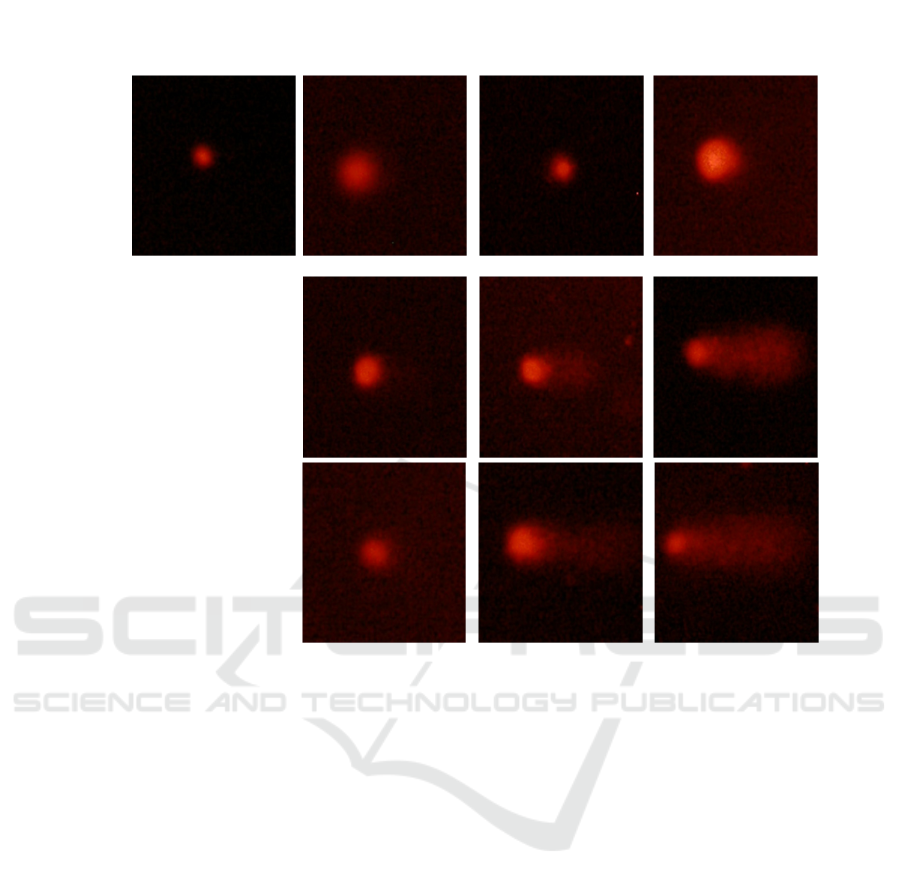
Figure 1: Fluorescence photomicrograph of in gill of clam Ruditapes philippinarum exposed to Ag NPs.
As can be seen from Figure 2, the OTM value in
the gills was elevated at the 3 days, but there was no
significant difference compared with the control
group. On the 7th day of the experiment, the OTM
value of each treatment group was significantly
increased (P<0.05), and the injury was most
significant in the treatment group with a
concentration of 40 μg/L, and the damage intensity
increased with the duration of exposed, and the
treatment group reached the maximum value on the
14th day. The above results show that the DNA
damage of clam gills is gradually more severe with
the increase doses and duration of exposed. The
genotoxicity of zebrafish (Danio rerio) exposed to
nTiO
2
(1 and 10 μg/L) for 14, 21 and 28 days was
assessed using RAPD-PCR technology, the genomic
stability decreased by 37% after 14 days of exposure
and increased with duration of exposed, and the
highest genotoxic effect was observed at the
maximum concentration of nTiO
2
(10 μg/L) after
exposure 21 days (Rocco, 2015).
The specific mechanism of genotoxicity of
nanoparticles to clams cells is unclear, but one study
suggests that one possibility is due to their small size
(1–100 nm), Ag NPs can penetrate the nucleus
through the nuclear pores, and the Ag NPs are highly
reactive and surface-charged, interacting directly
with DNA or nuclear proteins (Joubert, 2013).
Another possibility is the release of metal ions within
the cell to induce the production of excess reactive
oxygen species, and ROS reacts with DNA molecules
to cause damage to purine and pyrimidine bases, as
well as the DNA backbone, leading to DNA damage
(Rocha, 2014). This indirect damage caused by ROS
is the main pathway to DNA damage and can lead to
physiological damage, including damage to the
reproductive system, inhibition of growth, and
damage to various organelles such as lysosomals and
mitochondria (Gomes, 2013).
10 μg/L
40 μg/L
0
3d
7d
14d
0d
Effects of Silver Nanoparticles on DNA Damage in Gills of the Ruditapes Philippinarum
165

Figure 2: Changes of DNA damage (OTM) in gill of clam Ruditapes philippinarum exposed to Ag NPs.
4 CONCLUSION
In this study, the effects of DNA damage in clams
exposed to Ag NPs were analyzed by comet
experiments, it can be concluded that Ag NPs were
considered to cause genotoxic effect on clam gills,
and induced a time-dependent increase of DNA
damage. The gills are more sensitive to high Ag NPs
concentration. The genotoxicity developed in a dose-
and time-dependent manner.
REFERENCES
Blaser S A, Scheringer M, MacLeod M, et al. (2008)
Estimation of cumulative aquatic exposure and risk due
to silver: Contribution of nano-functionalized plastics
and textiles. J. Science of The Total Environment,
390(2): 396-409.
Buffet P E, Zalouk-Vergnoux A, Châtel A, et al. (2014) A
marine mesocosm study on the environmental fate of
silver nanoparticles and toxicity effects on two
endobenthic species: the ragworm Hediste diversicolor
and the bivalve mollusc Scrobicularia plana. J. Science
of the Total Environment, 470: 1151-1159.
Canesi L, Ciacci C, Fabbri R, et al. (2012) Bivalve molluscs
as a unique target group for nanoparticle toxicity. J.
Marine Environmental Research, 76:16-21.
Chernousova S, Epple M. (2013) Silver as antibacterial
agent: ion, nanoparticle, and metal. J. Angewandte
Chemie International Edition, 52(6): 1636-1653.
Collins A R, Oscoz A A, Brunborg G, et al. (2008) The
comet assay: topical issues. J. Mutagenesis, 23(3): 143-
151.
Dhawan A, Bajpayee M, Parmar D. (2009) Comet assay: a
reliable tool for the assessment of DNA damage in
different models. J. Cell biology and toxicology, 25(1):
5-32.
Faggio C, Tsarpali V, Dailianis S. (2018) Mussel digestive
gland as a model tissue for assessing xenobiotics: An
overview. J. Science of The Total Environment,
636:220-229.
Gomes T, Araújo O, Pereira R, et al. (2013) Genotoxicity
of copper oxide and silver nanoparticles in the mussel
Mytilus galloprovincialis. J. Marine Environmental
Research, 84:51-59.
Joubert Y, Pan J F, Buffet P E, et al. (2013) Subcellular
localization of gold nanoparticles in the estuarine
bivalve Scrobicularia plana after exposure through the
water. J. Gold Bulletin, 46(1): 47-56.
McGillicuddy E, Murray I, Kavanagh S, et al. (2017) Silver
nanoparticles in the environment: Sources, detection
and ecotoxicology. J. Science of The Total
Environment, 575:231-246.
Moore M N. (2006) Do nanoparticles present
ecotoxicological risks for the health of the aquatic
environment?. J. Environment International, 32(8):
967-976.
Rocco L, Santonastaso M, Mottola F, et al. (2015)
Genotoxicity assessment of TiO
2
nanoparticles in the
teleost Danio rerio. J. Ecotoxicology and
environmental safety, 113: 223-230.
Rocha T L, Gomes T, Cardoso C, et al. (2014)
Immunocytotoxicity, cytogenotoxicity and
genotoxicity of cadmium-based quantum dots in the
marine mussel Mytilus galloprovincialis. J. Marine
Environmental Research, 101:29-37.
Tice R R, Agurell E, Anderson D, et al. (2000) Single cell
gel/comet assay: Guidelines for in vitro and in vivo
genetic toxicology testing. J. Environmental and
Molecular Mutagenesis, 35(3): 206-221.
Zuykov M, Pelletier E, Demers S. (2011) Colloidal
complexed silver and silver nanoparticles in
extrapallial fluid of Mytilus edulis. J. Marine
environmental research,71(1): 17-21.
03714
0
10
20
30
40
50
60
b
b
c
b
a
a
a
a
a
OTM
Exposure time/da
y
Control
AgNPs-10μg·L
−1
AgNPs-40μg·L
−1
ICBB 2022 - International Conference on Biotechnology and Biomedicine
166
